Abstract
Purpose: To investigate the feasibility and value of three-dimensional ultrasound/contrast-enhanced ultrasound (3D US-CEUS) fusion imaging for the immediate evaluation of technical success and the guidance of supplementary ablation during the liver cancer thermal ablation procedure.
Materials and methods: Patients diagnosed with malignant liver cancer intending to receive thermal ablation including radiofrequency ablation (RFA) or microwave ablation (MWA) were enrolled. 3D US-CEUS fusion imaging was used to immediately assess the technical success and guide supplementary ablation. Contrast-enhanced computed tomography/magnetic resonance imaging (CECT/CEMRI) was performed one month after ablation to assess the technique effectiveness of the ablation. The registration success rate, duration time of 3D US-CEUS fusion imaging, technique effectiveness rate and major complications were recorded.
Results: In total, 76 patients with 95 tumours who underwent RFA or MWA and assessed by 3D US-CEUS fusion imaging were enrolled. The registration success rate of 3D US-CEUS fusion imaging was 93.7% (89/95), and the duration time was 4.0 ± 1.1 min. Thirty lesions received supplementary ablation immediately during the procedure. The technique effectiveness rate of the ablation was 98.8% (81/82). There were no major complications related to ablation.
Conclusions: 3D US-CEUS fusion imaging is a feasible and valuable technique for the immediate evaluation and guidance of supplementary ablation during the liver cancer thermal ablation procedure.
Introduction
Recently developed fusion imaging techniques allow two or more images to be overlapped. The most common mode is fusion imaging between real-time ultrasound (US) and computed tomography (CT)/magnetic resonance imaging (MRI), which is called US-CT/MRI fusion imaging. This technique has been successfully applied to liver tumour detection, targeting, biopsies, and ablation efficacy evaluation [Citation1–9]. After liver tumour ablation, US-CT/MRI can show the relative position of the lesion and the ablation zone exactly, and the ablative margin (AM) can also be evaluated precisely [Citation8,Citation9]. We have successfully reported the application of contrast-enhanced US (CEUS)-CT/MRI fusion imaging for determining AM after the radiofrequency ablation (RFA) of hepatocellular carcinoma (HCC). CEUS-CT/MRI fusion imaging is helpful in the guidance and assessment of the RFA procedure [Citation10,Citation11]. However, our previous experience has shown that US-CT/MRI fusion imaging is not sufficiently convenient during the ablation procedure. Recent Digital Imaging and Communications in Medicine (DICOM) image data for CT/MRI cannot be easily obtained during US-guided thermal ablation in certain circumstances.
US is the most popular guidance and monitoring tool for the thermal ablation of liver cancer because it provides information in real time and is convenient, free of radiation and low-cost. CEUS is recognised as an important method for the assessment of the immediate treatment response to liver ablation according to the microvascular perfusion of the ablation zone [Citation12]. Three-dimensional (3D) US has been successfully used in thermal ablation to provide more intuitive spatial structure information [Citation10,Citation13,Citation14]. The fusion imaging technique is a useful tool for precise image comparison [Citation6]. We therefore hypothesised that US can be used as mono-modality fusion imaging to assess technical success during the ablation procedure. 3D US images of the liver tumour before ablation during the procedure were used as reference images and fused with real-time CEUS images of the ablation zones after ablation. Depending on the evaluation results, supplementary ablation was immediately implemented. This method is cost-effective, radiation free, requires minimal CT/MRI resources and can easily be performed in the operation room. There are few clinical literature reports on the application of 3D US-CEUS fusion imaging to evaluate the technical success of liver cancer ablation during the procedure.
Thus, the purpose of this clinical study was to investigate the feasibility and value of 3D US-CEUS fusion imaging in evaluating technical success and guiding supplementary ablation during the procedure.
Materials and methods
Study subjects
This prospective study was approved by the institutional ethics review board and was performed in compliance with the Declaration of Helsinki. Informed consent was obtained from all participants. From August to December 2015, all patients diagnosed with malignant liver cancer who intended to receive thermal ablation in our department were enrolled. The diagnosis of HCC was based on clinical criteria [Citation15] or pathological results. Other malignant liver cancers were diagnosed using pathological results. The inclusion criteria for liver thermal ablation in this study were (1) no more than 5 lesions in each patient; (2) maximum diameter of each lesion ≤7.0 cm; (3) Child-Pugh class A or B; and (4) platelet count >50 × 109/L [Citation16]. The exclusion criteria were (1) invisible lesions on conventional US (i.e., 3D US would not be suitable for the reference data set); (2) being allergic to the US contrast agents (UCAs); and (3) undergoing thermal ablation with palliative intent.
Thermal ablation
Equipment: Two types of thermal ablation were used in this study, RFA and microwave ablation (MWA). The cooled-tip RFA system (Covidien, Mansfield, MA) and an internally cooled-tip electrode with a 3 cm tip were used for RFA. A microwave (MW) generator (Kangyou Cor., Nanjing, China) of 2450 MHz and an internally cooled microwave antenna were used for MWA.
Ablation strategy: Two ultrasound interventional physicians (X.E.J. and L.K.) with 10 years of experience in liver ablation performed all of the ablation procedures. Generally, the RF generator was set in impedance mode with a maximum output. Each RF electrode insertion required approximately 12 min. The MW generator was set at 60 watts and maintained for up to 6 min in each MW antenna insertion. Multiple insertions of the RF electrode or MW antenna were required to obtain sufficient ablation and a 5 mm AM as far as possible. When necessary, assisted means such as artificial ascites, artificial pleural effusion, laparoscopy or open operation were performed to improve the visualisation of the lesions and prevent damage to the surrounding critical organs and structures. All applicator placement and repositioning were guided by US. In some patients, the target tumour was affected by gasification and was unclear after ablation. The subsequent insertions were made according to the reference 3D US images acquired before ablation.
Immediate assessment of technical success and guidance of supplementary ablation via 3D US-CEUS fusion imaging
Equipment: A MyLab Twice US machine (Esoate, Genoa, Italy), a convex array probe CA541 (frequency range from 1 to 8 MHz) with fusion imaging (Virtual Navigator) and 3D software were employed for US guidance and exploration.
UCAs: SonoVue (Bracco, Milan, Italy) was used to evaluate the ablation effect via CEUS. SonoVue was injected as a rapid bolus of 1.5–2.0 mL via an antecubital vein followed by 5 mL of saline solution. When necessary, SonoVue was injected repeatedly.
Acquisition and registration: The experienced ultrasound interventional physicians, who also had more than 5 years fusion imaging experience, performed the fusion imaging procedures. Before ablation, 3D US volume data of the target liver cancer of the patient were acquired in free-hand scanning mode at the end of an expiratory breath. These 3D US volume images were taken as reference images and used to outline the margin of the tumour and 5 mm AM. The cross-sections of the 3D US volume were selected based on the largest diameter dimension of the cancer in three perpendicular planes. For the regular lesions, the margin of cancer and its virtual AM were outlined by two concentric spheres in different colours (the radius difference was 5 mm). For the irregular lesions, the margin of cancer was outlined by depicting its contour in three axial planes, and its 5 mm virtual AM could be acquired automatically by the Virtual Navigator. Because the 3D US volume images were acquired immediately before the ablation procedure and the position of the patient was fixed, the 3D US volume images could automatically be fused with the real-time two-dimensional (2D) US images with the aid of the magnetic positioning system. After ablation, the liver and lesion exhibited a certain degree of displacement. To ensure the accuracy of subsequent assessments, manual fine-tuning was required to obtain satisfactory registration in which the adjacent alignment markers (e.g., vessels) were fully matched.
Assessment of technical success and supplementary ablation: After the vaporisation weakened following ablation in 5 ∼ 10 min, real-time CEUS was performed in the same place and fused with the reference 3D US images. Then, the technical success of the liver cancer was assessed based on whether the non-perfusion area covered the entire cancer and virtual AM. The assessment results were divided into three grades. Grade A denoted complete ablation and sufficient AM (AM ≥5 mm). If the distance between the cancer and large vessels or liver surface was less than 5 mm, AM was still considered as obtained. Grade B denoted complete ablation but insufficient AM (AM <5 mm). Grade C denoted incomplete ablation. For cancers assessed as Grade A, the ablation procedure was finished. For cancers assessed as Grade B, supplementary ablation was performed immediately under US guidance to achieve Grade A as best as possible. For cancers assessed as Grade C, supplementary ablation was performed immediately under US guidance to achieve either Grade A or B.
Follow-up
All patients underwent conventional US examination within 24–72 h after ablation to exclude peri-procedural complications. Contrast-enhanced CT/MRI (CECT/CEMRI) was performed one month after ablation to assess the technique effectiveness of the ablation. If the ablation was considered technically effective, the patient was followed up by CEUS or CECT/CEMRI imaging every three months after thermal ablation.
Data analysis
Registration success rate. The registration success rate was assessed after manual fine-tuning after ablation during the procedure. The registration was considered successful when the adjacent alignment markers were fully matched between the real-time 2D US images and the 3D US volume images.
The duration time of 3D US-CEUS fusion imaging. This measurement included the acquisition of the 3D US images, the outline of the liver cancer margin and its virtual AM, registration and the assessment of the ablation effect.
Technique effectiveness rate. A tumour was considered to have been effectively ablated when there were no longer any enhancements within the ablation zone during the arterial phase on CECT/CEMRI. The CECT/CEMRI results were evaluated by the radiologists, who were blinded to the assessment results of 3D US-CEUS fusion imaging.
Major complications. The major complications related to ablation were also recorded. A major complication was defined as an event that led to substantial morbidity and disability requiring an improved level of care or resulting in hospital admission or a substantially lengthened hospital stay. All other complications were considered minor [Citation17].
Statistical analysis
All statistical analyses were performed using SPSS for Microsoft Windows version 13.0 (SPSS Inc., Chicago, IL). The measurement data were reported as the mean ± standard deviation (range).
Results
General information
In total, 76 patients with 95 tumours were enrolled. A flow chart of the trial is presented in . The baseline characteristics of the sample are summarised in .
Figure 1. Flow chart of the trial. After ablation was finished, CECT or CEMRI was obtained at one month. *For the 3 Grade B tumours, additional treatment was not obtained due to the high risk to damage the surrounding vital structures (n = 1) or poor liver function reserve (n = 2).
US: ultrasound; RFA: radiofrequency ablation; MWA: microwave ablation; CEUS: contrast-enhanced ultrasound.
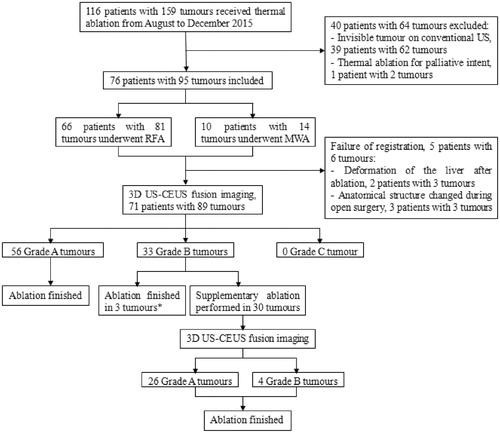
Table 1. Baseline characteristics of the sample.
Registration of 3D US-CEUS fusion imaging
The registration success rate was 93.7% (89/95), and the duration time of 3D US-CEUS fusion imaging was 4.0 ± 1.1 min (range, 2.5–10.0 min). Six lesions were not registered because of deformation of the liver after ablation (n = 3) or during open surgery (n = 3).
Evaluation of technical success with 3D US-CEUS fusion imaging
The initial assessment results included 56 Grade A tumours (), 33 Grade B tumours and 0 Grade C tumours. The ablation strategies were changed for 30 Grade B tumours (), and the supplementary ablation rate was 33.7% (30/89). One, two and three supplementary insertions were performed in 23, 4 and 3 tumours, respectively. After supplementary ablation, 26 of 30 tumours were assessed as Grade A. Ultimately, seven tumours did not achieve sufficient AM because of adjacency of bile duct (n = 1), bowel (n = 1) or heart (n = 1), a difficult puncture path (n = 1) or poor liver function reserve (n = 3).
Figure 2. A 82-year-old female patient had a 24 mm HCC tumour in segment 8, which was shown (arrow) on (A) 2D US and (B) CEMRI. (C) The 3D US-US fusion imaging of the lesion before RFA. The real-time US image is shown at left, and the pre-ablation 3D US image is shown at right. (D) Assessment of the ablation effect on 3D US-CEUS fusion imaging after RFA. The non-perfusion area covered the entire lesion with 5 mm AM. The assessment result was Grade A. (E) CEMRI one month after RFA demonstrated the technique effectiveness of the ablation (arrow).
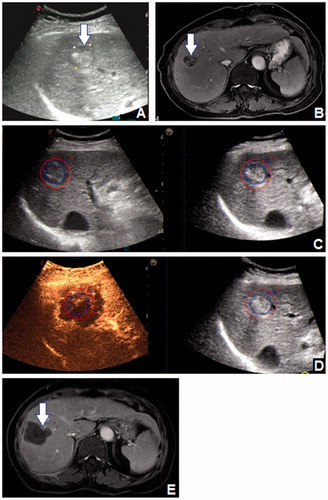
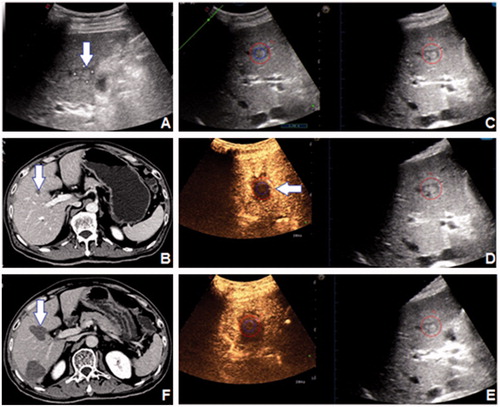
Figure 3. A 61-year-old male patient had a 16 mm HCC tumour in segment 5, which was shown (arrow) on (A) 2D US and (B) CECT. (C) The 3D US-US fusion imaging of the lesion before RFA. The real-time US image is shown at left, and the pre-ablation 3D US image is shown at right. (D) Initial assessment of the ablation effect on 3D US-CEUS fusion imaging after RFA. The non-perfusion area covered the entire lesion, but part of the AM was not covered (arrow). The assessment result was Grade B. (E) Ultimate assessment of the ablation effect on 3D US-CEUS fusion imaging after supplementary ablation. The non-perfusion area covered the entire lesion and the 5 mm AM. The assessment result was Grade A. (F) CECT one month after RFA demonstrated the technique effectiveness of the ablation (arrow).
Technique effectiveness as determined by CECT/CEMRI
CECT/CEMRI results were obtained in 66 patients with 82 tumours. One lesion was incompletely ablated; thus, the technique effectiveness rate was 98.8% (81/82). The residual lesion was assessed as Grade B during the ablation procedure because of adjacency of the heart. The lesion was later ablated with RFA, and the ablation was subsequently confirmed to be technically effective by CEMRI.
Complications
During the follow-up period, there were no major complications related to ablation.
Discussion
3D US-CEUS fusion imaging is a new single-modality fusion-imaging technique that combines the advantages of 3D US, CEUS and fusion imaging. 3D US-CEUS fusion imaging overcomes some of the shortcomings of multi-modality fusion imaging. First, this technique can be performed without CT/MRI images. The assessment can also be performed in the absence of CT/MRI DICOM image data, which is more convenient for US-guided liver ablation. Toshikuni et al. [Citation18] reported the use of 3D US-CEUS fusion imaging to assess the effects of RFA on HCC in a small sample. Their results preliminarily proved the feasibility of 3D US-CEUS in the evaluation of the effects of RFA on HCC. However, the total time of the 3D US-CEUS assessment in their research ranged from 20 to 30 min and was thus obviously longer than in our study. The main reasons for this discrepancy are that they performed the evaluation 1 to 2 d after ablation and used manual registration mainly based on anatomical or contour features as the fusion imaging technique. By contrast, our evaluation was performed immediately during the ablation procedure, and automatic registration based on electromagnetic positioning was used. This process might improve the efficiency and convenience of 3D US-CEUS fusion imaging evaluation. Moreover, immediate evaluation during the ablation procedure may be helpful to reduce the need to perform a second session of ablation. Second, ultrasound interventional physicians can obtain ultrasonic images more easily than CT/MRI images and readily recognise the corresponding point on US images for registration. Our results showed that the registration success rate was 93.7% (89/95) using 3D US-CEUS fusion imaging, higher than the rate of 81.6% in our previous report using 3D CEUS-CT/MRI fusion imaging [Citation10]. However, six lesions could not be registered because of the severe deformation of the liver after ablation and the changes in anatomical structure during open surgery. Although many factors can affect fusion imaging procedures, the duration of 3D US-CEUS fusion imaging was only 4.0 ± 1.1 min. Third, when the anatomical position of the tumour changed because adjuvant methods were employed, the pre-ablation 3D US images could still be fused with the post-ablation 2D US images because the 3D US images were obtained immediately during the procedure. In this study, 37 tumours were ablated with the adjuvant methods, such as artificial ascites, artificial pleural effusion, open operation and laparoscopy. Most obtained satisfactory registration. The above results suggest that 3D US-CEUS fusion imaging is a convenient technique to evaluate the effect of ablation.
In some previous reports, 3 D US – US fusion imaging was used in the RFA procedure. When the ablative hyperechoic zone expanded over the tumour with the AM, the RFA procedure was terminated [Citation14,Citation19]. However, CEUS is more accurate than US in the assessment of the ablation zone [Citation20,Citation21]. In our study, the margin of the index tumour and its virtual AM were outlined on 3D US-CEUS fusion imaging before ablation. The ablation effects were evaluated quantitatively and comprehensively simply by observing whether the non-perfusion ablation zone completely covered the index tumour and its virtual AM in all axial planes. Further, if the non-ablated zone inside the outlined margin was detected, supplementary ablation could be performed immediately to enlarge the ablation zone according to the 3D US-CEUS fusion images. With the aid of 3D US-CEUS fusion imaging, 33.7% (30/89) of the lesions estimated as Grade B received supplementary ablation, thus reducing the proportion of Grade B lesions to 7.9% (7/89). Finally, the technique effectiveness rate was 98.8% (81/82), higher than the range of 67.7–93.5% reported in previous studies under guidance by conventional US [Citation22–27] and identical to the rate reported by Park et al. (100%), who evaluated the usefulness of 3D US-US fusion imaging for the guidance of RFA of HCC [Citation28]. In addition, no major complications occurred in these patients. Thus, our results indicate that 3D US-CEUS fusion imaging is a convenient and precise method to assess the liver ablation effect, guide supplementary ablation and improve technique effectiveness. In addition, the safety of ablation is also guaranteed.
This study has several limitations. First, this was a single-arm study without a control group, and the follow-up period was short. The assessment of immediate treatment response during the liver ablation procedure by 3D US-CEUS fusion imaging is a new field, and this study is a preliminary report. Further studies with control groups and longer follow-up periods will be performed. Second, this study was performed using a specific type of US system, and the results may not be generalisable to other institutions or countries where other fusion imaging systems are used. However, other manufacturers (such as GE, Philips, and Hitachi) are promoting their own 3D US-CEUS fusion imaging techniques. Thus, 3D US-CEUS fusion imaging has bright prospects. Third, 3D US-CEUS fusion imaging was not suitable for invisible lesions on US because clear pre-ablation 3D US volume images could not be obtained as the reference images for fusion with the post-ablation CEUS images. In this situation, US-CT/MRI fusion imaging should be used instead.
Conclusion
3D US-CEUS fusion imaging is a feasible and valuable technique in the immediate evaluation and guidance of supplementary ablation during the liver cancer thermal ablation procedure.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Additional information
Funding
References
- Bo XW, Xu HX, Wang D, et al. (2016). Fusion imaging of contrast-enhanced ultrasound and contrast-enhanced CT or MRI before radiofrequency ablation for liver cancers. Br J Radiol 89:20160379.
- Lee MW, Rhim H, Cha DI, et al. (2013). Planning US for percutaneous radiofrequency ablation of small hepatocellular carcinomas (1–3 cm): value of fusion imaging with conventional US and CT/MR images. J Vasc Interv Radiol 24:958–65.
- Song KD, Lee MW, Rhim H, et al. (2013). Fusion imaging-guided radiofrequency ablation for hepatocellular carcinomas not visible on conventional ultrasound. AJR Am J Roentgenol 201:1141–7.
- Mauri G, Cova L, De Beni S, et al. (2015). Real-time US-CT/MRI image fusion for guidance of thermal ablation of liver tumors undetectable with US: results in 295 cases. Cardiovasc Intervent Radiol 38:143–51.
- Liu FY, Yu XL, Liang P, et al. (2012). Microwave ablation assisted by a real-time virtual navigation system for hepatocellular carcinoma undetectable by conventional ultrasonography. Eur J Radiol 81:1455–9.
- Toshikuni N, Tsutsumi M, Takuma Y, Arisawa T. (2014). Real-time image fusion for successful percutaneous radiofrequency ablation of hepatocellular carcinoma. J Ultrasound Med 33:2005–10.
- Yu XL, Liu FY, Liang P, et al. (2011). Microwave ablation assisted by a computerised tomography-ultrasonography fusion imaging system for liver lesions: an ex vivo experimental study. Int J Hyperthermia 27:172–9.
- Makino Y, Imai Y, Igura T, et al. (2013). Usefulness of the extracted-overlay function in CT/MR-ultrasonography fusion imaging for radiofrequency ablation of hepatocellular carcinoma. Dig Dis 31:485–9.
- Lee JY, Choi BI, Chung YE, et al. (2012). Clinical value of CT/MR-US fusion imaging for radiofrequency ablation of hepatic nodules. Eur J Radiol 81:2281–9.
- Zhong-Zhen S, Kai L, Rong-Qin Z, et al. (2012). A feasibility study for determining ablative margin with 3D-CEUS-CT/MR image fusion after radiofrequency ablation of hepatocellular carcinoma. Ultraschall Med 33:E250–5.
- Li K, Su ZZ, Xu EJ, et al. (2016). Improvement of ablative margins by the intraoperative use of CEUS-CT/MR image fusion in hepatocellular carcinoma. BMC Cancer 16:277.
- Claudon M, Dietrich CF, Choi BI, et al. (2013). Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver - update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound. Med Biol 39:187–210.
- Su ZZ, Li K, Xu EJ, et al. (2015). A clinical validation study for the feasibility and reliability of three-dimensional ultrasound-ultrasound automatic image registration. Int J Hyperthermia 31:875–82.
- Hiraoka A, Hirooka M, Koizumi Y, et al. (2010). Modified technique for determining therapeutic response to radiofrequency ablation therapy for hepatocellular carcinoma using US-volume system. Oncol Rep 23:493–7.
- Bruix J, Sherman M. American Association for the Study of Liver Diseases (2011). Management of hepatocellular carcinoma: an update. Hepatology 53:1020–2.
- Liu M, Huang GL, Xu M, et al. (2017). Percutaneous thermal ablation for the treatment of colorectal liver metastases and hepatocellular carcinoma: a comparison of local therapeutic efficacy. Int J Hyperthermia 33:1–11.
- Ahmed M, Solbiati L, Brace CL, et al. (2014). Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology 273:241–60.
- Toshikuni N, Shiroeda H, Ozaki K, et al. (2013). Advanced ultrasonography technologies to assess the effects of radiofrequency ablation on hepatocellular carcinoma. Radiol Oncol 47:224–9.
- Minami Y, Minami T, Chishina H, et al. (2016). US-US fusion imaging in radiofrequency ablation for liver metastases. Dig Dis 34:687–91.
- Lekht I, Gulati M, Nayyar M, et al. (2016). Role of contrast-enhanced ultrasound (CEUS) in evaluation of thermal ablation zone. Abdom Radiol (NY) 41:1511–21.
- Meloni MF, Andreano A, Franza E, et al. (2012). Contrast enhanced ultrasound: should it play a role in immediate evaluation of liver tumors following thermal ablation? Eur J Radiol 81:e897–902.
- Ierardi AM, Giorlando F, Piacentino F, et al. (2017). Factors predicting outcomes of microwave ablation of small hepatocellular carcinoma. Radiol Med 122:81–7.
- Kawasaki T, Hata KY, Kinoshita D, et al. (2016). Radiofrequency ablation guided by contrast-enhanced sonography versus B-mode sonography for hepatocellular carcinoma after transcatheter arterial chemoembolization. Dig Dis 34:692–5.
- Ayav A, Germain A, Marchal F, et al. (2010). Radiofrequency ablation of unresectable liver tumors: factors associated with incomplete ablation or local recurrence. Am J Surg 200:435–9.
- Wang T, Lu XJ, Chi JC, et al. (2016). Microwave ablation of hepatocellular carcinoma as first-line treatment: long term outcomes and prognostic factors in 221 patients. Sci Rep 6:32728.
- Zhai H, Liang P, Yu XL, et al. (2015). Microwave ablation in treating intrahepatic recurrence of hepatocellular carcinoma after liver transplantation: an analysis of 11 cases. Int J Hyperthermia 31:863–8.
- Zhang D, Liang P, Yu X, et al. (2013). The value of artificial pleural effusion for percutaneous microwave ablation of liver tumour in the hepatic dome: a retrospective case-control study. Int J Hyperthermia 29:663–70.
- Park HJ, Lee MW, Rhim H, et al. (2015). Percutaneous ultrasonography-guided radiofrequency ablation of hepatocellular carcinomas: usefulness of image fusion with three-dimensional ultrasonography. Clin Radiol 70:387–94.

