Abstract
Purpose: This study aims to evaluate the effects of Capacitive and Resistive electric transfer (CRet) and hotpack (HP) on haemoglobin saturation and tissue temperature.
Materials and methods: The participants were 13 healthy males (mean age 24.5 ± 3.0). They underwent three interventions on different days: (1) CRet (CRet group), (2) HP (HP group) and (3) CRet without power (sham group). The intervention and measurement were applied at the lower paraspinal muscle. Indiba® active ProRecovery HCR902 was used in the CRet group, and the moist heat method was used in the HP group. Oxygenated, deoxygenated and total haemoglobin (oxy-Hb, deoxy-Hb, total-Hb) counts were measured before and after the 15-min interventions, together with the temperature at the skin surface, and at depths of 10 mm and 20 mm (ST, 10mmDT and 20mmDT, respectively). The haemoglobin saturation and tissue temperature were measured until 30 min after the intervention and were collected at 5-min intervals. Statistical analysis was performed for each index by using the Mann–Whitney U test for comparisons between all groups at each time point.
Results: Total-Hb and oxy-Hb were significantly higher in the CRet group than in the HP group continuously for 30 min after the intervention. The 10mmDT and 20mmDT were significantly higher in the CRet group than in the HP group from 10- to 30 min after intervention.
Conclusions: The effect on haemoglobin saturation was higher in the CRet group than in the HP group. In addition, the CRet intervention warmed deep tissue more effectively than HP intervention.
Introduction
Thermotherapy is widely used as a component of physical therapy in clinical practice. Thermal stimulation to human body causes vasodilation and improves blood circulation [Citation1–4]. In particular, thermotherapy has a number of beneficial effects in the treatment of musculoskeletal disorders such as pain and tissue injury (muscle, tendon and ligament) [Citation1–4]. Therefore, it is recognised as a major physical therapeutic method. Thermotherapy is usually classified into superficial and deep thermotherapy. In general, superficial thermotherapy is referred that which causes vasodilation and increases temperature only in the skin and superficial tissues; on the other hand, deep thermotherapy causes vasodilation and increases temperature of deep tissues [Citation5].
As a method of superficial thermotherapy, hotpack (HP) is used more frequently because of its convenience, low cost and safety than other devices such as paraffin bath, fluid therapy, and infra-red therapy [Citation6,Citation7]. Deep thermotherapy devices such as ultrasound and diathermy can improve haemoglobin saturation and increase the temperature of deep tissues more than superficial thermotherapy [Citation8–11]; however, these devices are infrequently used due to some demerits. Ultrasound energy concentrates around bony tissue and thus, ultrasound therapy has the risk of periosteal inflammation [Citation5,Citation12]. In addition, ultrasound therapy covers a relatively small area, because its effect reportedly reaches only twice as much as the irradiation area of the transducer. As most diathermy devices with frequencies of 8–14 MHz produce excessive heat during treatment, which is enough to cause a skin burn, a protective device, called a polus, must be kept between the skin and electrode [Citation13,Citation14]. Thus, these devices are used infrequently in clinical practice.
Recently, a system of Capacitive and Resistive electric transfer (CRet), which is one of the methods used in diathermy, was developed as a form of deep thermotherapy [Citation15]. This device delivers radiofrequency (RF) energy, which passes between active electrode and inactive electrode, and generates heat in the human body [Citation16,Citation17]. CRet does not require a polus and surface-cooling system because it uses 448 kHz, which is lower than that used in conservative diathermy, and does not cause excessive heat generation between the skin and the electrode; thus, this device is safer than other diathermy devices. In addition, earlier studies have revealed the clinical efficacy in the treatment of musculoskeletal disorders such as pain and tissue injury [Citation16,Citation17]. This is assumed to be caused by improvement in haemoglobin saturation and increased temperature; however, while previous studies revealed the change of tissue temperature, they did not reveal direct physiological effect of changes in haemoglobin saturation by the CRet intervention [Citation18]. Additionally, the different effects of CRet and HP as superficial thermotherapy on haemoglobin saturation and tissue temperature change are not known. Therefore, this study aimed to evaluate the effects of CRet and HP on haemoglobin saturation and tissue temperature.
Materials and methods
Participants
Thirteen healthy males participated in this study (mean ± SD, age 24.5 ± 3.0 years, height 172.3 ± 2.9 cm, weight 65.8 ± 6.0 kg). Participants were non-athletes and had not currently performed any excessive exercise. Participants with a history of orthopaedic or nervous system disease in their lower back were excluded. Written informed consent was obtained from each participant in accordance with the guidelines approved by the Kyoto University Graduate School of Medicine and the Declaration of Human Rights, Helsinki, 1975. The study protocol was approved by the Ethical Committee of the Kyoto University Graduate School of Medicine (C1150).
We calculated the sample size needed for two-way analysis of variance (ANOVA) with repeated measures (effect size = 0.40, α error = 0.05, power = 0.8) using G* power 3.1 software (Heinrich Heine University, Dusseldorf, Germany). The result showed that 13 subjects were required.
Apparatus
CRet intervention
Indiba® activeProRecovery HCR902 (Indiba S. A., Barcelona, Spain) was used for CRet intervention. This device operates at a frequency of 448 kHz. Rigid circular metallic electrode with a diameter of 65 mm was used as active electrode and a large flexible rectangular metallic plate (measuring 200 × 260 mm) was used as the inactive electrode. It delivered radiofrequency (RF) energy in two modes at active electrode: capacitive (CAP) and resistive (RES). The CAP electrode has a polyamide coating that acts as a dielectric medium, insulating its metallic body from the skin surface, thus it generated heat near the skin externally. The RES electrode is uncoated and RF energy travels directly through the body into the inactive electrode; thus, it generates heat in the deeper parts of the body. There are several contraindications of CRet intervention (e.g. pregnancy, deep vein thrombosis, hypoesthesia, damaged skin and implanted pacemaker).
Measurement
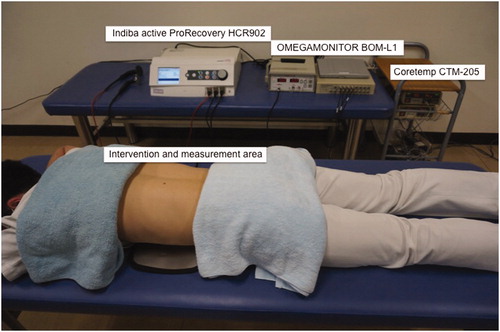
Haemoglobin saturation was measured using an OMEGAMONITOR BOM-L1 TR W (Omegawave Co., Ltd., Tokyo, Japan). Two photo-detectors of this device absorb three wavelengths of light (780, 810, 830 nm) from a light source. The absorbance is analysed using a modified version of the Lambert–Beer law, yielding a Hb content under the photo-detectors. By using these three wavelengths, it is possible to differentiate between oxygenated and deoxygenated haemoglobin (oxy-Hb and deoxy-Hb). The sum of oxy-Hb and deoxy-Hb reflects total amount of haemoglobin (total-Hb), which is related to the blood volume within the tissues. The light source and detectors were covered with a black rubber shield and affixed to the skin over the centre of the elector spinae muscle belly on the right side at L2–3. The distances between the light source and the two detectors were 15 and 30 mm; the light source was placed at the cranial end, while the detectors were placed at the caudal end. The distance was selected based on a previous study that showed the maximum measuring depth for the tissue blood volume and its oxygenation of the intra-soft tissue were approximately 15 and 30 mm from the surface of the skin when the source–detector separation was 15 and 30 mm, respectively [Citation19]. A differential calculation in the measurements obtained from the two detectors demonstrated that the subcutaneous fatty tissue had little influence on the measurements of the intramuscular haemodynamics. The instruments used in this study apply the differences in values from two detectors, because the data obtained from only one detector are probably not accurate due to the influence of superficial tissue.
Tissue temperatures were measured using a Coretemp CTM-205 (Terumo Co., Ltd., Tokyo, Japan). This instrument is an electronic thermometer for non-invasive measurement of body temperature. We measured temperatures at three depths (skin surface, 10 mm and 20 mm below the skin surface) each simultaneously. Skin superficial temperature (ST) was measured by a thermistor probe PD-K161, which measures body temperature by detecting electrical resistance, using a thermistor built into the probe. In addition, deep temperatures (10mmDT and 20mmDT) were measured by a temperature-compensated probe, PD-11 and PD-51, that enables measurement of the temperature 10 mm and 20 mm below the skin surface, respectively, using a zero-heat-flow method. The measurement accuracy according to the instrument manual was as follows: ± <0.1 °C at 30–40 °C, ± <0.2 °C at 10–30 °C or 40–45 °C and ± <0.5 °C at 0–10 °C or <45 °C.
Experimental procedure
The participants underwent each intervention for 15-min duration: (1) CRet (CRet group), (2) hotpack (HP group) and (3) CRet without power (sham group). These interventions were applied by a skilled physical therapist. The intervention order was randomised, and each condition was tested more than 24 h apart. On the day of the experiment, participants were instructed not to consume alcohol, avoid smoking and performing any intense activities for at least 24 h before experiment. The experiment was conducted in a controlled environment with the temperature maintained between 24 °C and 26 °C. The intervention and measurement were applied at the lower paraspinal muscle, therefore the participants were asked to lie down in a prone position on a bed to receive intervention and measurement ().
In CRet group, the participants were applied total of 15-min intervention (5 min in CAP and 10 min RES). We selected the intervention time according to the previous study in which lumbar and knee musculoskeletal injuries were treated [Citation20]. A manufacturer-supplied conductive cream was employed as a coupling medium between the electrode and the skin surface during the intervention. The inactive electrode was placed on the skin of epigastric area. The intensity was defined as 6 or 7 on a subjective 11-point analogue scale that the participants used to self-report thermal sensing (0, no thermal sensing; 10, worst possible thermal sensing) according to the manufacturer’s recommended safety interventions. Conductive cream was removed after the thermal intervention. In sham group, CRet was unpowered and was performed using the same method and conditions as those in the CRet intervention. In HP group, moist heat method of hotpack was applied for 15 min. Hot pack (S – PACK CLS-12: SAKAI medical Co., Ltd., Tokyo, Japan) was heated to 80 °C in a hydro-collator tank (PACKWARMER CL – 15: SAKAI Medical Co., Ltd., Tokyo, Japan) and wrapped in eight layers with towels.
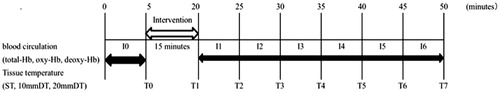
Haemoglobin saturation and tissue temperature were measured before 5 min and 30 min after the intervention (). The haemoglobin saturation data were divided into 5-min intervals, and analysed by averaging each 5-min interval (I0: before the intervention, I1–I6: after the intervention). Tissue temperature data were analysed and compared between the groups measured at 5-min intervals (T0: before the intervention, T1–T7: after the intervention).
Figure 2. Experimental procedure. Haemoglobin saturation and tissue temperature were measured during 5 min before the intervention (I0, T0). Then, the interventions were performed for 15 min. After the intervention, haemoglobin saturation continuously measured for 30 min, and it was divided into six intervals; from I1 to I6. Tissue temperatures were measured seven times (T2–T7) after the intervention every 5 min.
Statistical analysis
Statistical analysis was performed for each index by using the Mann–Whitney U test for comparisons between all groups at each time point. Statistical analyses were performed using the SPSS version 20.0 (IBM Corp., Armonk, NY), with a significance threshold of 0.016 for the comparison of the three groups.
Results
Haemoglobin saturation in each group ()
The analysis revealed that total-Hb and oxy-Hb were significantly higher in the CRet and HP groups than in the sham group from I1 to I6 (p < 0.016), moreover, these were significantly higher in the CRet group than in the HP group from I1 to I6 (p < 0.016).
Table 1. Haemoglobin saturation in each group.
Tissue temperature in each group ()
The analysis revealed that ST, 10mmDT and 20mmDT were significantly higher in the CRet and HP groups than in the sham groups from T1 to T7 (p < 0.016). As for 10mmDT and 20mmDT, the difference was significantly higher in the CRet group than in the HP group after intervention (10 mmDT: T2 to T7 p < 0.016, 20mmDT: T1 to T7 p < 0.016).
Table 2. Tissue temperature in each group.
Discussion
In this study, we investigated the effects of CRet and HP on haemoglobin saturation and tissue temperature. The results showed that total-Hb and oxy-Hb were significantly higher after the intervention in the CRet and HP group than in the sham group. In addition, these were significantly higher in the CRet group than in the HP group. As for tissue temperatures: ST, 10mmDT and 20mmDT were significantly higher in the CRet and HP groups than in the sham group. Moreover 10mmDT and 20mmDT were almost significantly higher in the CRet group than in the HP group.
The present study revealed that the total-Hb and oxy-Hb were significantly higher during 30 min after the intervention in the CRet and HP groups than in the sham group. The oxy-Hb, which is a component of total-Hb, increased in the CRet and HP group, whereas no change was detected in deoxy-Hb. This suggested that the change of total-Hb was due to the increase in oxy-Hb, and haemoglobin saturation improved after thermal intervention. The depth-resolved assessment of total haemoglobin and haemoglobin saturation could be acquired by varying the distance between the probes. In this study, the 15-mm penetration depth was thought to reflect the status of the fat and fascia of the subcutaneous layer, while the 30-mm depth was thought to reflect the status of the muscle layer. Thus, the change in total-Hb and oxy-Hb in this study was used to define muscle condition. Some previous studies demonstrated that the blood circulation and haemoglobin saturation improved by thermotherapy. The mechanisms of thermal effects improving blood circulation and haemoglobin saturation and can be attributed to the relaxation of vascular smooth muscles via skin temperature receptors, suppression of the sympathetic nervous system through indirect activation of local spinal reflexes, and increases in the local release of inflammatory vasoactive compounds such as prostaglandin and histamine, and the compound effect would result in vasodilation and increase in blood flow [Citation21–24]. Additionally, Moon reported that heat activates HIF-1 via ERK-NADPH oxidase-ROS pathways. Up-regulation of HIF-1 target genes results in increased tumour perfusion/vascularisation and partially decreased oxygen consumption, which results in decreased hypoxia in tumors [Citation25]. The reaction might be different between muscle and tumour tissues, but tissue oxygenation in non-tumoral tissues may improve through a similar mechanism.
Moreover, the total-Hb and oxy-Hb were significantly higher after the intervention in the CRet group than in the HP group; thus, CRet had better efficacy in improving haemoglobin saturation than HP. We assume that this phenomenon was caused by increasing temperature of the deep tissue. The results of this study showed no significant difference in ST between CRet group and HP group; however, 10mmDT and 20mmDT were significantly higher after the intervention in the CRet group than in the HP group. Earlier, a study reported that there was a 0.7 °C increase at the 30 mm below the skin superficial level after HP intervention [Citation8]. In this study, the mean increments in temperatures in this study were 3.6 °C and 3.0 °C at 20 mm below the superficial skin immediately after CRet and HP interventions, respectively. Accordingly, the temperature deeper than 20 mm below the skin surface is also presumed to be higher in CRet group than in the HP group. Previous studies revealed that shortwave and microwave diathermy provides deep thermotherapy, which is similar to that provided by CRet, and increases blood flow in the muscle [Citation26,Citation27]. In addition, the temperatures decreased faster in the HP group than in the CRet group in our study. Although heat was lost by vaporisation in both the CRet and HP groups, the temperature was kept relatively higher by heat that was gradually conducted from deeper tissue towards the surface layer in the CRet group. Thus, we concluded that CRet intervention warmed deeper tissue more than HP intervention, and improved haemoglobin saturation.
In contrast, most of the published research in this area has been conducted on laboratory animals such as rats and dogs. These small mammals have different physical characteristics from humans, with limited physiological heat-loss mechanisms. For example, rats have a higher thermoneutral zone of around 30 °C, which for humans is equivalent to 22–25 °C [Citation28,Citation29]. Song et al. reported that the blood flow in rat normal tissue, e.g. skin and muscle, increases by a factor of 3–20 upon heating to 42–45 °C [Citation30]. Based on this information, the blood flow in humans may increase at a temperature lower than 42–45 °C. Actually, ST increased to 36–38 °C and DT at 10 mm and 20 mm increased to 37–39 °C, with improved haemoglobin saturation in our study. In addition, Roemer et al. reported that in canine thigh muscle subjected to stepwise changes in microwave heating, four types of responses were identified [Citation31]:
Type I: at low power levels, temperatures increased monotonically to elevated steady state values;
Type II: at higher power levels, when temperatures increased above some critical point, increased blood perfusion was activated to yield heavily damped temperature oscillations;
Type III: at even higher powers, temperature responded to lightly damped or self-sustained large oscillations, with a maximum oscillation of 7 °C, and;
Type IV: temperatures rapidly increased above physiologically safe levels at yet higher powers.
In our study, the temperature at the skin surface and at 10 mm and 20 mm deep in tissues increased by 3–4 °C with CRet and HP, and the haemoglobin saturation improved after 30 min of intervention. The critical temperature in humans may be different from that of dogs, but the type of blood circulation response in this study is considered to be Type I or II according to Roemer’s study. Physiological changes due to thermal stimulation in humans will be revealed in more detail by future studies that apply variable amounts of heat to the body.
In our study, CRet increased temperature at deep tissue and improved haemoglobin saturation more than HP, thus CRet was assumed to have better efficacy than HP clinically. The average difference in temperature between the two groups was less than 1 °C. However, a temperature rise of 1 °C can have various effects in the human body, such as changes in nerve conduction velocity, enzyme activity and oxy-Hb release [Citation32–35]. Tissue oxygenation insufficiency gives rise to hypoxic conditions in tissues, the production and release of algesic substance and tissue fibrosis, thereby causing pain, muscle spasms and joint contracture [Citation24]. Therefore, increasing temperature and improving haemoglobin saturation to affected sites and promoting tissue oxygenation are clinically important for improvement of the above conditions; moreover, it would also enhance muscle fatigue recovery, tissue repair and wound healing [Citation36,Citation37]. CRet intervention is considered to be capable of enhancing muscle fatigue recovery, tissue repair and wound healing; further studies are needed in this subject.
This study had some limitations. First, the intensity of CRet intervention was defined on a subjective analogue scale. In this study, the tissue temperatures at T1 were not different between the CRet and HP groups at all depths, thus it is estimated that the amount of thermal stimulation is almost same among these groups. We performed additional experiments to measure the applied power, and found that 5 min of CAP and 10 min of RES intervention provided 7.1 ± 1.2 kJ and 60.3 ± 5.3 kJ, respectively (unpublished data: 10 persons). Despite its limitations, the results are meaningful because HP and CRet intensities and intervention time are commonly used in clinical practice to assess musculoskeletal injuries. However, the result might be different if lower intensity CRet is used. Thus, further studies are needed. Second, the measurement time was limited. In this study, total-Hb and oxy-Hb were significantly higher during 30 min after the intervention in the CRet and HP groups than in the sham group. Nevertheless, prolonged effect after more than 30 min is not known. Third, we did not record a clinical parameter such as range of motion, and muscle strength. In spite of these limitations, this study suggested the effectiveness of CRet intervention on improving haemoglobin saturation. Although we compared the physiological responses between CRet and HP in this study, it has not revealed the result of comparison with other devices such as ultrasound and diathermy. Further comparison studies are needed in future. Fourth, we did not include female participants and participants of various ages, and the population were restricted to young healthy male participants. Adding additional subjects could reveal sources of variation in tissue response; therefore, further analysis is required. Fifth, we did not measure activity of the autonomic nervous system. If thermotherapy affects the parasympathetic nervous system, the resulting decrease in respiration rate could also increase haemoglobin saturation. Sixth, while changes in microvessel haematocrit can also affect haemoglobin concentration, haematocrit was not measured in this study. Neeman et al. have shown that haematocrit can fluctuate, and predicted that this fluctuation would affect blood oxygenation level-dependent magnetic resonance imaging (BOLD MRI) signals [Citation38]. It may be possible to identify changes in the blood condition in detail using BOLD MRI. Seventh, we used a non-invasive device to monitor deep tissue temperature instead of an invasive method using needles. The system uses a servo-controlled heater to null cutaneous heat flux, and then makes the assumption that subcutaneous temperature, which reflects core temperature in specific locations, is equal to heater temperature. The system has generally proven reliable and is an established measurement method for deep tissue temperature. Studies using this method to examine the thermal effect of exercise and thermotherapy have been published [Citation39,Citation40]. In addition, the measurement accuracy according to the instrument manual is ± <0.1 °C at 30–40 °C. The temperatures of each point in this study were within 30–40 °C; thus, measurement accuracy is not the major problem. Given the accumulating knowledge, the system can be used for the purposes of this study to compare group differences with accuracy comparable to that of previous studies, in spite of minor concerns related to the change in perfusion. Despite these limitations, the findings from the present study provide valuable information on the effects of CRet intervention.
Conclusions
The effect on haemoglobin saturation was higher in the CRet group than HP group. In addition, the CRet intervention warmed deep tissue more effectively than HP intervention. CRet, which is a form of deep thermotherapy, is assumed to be capable of treating musculoskeletal disorders more effectively than HP, which is a form of superficial thermotherapy.
Acknowledgements
We would like to thank the students of the Human Health Sciences at Kyoto University for their help with data collection.
Disclosure statement
The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Gam AN, Johannsen F. Ultrasound therapy in musculoskeletal disorders: a meta-analysis. Pain 1995;63:85–91.
- van der Windt DA, van der Heijden GJ, van den Berg SG, et al. Ultrasound therapy for musculoskeletal disorders: a systematic review. Pain 1999;81:257–71.
- Furlan RM, Giovanardi RS, Britto AT, et al. The use of superficial heat for treatment of temporomandibular disorders: an integrative review. Codas 2015;27:207–12.
- Malanga GA, Yan N, Stark J. Mechanisms and efficacy of heat and cold therapies for musculoskeletal injury. Postgrad Med 2015;127:57–65.
- Cameron MH. (2009). Physical agents in rehabilitation from research to practice. 3rd ed. Philadelphia: Elsevier, 160–1.
- Sumida M, Senda M, Ochi F, et al. Survey result of frequency of use and the effect of exercise therapy equipment and occupational therapy equipment. Jpn J Rehabil Med 2008;9:559–68.
- Mayer JM, Mooney V, Matheson LN, et al. Continuous low-level heat wrap therapy for the prevention and early phase treatment of delayed-onset muscle soreness of the low back: a randomized controlled trial. Arch Phys Med Rehabil 2006;87:1310–17.
- Draper DO, Harris ST, Schulthies S, et al. Hot-pack and 1-MHz ultrasound treatments have an additive effect on muscle temperature increase. J Athl Train 1998;33:21–4.
- Draper DO, Knight K, Fujiwara T, et al. Temperature change in human muscle during and after pulsed short-wave diathermy. J Orthop Sports Phys Ther 1999;29:13–8. discussion 9–22.
- Hayes BT, Merrick MA, Sandrey MA, et al. Three-MHz ultrasound heats deeper into the tissues than originally theorized. J Athl Train 2004;39:230–4.
- Lehmann JF, Stonebridge JB, deLateur BJ, et al. Temperatures in human thighs after hot pack treatment followed by ultrasound. Arch Phys Med Rehabil 1978;59:472–5.
- Batavia M. Contraindications for superficial heat and therapeutic ultrasound: do sources agree? Arch Phys Med Rehabil 2004;85:1006–12.
- Carr JM, Levine DB, Bennett AP, et al. A feasibility study for removing tissue contamination from porous implants. Biomed Instrum Technol 1995;29:220–5.
- Hui CF, Chan CW, Yeung HY, et al. Low-intensity pulsed ultrasound enhances posterior spinal fusion implanted with mesenchymal stem cells-calcium phosphate composite without bone grafting. Spine 2011;36:1010–16.
- Kato S, Saitoh Y, Miwa N. Repressive effects of a capacitive-resistive electric transfer (CRet) hyperthermic apparatus combined with provitamin C on intracellular lipid-droplets formation in adipocytes. Int J Hyperthermia 2013;29:30–7.
- Osti R, Pari C, Salvatori G, et al. Tri-length laser therapy associated to tecar therapy in the treatment of low-back pain in adults: a preliminary report of a prospective case series. Lasers Med Sci 2015;30:407–12.
- Hawamdeh MM. The effectiveness of capacitive resistive diathermy (Tecartherapy®) in acute and chronic musculoskeletal lesions and pathologies. Eur J Sci Res 2014;118:336–40.
- Kumaran B, Watson T. Thermal build-up, decay and retention responses to local therapeutic application of 448 kHz capacitive resistive monopolar radiofrequency: a prospective randomised crossover study in healthy adults. Int J Hyperthermia 2015;31:883–95.
- Kashima S. Spectroscopic measurement of blood volume and its oxygenation in a small volume of tissue using red laser lights and differential calculation between two point. Optics Laser Technol 2003;35:485–9.
- Benitsz MP, Colomer JF. TECAR therapy in knee and spinal pathologies. MKT 2009;1.
- Crockford GW, Hellon RF, Parkhouse J. Thermal vasomotor responses in human skin mediated by local mechanisms. J Physiol 1962;161:10–20.
- Sekins KM, Lehmann JF, Esselman P, et al. Local muscle blood flow and temperature responses to 915MHz diathermy as simultaneously measured and numerically predicted. Arch Phys Med Rehabil 1984;65:1–7.
- Wessman HC, Kottke FJ. The effect of indirect heating on peripheral blood flow, pulse rate, blood pressure, and temperature. Arch Phys Med Rehabil 1967;48:567–76.
- Morishita K, Karasuno H, Yokoi Y, et al. Effects of therapeutic ultrasound on intramuscular blood circulation and oxygen dynamics. J Jpn Phys Ther Assoc 2014;17:1–7.
- Moon EJ, Sonveaux P, Porporato PE. NADPH oxidase-mediated reactive oxygen species production activates hypoxia-inducible factor-1 (HIF-1) via the ERK pathway after hyperthermia treatment. Proc Natl Acad Sci USA 2010;107:20477–82.
- Chastain PB. The effect of deep heat on isometric strength. Phys Ther 1978;58:543–6.
- McMeeken JM, Bell C. Effects of microwave irradiation on blood flow in the dog hindlimb. Exp Physiol 1990;75:367–74.
- Kokolus KM, Capitano ML, Lee CT, et al. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc Natl Acad Sci USA 2013;110:20176–81.
- Lodhi IJ, Semenkovich CF. Why we should put clothes on mice. Cell Metab 2009;9:111–2.
- Song CW, Lokshina A, Rhee JG, et al. Implication of blood flow in hyperthermic treatment of tumors. IEEE Trans Biomed Eng 1984;31:9–16.
- Roemer RB, Oleson JR, Cetas TC. Oscillatory temperature response to constant power applied to canine muscle. Am J Physiol 1985;249:R153–8.
- Kelly R, Beehn C, Hansford A, et al. Effect of fluidotherapy on superficial radial nerve conduction and skin temperature. J Orthop Sports Phys Ther 2005;35:16–23.
- Halle JS, Scoville CR, Greathouse DG. Ultrasound’s effect on the conduction latency of the superficial radial nerve in man. Phys Ther 1981;61:345–50.
- Mace TA, Zhong L, Kokolus KM, et al. Effector CD8+ T cell IFN-γ production and cytotoxicity are enhanced by mild hyperthermia. Int J Hyperthermia 2012;28:9–18.
- Knippertz I, Stein MF, Dorrie J, et al. Mild hyperthermia enhances human monocyte-derived dendritic cell functions and offers potential for applications in vaccination strategies. Int J Hyperthermia 2011;27:591–603.
- Baker RJ, Bell GW. The effect of therapeutic modalities on blood flow in the human calf. J Orthop Sports Phys Ther 1991;13:23–7.
- Robinson SE, Buono MJ. Effect of continuous-wave ultrasound on blood flow in skeletal muscle. Phys Ther 1995;75:145–9. discussion 9–50.
- Neeman M, Dafni H, Bukhari O, Braun RD, Dewhirst MW. In vivo BOLD contrast MRI mapping of subcutaneous vascular function and maturation: validation by intravital microscopy. Magn Reson Med 2001;45:887–98.
- Takemura M, Iwai K, Miyakawa S. Effects of Cooling on Tissue Temperature and Hemodynamics after Exercises. Bull Facul Health Sci Univ Tsukuba 2014;37:123–7.
- Demachi K, Yoshida T, Tsuneoka H. Relationship between the deep forehead temperature measured by the zero-heat-flow method and esophageal temperature during exercise in humans. Jpn J Biometeor 2011;48:119–27.