Abstract
Purpose: To validate a nomogram for the prediction of treatment outcomes after preoperative radiochemotherapy and surgery for locally advanced rectal cancer with a cohort of patients treated with additional deep regional hyperthermia.
Patients and methods: A total of 86 patients were treated with preoperative radiochemotherapy and deep regional hyperthermia at our institution. For every patient, the 5-year probability for death, distant metastases and local failure based on a previously published nomogram were calculated and patients were divided into three risk groups.
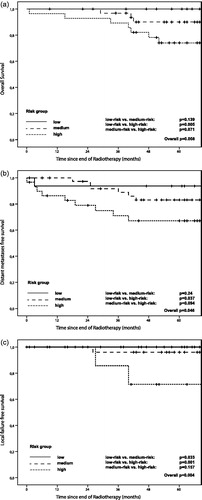
Results: Low-lying and clinically lymph node positive tumours were more frequent in the validation cohort. Five-year Kaplan–Meier estimates for overall survival (OS), distant metastases-free survival (DMFS) and local control (LC) were 87.3%, 79.9%, 95.8% (observed) and 75.5%, 71%, 90% (predicted), respectively. Discrimination between low- and high-risk groups was at a significant level for all endpoints. The c-index was 0.81 (OS), 0.67 (DMFS) and 0.92 (LC), respectively.
Conclusions: The nomogram showed reasonable performance when deep regional hyperthermia is incorporated into preoperative therapy. The higher than predicted rates seen for OS and DMFS in particular in the high-risk groups warrant further prospective validation and subsequent investigation of the underlying mechanisms.
Introduction
Preoperative radiotherapy followed by surgical resection and adjuvant 5-fluorouracil (5-FU) based chemotherapy according to the CAO/ARO/AIO 94 trial is standard of care for locally advanced rectal cancer [Citation1]. However, based on individual factors, treatment outcomes vary between patients. For instance, in patients achieving a pathological complete response (pCR) significantly higher overall survival (OS) rates have been reported [Citation2]. In order to estimate the risk of death or disease recurrence within 5 years after standard treatment, Valentini et al. developed a nomogram for patients with locally advanced rectal cancer based on clinical, pathological and treatment related parameters [Citation3]. Individual patient data from five large-scale European rectal cancer trials were pooled in order to generate a predictive model [Citation1,Citation4–6]. Patients were then stratified in three risk groups for local failure, distant failure and death within 5 years. The authors consider the identification of patients who are likely to benefit from adjuvant chemotherapy and risk adjusted follow-up intervals as the primary scope of nomograms [Citation3]. Shen et al. validated the model with a retrospective cohort from a single centre in China [Citation7]. Their results suggest favourable discrimination of risk groups considering local control (LC) but suboptimal distinction regarding distant metastases-free survival (DMFS) and OS especially in the low- and medium-risk groups. In terms of calibration, there was a trend towards underestimating the likelihood of local or distant failures in particular in the high-risk groups.
Deep regional hyperthermia has been applied in combination with preoperative radiotherapy in several prospective randomised trials [Citation8–10]. While a meta-analysis by the Cochrane Collaboration showed a significant increase in the pCR rate with combined modality treatment consisting of preoperative radiotherapy and hyperthermia, randomised long-term outcome data for the combination of hyperthermia with preoperative radiochemotherapy has not been published so far [Citation11]. In the absence of such data, the validation of the nomograms published by Valentini et al. is an interesting tool to compare the outcome of patients treated with preoperative radiochemotherapy plus deep regional hyperthermia with a large cohort treated with radiochemotherapy alone. Furthermore, this study aims to investigate the performance of the predictive model when a trimodal instead of a bimodal preoperative regimen is used. In addition, we sought to identify subgroups of patients that could particularly benefit from the more intensified local treatment.
Patients and methods
The validation cohort consisted of 86 patients with locally advanced rectal adenocarcinoma treated at the Department of Radiation Oncology, University Hospital, Tübingen, Germany, between 2007 and 2011. Patients with UICC Stage II or III treated with preoperative radiochemotherapy and concomitant deep regional hyperthermia were included. For local tumour staging, endoscopic ultrasound and/or MRI were performed. Long-course neoadjuvant 3D conformal radiotherapy or intensity-modulated radiotherapy with 50.4 Gy (1.8 Gy per fraction, 5 fractions per week) was planned. The clinical target volume included the primary tumour, the mesorectum and at least the internal iliac lymphatics. Continuous venous infusions of 5-FU 1000 mg/m2 per day for 120 h were given during the first and fifth week of treatment. Deep regional hyperthermia to the pelvis was prescribed once or twice weekly. Four cycles of 5-FU based adjuvant chemotherapy after total mesorectal excision were planned. Deep regional hyperthermia was performed according to the quality assurance guidelines of the European Society of Hyperthermic Oncology and as previously described [Citation12]. T90 and CEMT43 were calculated as reported previously [Citation13].
Follow-up data were extracted from patient records and the regional cancer registry. Local recurrence during follow-up was defined as histologically proven recurrence or evidence of tumour regrowth in imaging studies. Distant recurrence was defined as manifestation of tumour outside the pelvis.
The training cohort was described before [Citation3]. Briefly, the training cohort consisted of pooled data of 2795 patients from five prospective European rectal cancer trials. A multivariate nomogram for 5-year OS, DMFS and LC was developed by Cox regression. For each endpoint, three distinct risk groups were identified.
For each patient the probabilities for 5-year OS, DMFS and LC based on the nomogram were calculated using an online template (www.predictcancer.org). Previously published probability thresholds were used to group patients into a low-, medium- and high-risk group for the respective endpoints [Citation3]. Survival estimates were calculated according to the Kaplan–Meier method. Median follow-up was calculated based on the reverse Kaplan–Meier method [Citation14]. Survival curves were compared using the log-rank test. Patient and tumour characteristics were compared between the training and validation cohort using the chi-square test. For all tests p < 0.05 was considered statistically significant. Statistics were calculated with SPSS (IBM, Armonk, NY). The concordance index (c-index) was calculated using ‘R’ version 3.3.2. and the package ‘compareC 1.3.1’.
Results
Patient und tumour characteristics are shown in . Compared with the training cohort from Valentini et al., our validation cohort had a higher number of low-lying tumours and a higher rate of clinically lymph node positive patients [Citation3].
Table 1. Patient and treatment characteristics in the training and validation cohort.
A total of 86 patients (52 males, 34 females) with a median age of 64 years and a median follow-up of 68 months started radiochemotherapy with concomitant deep regional hyperthermia. All but one patient completed radiotherapy as prescribed. This patient developed symptoms of a paralytic ileus after 19.8 Gy, one cycle of chemotherapy and two hyperthermia treatments and required surgical resection thereafter. Another patient required dose reduction of the second cycle of 5-FU to 75%. The median number of hyperthermia treatments was 5. Mean T90 and CEMT43 were 39.4 °C and 2.36 min, respectively.
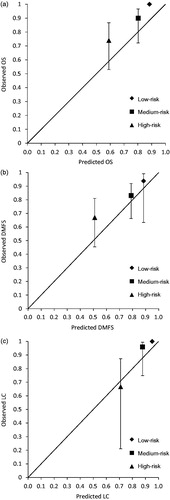
The predicted and observed 5-year Kaplan–Meier estimates for OS, DMFS and LC were 75.5%, 71%, 90% and 87.3%, 79.9%, 95.8%, respectively. In terms of DMFS the difference between predicted and observed estimates were more pronounced for patients with distal rectal cancer (predicted: 69.9%, observed: 84%, n = 46) than for patients with tumours in the middle and upper rectum (predicted: 72.1%, observed: 75%, n = 40). After dividing patients into a low-, medium- and high-risk group, we tested the ability of the nomogram to discriminate between these risk groups. No events were scored in the low-risk groups for LC and OS. The difference between Kaplan–Meier curves when comparing low- and high-risk groups was significant for OS, DMFS and LC (). Calibration plots with the predicted and observed outcome for all endpoints and risk groups are shown in . For OS and DMFS, the observed rates were higher than predicted in all risk groups. In terms of discrimination, the c-index for OS, DMFS and LC was 0.81, 0.67 and 0.92, respectively.
Discussion
Our group had previously reported excellent long-term outcome data for patients treated with a trimodal preoperative regimen of radiochemotherapy and deep regional hyperthermia. Moreover, there was a trend towards improved OS and LC compared with a group of patients treated with radiochemotherapy only. DMFS was similar between the two groups. At the same time, we had pointed out the limitations and potential biases with the comparison of two retrospective and non-randomised cohorts. For instance, patients in the hyperthermia group were approximately 4 years younger and had more low lying tumours [Citation15]. The nomograms published by Valentini et al. allow to estimate an individual patient’s probability of being alive, metastases and local failure free after 5 years based on risk factors determined by Cox regression from pooled data of almost 3000 patients [Citation3]. We used these nomograms to calculate the mentioned probabilities for a cohort of 86 patients treated with additional deep regional hyperthermia. This approach has two major advantages compared with a non-randomised control group from the same institution: First, our data can be compared with a larger set of patients. Furthermore, the risk for imbalances is reduced since parameters such as age, tumour location and the resulting surgical technique and tumour stages are included in the model.
With a c-index of 0.81 and 0.92 the nomogram provides good discrimination for the endpoints OS and LC. It appears counterintuitive that the lowest c-index of 0.67 is seen for DMFS since hyperthermia is primarily considered a modality used for local treatment intensification. For all risk groups, DMFS is higher than predicted, which is reassuring since there have been concerns that hyperthermia might increase the risk of distant metastases. Our data do not support this hypothesis [Citation16,Citation17]. On the contrary, while calibration for DMFS in the low- and medium-risk group is close to perfect, the rate of distant metastases in the high-risk group in our cohort is approximately 17% lower than predicted. Moreover, the difference between the predicted and observed frequency of distant failures was more pronounced in the subgroup with low-lying tumours. It can be hypothesised that this might be due to more effective hyperthermic treatment in distal tumours without bony structures surrounding the target volume. However, an optimal threshold for tumour height beyond which sufficient heating is unlikely to be achieved has not been established yet. Two studies report rectal temperatures relative to the distance from the catheter tip during deep regional hyperthermia for bladder and cervical cancer, respectively. In both studies temperatures around 40.5 °C were achieved in 10 cm depth [Citation18,Citation19]. Using MR-thermometry Gellermann et al. observed lower temperatures in tumours located more than 2 cm above the symphysis compared with lower seated tumours [Citation20]. However, tumour location is not the only factor that could determine the ‘heatability’ of a tumour as illustrated by Sreenivasa et al. who showed a very different heatability of tumours in patients with comparable habitus and tumour location [Citation21].
Follow-up at our institution was performed based on the recommendation of the German Cancer Society. Thus, similar to the studies in the training cohort the use of cross-sectional imaging during follow-up was not mandatory. It is therefore unlikely that the lower rate of distant metastases in the high-risk group of our validation cohort can be explained by different intensities of follow-up. Hyperthermia per se is a treatment modality primarily used to enhance local effects of radiotherapy. The properties of hyperthermia as a stand-alone cytotoxic treatment and in combination with both radiation and chemotherapy have long been known [Citation22]. At the thermal dose range usually applied in clinical practice, the main gain expected by the addition of hyperthermia to radiotherapy is pure radio-sensitisation. Despite the fact that this synergistic effect has been clinically exploited for several years in different tumour types [Citation9,Citation11], the underlying molecular mechanisms which drive heat-induced cytotoxicity have yet to be fully elucidated. Hyperthermia may enhance the tumoricidal impact of radiation by exerting pleiotropic effects on different DNA repair pathways. Although still controversial, some authors showed in vitro that the impairment of homologous recombination could play a central role [Citation23,Citation24]. In recent years, a growing interest has also emerged in the potential immune modulation triggered by hyperthermia. Overall, hyperthermia may enhance the immunogenicity of the irradiated microenvironment by favouring tumoural antigens presentation to dendritic cells (DCs) and T-cell priming. Schildkopf et al. showed in human colorectal cancer cell lines that the addition of hyperthermia to radiation promotes a necrotic pattern of cell death with higher levels of chromatin associated high-mobility group box1 (HMGB1) protein in the extracellular matrix [Citation25]. Furthermore, the same group demonstrated that hyperthermia combined with radiotherapy increases the amount of heat-shock protein 70 in the extracellular matrix compared with radiotherapy alone, able to foster the maturation of DCs [Citation26]. Taking all together, it was suggested that hyperthermia could synergise radiation through an immune-mediated abscopal effect, beside its local activity [Citation27–29]. Therefore, it could be hypothesised that the lower than predicted rate of distant failure observed in our high-risk group may be associated with a potential systemic immunostimulation of hyperthermia.
In terms of LC, outcome is excellent for the low- and medium-risk group with no event in the low-risk group and a single event in the intermediate-risk group. Among the six parameters that determine the 5-year probability for LC are postoperative T- and N-stages. A total of 50% of the patients in our cohort were categorised in the low-risk group for local failure while in the original training cohort this was the case only for 33% of patients. Since pretherapeutic T-stages were comparable between the training cohort and our validation cohort, the higher percentage of patients in the low-risk group is possibly caused by the intensified preoperative treatment regimen.
There are some limitations to our analysis. First, we cannot rule out imbalances in terms of comorbidities between the training and the validation cohort that could explain the trend towards higher OS than predicted in all three risk groups. In particular, patients with cardiovascular comorbidities are usually not considered suitable for hyperthermia. On the other hand, the same comorbidities are a frequent exclusion criterion in prospective studies of rectal cancer due to the cardiotoxic side effects of 5-FU. Furthermore, the first patients in our validation cohort were treated approximately 3 years after the last patient in the training cohort. The approval of targeted agents for patients with metastatic colorectal cancer falls in the time interval between the two cohorts and could to some extend explain the trend towards an improved OS in all three risk groups of the validation cohort [Citation30,Citation31]. While the nomogram validated in our study is useful in order to compare treatment results between two groups of patients (training and validation cohort) and categorise them into distinct risk groups, its use is limited for the prospective selection of patients who might benefit from trimodal treatment since the model also includes postoperative parameters. For this purpose, the detection of novel biological or imaging based biomarkers is necessary.
In summary, our validation study showed a good discriminative performance for OS and LC of the previously published nomogram when a trimodal instead of a bimodal neoadjuvant treatment protocol was applied. The trend towards a higher DMFS than predicted in patients at high-risk for distant failure warrants further preclinical investigations of the underlying mechanisms. While local failures after preoperative radiochemotherapy followed by ‘perfect’ total mesorectal excision have become a rare event, there is still a great interest in establishing therapeutic regimens that can increase tumour downstaging and therefore qualify a relevant number of patients for organ-preservation strategies. Besides dose-escalated radiotherapy, intensified chemotherapy regimens and total neoadjuvant therapy, hyperthermia is a treatment modality that is currently under investigation in prospective phase II trials [Citation32–38].
Acknowledgements
Cihan Gani is supported by the Clinician Scientist Program of the Medical Faculty, Eberhard Karls Universität Tübingen [Funding number: 363–0-0]. We thank Joge Jossy Tonison for English language editing.
Disclosure statement
The authors declare that they have no conflict of interest.
References
- Sauer R, Becker H, Hohenberger W, et al.; German Rectal Cancer Study Group. (2004). Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351:1731–40.
- Maas M, Nelemans PJ, Valentini V, et al. (2010). Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 11:835–44.
- Valentini V, van Stiphout RG, Lammering G, et al. (2011). Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol 29:3163–72.
- Bosset JF, Collette L, Calais G, et al.; EORTC Radiotherapy Group Trial 22921. (2006). Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355:1114–23.
- Gerard JP, Conroy T, Bonnetain F, et al. (2006). Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol 24:4620–5.
- Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. (2006). Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 93:1215–23.
- Shen L, van Soest J, Wang J, et al. (2015). Validation of a rectal cancer outcome prediction model with a cohort of Chinese patients. Oncotarget 6:38327–35.
- Berdov BA, Menteshashvili GZ. (1990). Thermoradiotherapy of patients with locally advanced carcinoma of the rectum. Int J Hyperthermia 6:881–90.
- van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, et al.; Dutch Deep Hyperthermia Group. (2000). Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Lancet 355:1119–25.
- Kakehi M, Ueda K, Mukojima T, et al. (1990). Multi-institutional clinical studies on hyperthermia combined with radiotherapy or chemotherapy in advanced cancer of deep-seated organs. Int J Hyperthermia 6:719–40.
- De Haas-Kock DF, Buijsen J, Pijls-Johannesma M, et al. (2009). Concomitant hyperthermia and radiation therapy for treating locally advanced rectal cancer. Cochrane Database Syst Rev 3:CD006269.
- Bruggmoser G, Bauchowitz S, Canters R, et al.; Atzelsberg Research Group; European Society for Hyperthermic Oncology. (2012). Guideline for the clinical application, documentation and analysis of clinical studies for regional deep hyperthermia: quality management in regional deep hyperthermia. Strahlenther Onkol 188:198–211.
- Schroeder C, Gani C, Lamprecht U, et al. (2012). Pathological complete response and sphincter-sparing surgery after neoadjuvant radiochemotherapy with regional hyperthermia for locally advanced rectal cancer compared with radiochemotherapy alone. Int J Hyperthermia 28:707–14.
- Schemper M, Smith TL. (1996). A note on quantifying follow-up in studies of failure time. Control Clin Trials 17:343–6.
- Gani C, Schroeder C, Heinrich V, et al. (2016). Long-term local control and survival after preoperative radiochemotherapy in combination with deep regional hyperthermia in locally advanced rectal cancer. Int J Hyperthermia 32:187–92.
- Nathanson SD, Cerra RF, Hetzel FW, et al. (1990). Changes associated with metastasis in B16-F1 melanoma cells surviving heat. Arch Surg 125:216–19.
- Thrall DE, Prescott DM, Samulski TV, et al. (1996). Radiation plus local hyperthermia versus radiation plus the combination of local and whole-body hyperthermia in canine sarcomas. Int J Radiat Oncol Biol Phys 34:1087–96.
- Juang T, Stauffer PR, Craciunescu OA, et al. (2014). Thermal dosimetry characteristics of deep regional heating of non-muscle invasive bladder cancer. Int J Hyperthermia 30:176–83.
- Sapozink MD, Joszef G, Astrahan MA, et al. (1990). Adjuvant pelvic hyperthermia in advanced cervical carcinoma. I. Feasibility, thermometry and device comparison. Int J Hyperthermia 6:985–96.
- Gellermann J, Wlodarczyk W, Hildebrandt B, et al. (2005). Noninvasive magnetic resonance thermography of recurrent rectal carcinoma in a 1.5 Tesla hybrid system. Cancer Res 65:5872–80.
- Sreenivasa G, Gellermann J, Rau B, et al. (2003). Clinical use of the hyperthermia treatment planning system HyperPlan to predict effectiveness and toxicity. Int J Radiat Oncol Biol Phys 55:407–19.
- Horsman MR, Overgaard J. (2007). Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol (R Coll Radiol) 19:418–26.
- Krawczyk PM, Eppink B, Essers J, et al. (2011). Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerases-1 inhibition. Proc Natl Acad Sci USA 108:9851–6.
- Bergs JW, Krawczyk PM, Borovski T, et al. (2013). Inhibition of homologous recombination by hyperthermia shunts early double strand break repair to non-homologous end-joining. DNA Repair (Amst) 12:38–45.
- Schildkopf P, Frey B, Mantel F, et al. (2010). Application of hyperthermia in addition to ionizing irradiation fosters necrotic cell death and HMGB1 release of colorectal tumor cells. Biochem Biophys Res Commun 391:1014–20.
- Schildkopf P, Frey B, Ott OJ, et al. (2011). Radiation combined with hyperthermia induces HSP70-dependent maturation of dendritic cells and release of pro-inflammatory cytokines by dendritic cells and macrophages. Radiother Oncol 101:109–15.
- Lauber K, Brix N, Ernst A, et al. (2015). Targeting the heat shock response in combination with radiotherapy: sensitizing cancer cells to irradiation-induced cell death and heating up their immunogenicity. Cancer Lett 368:209–29.
- Eckert F, Gaipl US, Niedermann G, et al. (2017). Beyond checkpoint inhibition – immunotherapeutical strategies in combination with radiation. Clin Transl Radiat Oncol 2:29–35.
- Werthmoller N, Frey B, Ruckert M, et al. (2016). Combination of ionising radiation with hyperthermia increases the immunogenic potential of B16-F10 melanoma cells in vitro and in vivo. Int J Hyperthermia 32:23–30.
- Cunningham D, Humblet Y, Siena S, et al. (2004). Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337–45.
- Hurwitz H, Fehrenbacher L, Novotny W, et al. (2004). Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–42.
- Garcia-Aguilar J, Chow OS, Smith DD, et al.; Timing of Rectal Cancer Response to Chemoradiation Consortium. (2015). Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol 16:957–66.
- Appelt AL, Ploen J, Harling H, et al. (2015). High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol 16:919–27.
- Rodel C, Graeven U, Fietkau R, et al.; German Rectal Cancer Study Group. (2015). Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 16:979–89.
- Preoperative radiochemotherapy with hyperthermia for locally advanced rectal cancer (HT01/NCT02353858).
- Neoadjuvant chemoradiation with 5-FU (or capecitabine) and oxaliplatin combined with hyperthermia in rectal cancer (HyRec/NCT01716949).
- Gani C, Bonomo P, Zwirner K, et al. (2017). Organ preservation in rectal cancer – challenges and future strategies. Clin Transl Radiat Oncol 3:9–15.
- Rasulov AO, Gordeyev SS, Barsukov YA, et al. (2017). Short-course preoperative radiotherapy combined with chemotherapy, delayed surgery and local hyperthermia for rectal cancer: a phase II study. Int J Hyperthermia 33:465–70.