Abstract
Purpose: Radiotherapy (RT) treatment of locally-advanced and recurrent head and neck carcinoma (HNC) results in disappointing outcomes. Combination of RT with cisplatin or cetuximab improves survival but the increased toxicity and patient's comorbidity warrant the need for a less-toxic radiosensitizer. Stimulated by several randomized studies demonstrating the radio-sensitizing effect of hyperthermia, we developed the HYPERcollar. Here, we report early experience and toxicity in patients with advanced HNC.
Methods and materials: 119 hyperthermia treatments given to 27 patients were analyzed. Hyperthermia was applied once a week by the HYPERcollar aimed at achieving 39–43 °C in the target area, up to patients’ tolerance. Pre-treatment planning was used to optimize treatment settings. When possible, invasive thermometry catheters were placed.
Results: Mean power applied during the 119 hyperthermia treatments ranged from 120 to 1007 W (median 543 W). 15 (13%) hyperthermia treatments were not fully completed due to: pain allocated to hyperthermia (6/15), dyspnea from sticky saliva associated with irradiation (2/15) and unknown reasons (7/15). No severe complications or enhanced thermal or mucosal toxicities were observed. Excluding post-operative treatment, response rates after 3 months were 46% (complete) and 7% (partial).
Conclusion: Hyperthermia with the HYPERcollar proved to be safe and feasible with good compliance and promising outcome.
Introduction
Patients with locally advanced primary head and neck carcinoma (HNC) are usually treated with radiation therapy, often combined with systemic therapy i.e., cisplatin or cetuximab. However, combined treatment is not suitable for a proportion of HNC patients. In patients with loco-regional treatment only, a two-year loco-regional control rate of 50% and a two-year overall survival rate of 53% was reported in a meta-analysis study by Pignon et al. [Citation1]. In patients with loco-regional radiation treatment combined with cisplatin or cetuximab, a two-year loco-regional control rate of 53% for cetuximab and 80% for cisplatin; and a two-year overall survival rate of 68% for cetuximab and 78% for cisplatin was reported [Citation2].
For recurrent HNC, the treatment of choice is surgery or (re)irradiation based on tumor site and previous irradiation. However, only 20% of patients are fit for this treatment and local tumor control after retreatment is only 26–52% [Citation3,Citation4], leading to a two-year overall survival of 10–20% for re-irradiation and chemotherapy [Citation5]. Moreover, toxicity after re-treatment with concurrent chemo-radiotherapy is substantial (grade four toxicity 18–23% and grade five toxicity 5–8%) [Citation5,Citation6]. Hence, treatment outcome in patients suffering from locally advanced or recurrent HNC is insufficient. As most patients die because of local recurrence or residual disease, there is a need for improved loco-regional treatment [Citation7]. Hyperthermia is known to enhance the therapeutic ratio of radiation resistant tumors [Citation8] and several randomized trials demonstrated survival benefits by adding hyperthermia to radiation [Citation9–12]. In a systematic review and meta-analysis by Datta et al. [Citation13], a significant improvement in loco-regional control (LRC) was found for HNC patients, with an odds ratio of 2.92 (95% CI, 1.58–5.42) in favor of the combined treatment of hyperthermia plus radiation versus radiation [Citation14]. In a phase III trial of Huilgol et al., hyperthermia-enhanced radiation treatment resulted in a higher complete response rate (79% versus 42% complete response) [Citation15]. Given this ability of hyperthermia to enhance outcome without increasing toxicity [Citation16], it seems reasonable to further study the combination of radiotherapy with hyperthermia for HNC. The development of the HYPERcollar has made it possible to extend the application of hyperthermia from superficial to deeply located HNC [Citation17].
The aim of this study is to describe the early clinical experience with deep hyperthermia treatment using the HYPERcollar combined with radiotherapy. Hereto, we analyzed feasibility and toxicity of deep hyperthermia in a cohort of patients with locally-advanced or recurrent HNC.
Patients and methods
Patients
Patients eligible for hyperthermia treatment were adults of 18 years and older, with a Karnofsky Performance Score of ≥70 and histologically proven HNC. We included consecutive patients with either recurrence of HNC or patients with inability to receive chemotherapy. Data were retrospectively analyzed.
Exclusion criteria were systemic temperatures >39 °C, oxygen saturation <90%, claustrophobia, tumor caudally of a tracheostoma (this prevents penetration of the microwaves to the tumor) and the presence of a pacemaker.
Metal implants in the head and neck region were not a contraindication. All work was conducted with the approval of the local Medical Ethical Committee.
Radiotherapy
Radiotherapy was given using either external beam irradiation or interstitial irradiation. A computerized tomography (CT) scan was made while applying a patient-specific thermoplastic mask and intravenous contrast was injected for better tumor visualization. Target volumes were delineated by the HNC radiation-oncologist and used in both radiotherapy and hyperthermia treatment planning.
Preferably, external beam irradiation was delivered stereotactically using the Cyberknife (CBK) (Accuray Inc., Sunnyvale, CA), because the higher dose per fraction is expected to lead to a higher hyperthermia-induced enhancement. Intensity-modulated radiotherapy (IMRT) was given in a 2 Gy fraction dose to a total dose of 40–70 Gy, given 5 or 6 times a week (19 patients). The 40 Gy scheme was given with palliative intent. Multiple fractionation schemes were used for the 7 patients treated with CBK (5*5.5 Gy, 6*5 Gy, 6*5.5 Gy or 6*6 Gy, given twice a week) [Citation3,Citation18,Citation19]. For the interstitial radiotherapy, a total dose of 38 Gy in 12 fractions was given (1 patient), dosed at 5 mm depth. Brachytherapy (BT) and CBK treatment was given to patients with recurrent disease as a first re-treatment option; no elective nodal areas were treated in these patients.
Hyperthermia
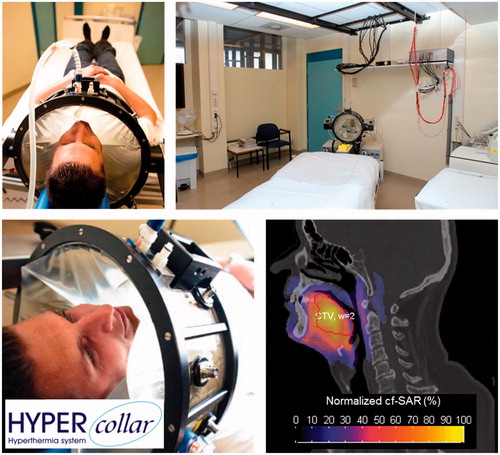
Hyperthermia was applied by radiofrequency (RF) electromagnetic energy using the HYPERcollar (), a twelve antenna applicator operating at 433 MHz for target selective heating [Citation20]. Treatment planning was applied for each patient to derive the settings per antenna (RF-power-weighting, phase-difference) leading to an optimum heat focusing at the target region. A full 3D patient model was generated based on the planning CT for radiotherapy and comprised of exterior, fat, muscle, bone, cartilage, white matter and grey matter [Citation20–22]. This 3D model was used for an electromagnetic simulation per antenna. Next, the RF-power absorption pattern, expressed in the specific absorption rate (SAR), was optimized using the hotspot-target-quotient [Citation22,Citation23]. Target coverage of 25% of the maximum SAR level (TC25%) [Citation22] was used to decide whether hyperthermia treatment was deemed possible:
Figure 1. Different views of the HYPERcollar, the clinical setup and an example simulated SAR pattern on top of a CT scan.
TC25% ≥ 75%: hyperthermia was always applied.
TC25% between 25 and 75%: inclusion depended on feasibility of invasive temperature measurement.
TC25% < 25%: hyperthermia was not applied.
Note that this criterion became more restrictive over time since initially tumor properties were assigned to the entire clinical target volume (CTV), leading to overestimated SAR in non-tumor CTV regions. This was corrected when gross tumor volume (GTV) delineations were introduced, which led to lower SAR values in the CTV margin and hence substantially lower TC25% values.
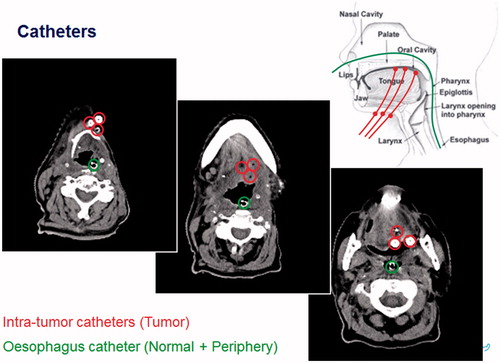
For thermometry purposes, closed-tip catheters were placed in the tumor or in normal tissue, using preferably local anesthesia, and/or at the skin (see ). A CT scan was made to document the location of all catheter tracts.
Just before treatment, multi-sensors temperature probes were inserted into the catheters for monitoring temperature in the tumor or surrounding organs at risk. The exact insertion length was measured for reconstructing the surrounding tissue type for each sensor. The HYPERcollar was placed around the target volume and the waterbolus was filled with deionized water. This water was circulated at a temperature of 20–30 °C, depending on target depth. Pre-optimized RF-power and phase settings were applied and RF-power was gradually increased until target temperature or hotspots were reached. Hyperthermia was applied for 60–75 min aimed at achieving 43 °C throughout the target region, but hotspots (normal tissue temperatures above 44 °C or pain-related discomfort) restricted this temperature. Initially, a library of three to six SAR distributions were pre-optimized for responding to hotspots [Citation20]. Later, we introduced our dedicated real-time complaint-adaptive treatment protocol [Citation20,Citation22]. During treatment, we continuously monitored heart rate, RF-power and phase per antenna, and temperature.
Evaluation
Over time, treatment time duration was increased from 60 to 75 min, to account for the 15 min heating-up phase, so tolerance was defined as the percentage of patients who completed 55 min (60-min group) or 70 min (75-min group).
The estimated SAR (SARest) [Citation22] was calculated using the real-time measured signals (power and phase) per channel and the respective predicted electric field per antenna. An experimentally defined applicator efficiency factor of 0.7 was applied to account for losses in the conductors and antennas. SARest was quantified by using the mean SARest in the target region averaged over all sessions.
Measured temperatures were quantified using the time-average of the T90 and T50, where T90 and T50 are the 90th and 50th percentile of the temperatures measured at each moment [Citation24]. Three HNC hyperthermia groups were distinguished to stratify the analysis for location dependent differences in treatment quality 1) head central: consisting of nasal cavity, paranasal sinus and nasopharynx; 2) head caudal: oral cavity and oropharynx; 3) Neck: larynx, hypopharynx and neck node metastases.
Follow-up and toxicity
Patients were weekly examined for the development of skin or subcutaneous burns within 24 h after treatment. Since no specific hyperthermia toxicity scoring system exists, toxicity was assessed according to the generic Common Terminology Criteria for Adverse Events (CTCAE) protocol, version 4.03 [Citation25]. Assessment was based on data from patient’s records and interviews, and responses were evaluated after three months.
After treatment completion, patients were seen in the outpatient clinic every 2 months during the first year, every 3 months for year 2, every 4 months for year 3 and every 6 months for years 4–5. Follow-up visits consisted of updating patient’s case history, physical examination and in case of suspicion for recurrence, a CT or magnetic resonance imaging (MRI) scan. Tumor response was analyzed by the WHO guidelines [Citation26] as complete, partial or no response.
Results
Patient statistics
Between February 2007 and July 2013, 27 patients were treated with irradiation in combination with deep hyperthermia in HNC. The primary tumor was located in the oropharynx [Citation10], the larynx [Citation5], the nasopharynx [Citation5], the sinonasal area [Citation3], the oral cavity [Citation3] and trachea [Citation1]. Eighteen patients were treated because of a recurrence and nine patients had a locally advanced tumor. Three patients received post-operative RT (PORT), of these two had recurrent disease and one locally advanced disease. Most patients (21/27) had squamous cell carcinomas, three had adenoid cystic carcinoma, one had mucoepidermoid lowgrade carcinoma and two poorly differentiated non-keratotic carcinoma. All patients had KPS >70, but did not receive concurrent chemo-radiation. Chemotherapy was not added in 18/27 patients with recurrent disease. In 9 patients with locally advanced disease the reason to omit chemotherapy was: age (4/9 patients), induction chemotherapy in stead of concurrent chemotherapy (2/9), patient's refusal (1/9), palliative intent of treatment (1/9) and unknown (1/9). Tumors had a median CTV size of 64 ml (range 28–185 ml).
The radiation dose ranged from BED 80 to 144 Gy (median BED 120 Gy). For locally advanced disease, median BED was 128.3 Gy, for recurrent disease 116 Gy. shows the demographics, tumor characteristics and treatment characteristics of the patients.
Table 1. Patient, tumor and treatment characteristics.
Hyperthermia
In total, 119 hyperthermia treatments were given to the 27 patients, with a median of 4 treatments. Hyperthermia treatments were given once or twice a week, 1–3 h after irradiation.
The median duration of hyperthermia was 60 min (range 18–75 min). Catheters for thermometry were placed in 16 patients, for measuring temperatures in the tumor (8/16) or organs at risk (8/16). Due to a strong confounding influence of swallowing and speaking, we abandoned intraluminal temperature measurements after the first two patients.
Median SARest for the whole group was 72.6 W/kg, which increased over time from 60.7 W/kg (60-min group) to 81.8 W/kg (75-min group). The increase in applied power by 21% (464 W to 595 W) led to an even higher increase of SARest of 35% (60.7 W/kg to 81.8 W/kg), indicating an improvement of the applied SAR pattern by the new steering method.
The median measured temperature in the tumor or normal tissue per patient was 38.8 °C (range 37.8–39.3 °C) (). Note that temperatures were measured predominantly in challenging cases since invasive thermometry was mandatory in our protocol when the predicted TC25% was below 75%.
Table 2. Thermometry, toxicity and response data of included patients.
Toxicity and early experience
Only 2 patients experienced grade 2 toxicity due to hyperthermia treatment, both of them were treated for a sinonasal carcinoma near the eye. This toxicity, a burning sensation of the eyeball, existed before treatment in one of these patients but increased.
Mean hyperthermia treatment time was 94% of planned duration. 15/119 (13%) hyperthermia treatments were stopped before the prescribed duration: Nine treatments in the 60-min group and six in the 75-min group. Six treatments were stopped due to additional pain caused by the tumor or treatment position, which we allocated to hyperthermia. In two cases treatment was interrupted by dyspnea, caused by sticky saliva allocated to radiotherapy. The earlier-mentioned burning sensation of the eyeball was also reason to prematurely stop hyperthermia treatment, but this pain manifested in only 1/6 treatments. No reason to prematurely stop treatment was given for four patients (six treatments), probably technical problems or patients discomfort was reason to stop treatment. One patient fulfilled hyperthermia treatment time, but developed cardiac arrhythmia that recovered spontaneously and did not recur.
One patient with a recurrent oropharyngeal carcinoma experienced pain caused by a metal implant in the mandible after the first hyperthermia treatment. A catheter was placed close to the implant and a new hyperthermia plan was made to reduce local power, which resulted in five complete hyperthermia sessions. No complaints during hyperthermia treatments were seen in another patient with metal implants within the CTV. The presence of dental fillings within the CTV also appeared tolerable.
Toxicity due to irradiation was present in most patients. Radiotherapy toxicity had a median score of grade 3, this was caused by tube feeding (11 patients), radiation dermatitis (Moist desquamation in areas other than skin folds and creases, in 2 patients), pneumonitis (2 patients) and fibrosis (1 patient).
Follow-up
The median follow-up time of this study was 13 months (range 2–107 months). After three months, an overall response rate of 59% was noted. provides MRI scans of an example patient with a cT4N0M0 base of tongue carcinoma that was successfully treated. After three months, complete response (CR) excluding the 3 patients with PORT and partial response (PR) were seen in 46% and 7%, respectively (). CR rates for locally-advanced and recurrent HNC were 78% and 39%, respectively. The 2-year Kaplan–Meier estimates of local control (LC) and overall survival (OS) were 40% and 44%, respectively. The 2-year rates for patients with locally advanced disease were of 50% (LC) and 67% (OS) and for re-irradiated patients 36% (LC) and 33% (OS). The 2-year rates for patients with PORT were of 100% (LC) and 67% (OS) and for no-PORT patients 32% (LC) and 42% (OS). Kaplan Meier figures are added as supplementary files.
Figure 3. Example of complete response after combined hyperthermia and irradiation in a cT4N0M0 carcinoma of the base of tongue.
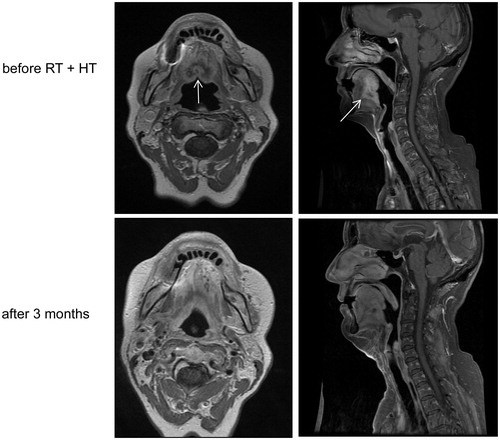
Table 3. Response and survival of included patients.
Discussion
In this study, we assessed the feasibility of hyperthermia using the HYPERcollar in HNC. The mentioned 27 patients showed good compliance since 87% of the treatments were completed. To ensure a 60-min hyperthermia treatment, treatment time was increased to 75 min, which did not reduce the completion rate and the power applied was even higher, probably due to a learning curve and the implementation of improved positioning and complaint-adaptive hyperthermia [Citation22]. The improvements are also reflected in the SARest, which increased from 60.7 W/kg (60-min group) to 81.8 W/kg (75-min group). Unfortunately, no improvement in measured median and maximum temperature were observed, which we allocate to a change in the catheter positioning protocol. Initially, we measured at locations of high-predicted SAR aimed at validating heating feasibility by measuring the highest temperature, but later we measured at low SAR locations to monitor and improve the lowest target temperatures. Note that only 8/27 patients received invasive thermometry, while dosimetry by SARest was performed in all patients and, although affected by waterbolus shape differences, provides a 3D assessment. We conclude that a higher time-temperature hyperthermia dose was achieved since at least equal, but probably higher temperatures, were obtained accompanied by an increased treatment time.
The quality of hyperthermia can be expressed in median temperature (T50), lowest temperature (T90) or thermal dose (CEM43T90) [Citation27]. In our study the median measured T50 was 39 °C in the 16/27 patients were invasive thermometry was available. Note that T90 and CEM43T90 were not assessed since reliable estimates of the lowest achieved temperature (T90), and hence lowest thermal dose (CEM43T90), in the target region requires over 10 probes, which was impossible based on the few probes used in this study. In addition, temperatures only served to verify our treatment approach since using accurate simulations are being used for 3D dosimetry. All temperatures were measured in tumor indicative normal tissue locations and predominantly in patients with low TC25% since invasive thermometry was mandatory. Also note that, although most clinical trials are aimed at achieving hyperthermia at 43 °C in the tumor, in reality the measured temperatures are much lower [Citation28]. Intratumoral T50/T90 temperatures of major successful multi-center phase III trials were on average 40.3 °C/39.2 °C [Citation29] and 39.4–40.9 °C/38.3–39.9 °C [Citation30,Citation31]. Note that these studies reported measured temperatures at the cervix entrance or in poorly perfused tumor regions; hence the highest temperature in the tumor is measured. Although the absence of 3D temperature data hampers a true comparison, we believe that the temperature levels obtained already were very close to those reported in literature in the large target volumes treated (median CTV size 63.5 ml).
Combining our simulation and measurement data, we observe a tremendous thermoregulatory response in the head and neck: delivery of power in one case even beyond 1 kW and SARest levels beyond 80 W/kg led to temperatures below 43 °C, i.e., the maximum measured temperature was 42.3 °C. Reflecting the fact that the HYPERcollar was initially developed for heating of the larynx, maximum temperature results indeed were higher in this region (39.0 °C vs 40.8 °C). To further improve the median temperatures we recently introduced the novel HYPERcollar3D into the clinic, which theoretically will double the SAR delivered to the target region [Citation23], as shown to be required in this study.
Simulation studies showed that patients with a tracheostoma could be treated with the HYPERcollar, as long as the tumor is not located caudally of the tracheostoma. The technical feasibility was confirmed by treatment of 2 patients with a tracheostoma, without positioning problems and side effects, and TC25% was sufficiently high (56.7 and 63.9 in these two patients).
Toxicity of deep hyperthermia treatment was mild (mainly grade 1). Most toxicity was due to metal implants >1 cm, which were not taken out and caused edema and pain. Toxicity due to metal dental fillings was not observed. Gradually we experienced that sticky saliva is a factor that can hamper hyperthermia treatment, and therefore, we evacuate saliva before treatment. Furthermore, adequate treatment with pain medication, sometimes with additional short-acting opioids just before hyperthermia, lengthened duration of treatment and eased patient positioning.
This study’s primary objective was to assess feasibility of deep H&N heating. Secondary, clinical response was evaluated and despite the limited follow-up, promising results were noticed, as 59% of our patients showed complete or partial response. The included patients were either re-irradiated, or had locally advanced disease. Other hyperthermia studies used a variety of superficial heating [Citation14,Citation15,Citation32,Citation33]. Datta et al. treated 32 patients with combined irradiation and local hyperthermia, for at least 20 min twice a week. They obtained a response in 63–76% for locally advanced HNC [Citation14]. Valdagni et al. treated metastatic lymph nodes in stage IV HNC patients, and this randomized trial showed an increase in 5 year nodal control of the hyperthermia and irradiation group, compared to irradiation alone (69% versus 24%) [Citation33]. The study of Hua et al. used nasopharyngeal cavity hyperthermia added to irradiation and chemotherapy for nasopharyngeal cancer. They showed improved 5 year local control for the hyperthermia group (91% versus 79%) [Citation32,Citation34]. Huilgol et al. showed improved complete response (79% versus 42%) and increased median survival time (241 vs 145 days) for the patients treated with irradiation combined with hyperthermia, compared to irradiation alone [Citation15].
A limitation of our study is the small sample size, the heterogeneous group, the short median follow-up time and the retrospective nature. Randomized trials with long-term follow-up are needed to assess the exact benefit of adding deep hyperthermia to irradiation. The strength of our study is that our data show that a variety of anatomic sites can be treated using the HYPERcollar, without major toxicity.
Hyperthermia causes a plethora of effects like the direct cytotoxic effects in hypoxic regions, inhibition of DNA double strand break repair and reversion of hypoxia by increased blood flow in tumors [Citation35]. The latter effect is specifically interesting in HNC scenarios since these tumors harbour hypoxic areas and randomized trials for dose-escalation to overcome this are ongoing [Citation36]. Our data in recurrent disease show that heating deeply located tumors in the head and neck region is feasible, which allows to exploit the specific benefits in a much larger group of patients then before. Future studies may investigate the use of hyperthermia as adjuvant to also primary irradiation, e.g., in patients above 70 that generally do not benefit from chemotherapy [Citation37] and HPV positive cases, where chemotherapy might be replaced by hyperthermia. The biological evidence and already existing clinical data [Citation13], supplemented with our data on hyperthermia feasibility in deeply located HNC, are a strong rationale for designing new clinical studies for exploitation of the large opportunities that this new technology provides in larger groups of patients.
Conclusion
This study shows that deep hyperthermia using the HYPERcollar is safe and feasible since no severe (>Grade II) hyperthermia-related toxicity was observed. In addition, deep hyperthermia using the HYPERcollar might be effective since 46% of patients without PORT showed complete or partial response. The high simulated SAR values combined with the measured temperatures at the target regions periphery indicate that adequate deep hyperthermia is achievable in a proportion of patients, but that improved technology is needed to achieve good heating in all patients. Given the promising dosimetric and early clinical results, the addition of deep H&N hyperthermia to radiotherapy is now part of the standard of care in our institution for patients with recurrent HNC and a study on hyperthermia for primary treatment of HNC is in preparation.
Acknowledgments
We thank all participating patients, Cobi van der Zee and Netteke van Holthe for their work.
Disclosure statement
MMP has financial interest in Sensius BV. No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Pignon JP, Le Maitre A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14.
- Magrini SM, Buglione M, Corvo R, et al. Cetuximab and radiotherapy versus cisplatin and radiotherapy for locally advanced head and neck cancer: a randomized phase II trial. J Clin Oncol. 2016;34:427–435.
- Roh KW, Jang JS, Kim MS, et al. Fractionated stereotactic radiotherapy as reirradiation for locally recurrent head and neck cancer. Int J Radiat Oncol Biol Phys. 2009;74:1348–1355.
- Yamazaki H, Kodani N, Ogita M, et al. Reirradiation of head and neck cancer focusing on hypofractionated stereotactic body radiation therapy. Radiat Oncol. 2011;6:98.
- Spencer SA, Harris J, Wheeler RH, et al. Final report of RTOG 9610, a multi-institutional trial of reirradiation and chemotherapy for unresectable recurrent squamous cell carcinoma of the head and neck. Head Neck. 2008;30:281–288.
- Langer CJ, Harris J, Horwitz EM, et al. Phase II study of low-dose paclitaxel and cisplatin in combination with split-course concomitant twice-daily reirradiation in recurrent squamous cell carcinoma of the head and neck: results of Radiation Therapy Oncology Group Protocol 9911. J Clin Oncol. 2007;25:4800–4805.
- Vargo JA, Ward MC, Caudell JJ, et al. A multi-institutional comparison of SBRT and IMRT for definitive reirradiation of recurrent or second primary head and neck cancer. Int J Radiat Oncol Biol Phys. 2018;100(3):595–605.
- Kampinga HH. Cell biological effects of hyperthermia alone or combined with radiation or drugs: a short introduction to newcomers in the field. Int J Hyperthermia. 2006;22:191–196.
- Amichetti M, Romano M, Busana L, et al. Hyperfractionated radiation in combination with local hyperthermia in the treatment of advanced squamous cell carcinoma of the head and neck: a phase I-II study. Radiother Oncol. 1997;45:155–158.
- Sugahara T, van der Zee J, Kampinga HH, et al. Kadota Fund International Forum 2004. Application of thermal stress for the improvement of health, 15-18 June 2004, Awaji Yumebutai International Conference Center, Awaji Island, Hyogo, Japan. Final report. Int J Hyperthermia. 2008;24:123–140.
- Jones EL, Oleson JR, Prosnitz LR, et al. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol. 2005;23:3079–3085.
- Franckena M. Review of radiotherapy and hyperthermia in primary cervical cancer. Int J Hyperthermia. 2012;28:543–548.
- Datta NR, Rogers S, Ordonez SG, et al. Hyperthermia and radiotherapy in the management of head and neck cancers: a systematic review and meta-analysis. Int J Hyperthermia. 2016;32:31–40.
- Datta NR, Bose AK, Kapoor HK, et al. Head and neck cancers: results of thermoradiotherapy versus radiotherapy. Int J Hyperthermia. 1990;6:479–486.
- Huilgol NG, Gupta S, Sridhar CR. Hyperthermia with radiation in the treatment of locally advanced head and neck cancer: a report of randomized trial. J Cancer Res Ther. 2010;6:492–496.
- Horsman MR, Overgaard J. Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol (R Coll Radiol). 2007;19:418–426.
- Paulides MM, Verduijn GM, Van Holthe N. Status quo and directions in deep head and neck hyperthermia. Radiat Oncol. 2016;11:21.
- Bonomo P, Cipressi S, Iermano C, et al. Salvage stereotactic re-irradiation with CyberKnife for locally recurrent head and neck cancer: a single center experience. Tumori. 2014;100:278–283.
- Cengiz M, Ozyigit G, Yazici G, et al. Salvage reirradiaton with stereotactic body radiotherapy for locally recurrent head-and-neck tumors. Int J Radiat Oncol Biol Phys. 2011;81:104–109.
- Paulides MM, Bakker JF, Linthorst M, et al. The clinical feasibility of deep hyperthermia treatment in the head and neck: new challenges for positioning and temperature measurement. Phys Med Biol. 2010;55:2465–2480.
- Verhaart RF, Fortunati V, Verduijn GM, et al. CT-based patient modeling for head and neck hyperthermia treatment planning: manual versus automatic normal-tissue-segmentation. Radiother Oncol. 2014;111:158–163.
- Rijnen Z, Bakker JF, Canters RA, et al. Clinical integration of software tool VEDO for adaptive and quantitative application of phased array hyperthermia in the head and neck. Int J Hyperthermia. 2013;29:181–193.
- Togni P, Rijnen Z, Numan WCM, et al. Electromagnetic redesign of the HYPERcollar applicator: toward improved deep local head-and-neck hyperthermia. Phys Med Biol. 2013;58:5997–6009.
- Franckena M, Fatehi D, de Bruijne M, et al. Hyperthermia dose-effect relationship in 420 patients with cervical cancer treated with combined radiotherapy and hyperthermia. Eur J Cancer. 2009;45:1969–1978.
- US Department of Health and Human Services; National Institutes of Health; National Cancer Institute [Internet]. Common Terminology Criteria for Adverse Events (CTCAE); Version 4.03 2010 June 14 [cited 2013 July]; Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247.
- Thrall D, Dewhirst M, Jones E, et al. A clinically proven, prospective, thermal dose descriptor exists. Clin Cancer Res. 2006;12:1944–1945.
- van Rhoon GC. Is CEM43 still a relevant thermal dose parameter for hyperthermia treatment monitoring? Int J Hyperthermia. 2016;32:50–62.
- Issels RD, Lindner LH, Verweij J, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol. 2010;11:561–570.
- van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, et al. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet. 2000;355:1119–1125.
- Fatehi D, van der Zee J, Notenboom A, et al. Comparison of intratumor and intraluminal temperatures during locoregional deep hyperthermia of pelvic tumors. Strahlenther Onkol. 2007;183:479–486.
- Hua Y, Ma S, Fu Z, et al. Intracavity hyperthermia in nasopharyngeal cancer: a phase III clinical study. Int J Hyperthermia. 2011;27:180–186.
- Valdagni R, Amichetti M. Report of long-term follow-up in a randomized trial comparing radiation therapy and radiation therapy plus hyperthermia to metastatic lymph nodes in stage IV head and neck patients. Int J Radiat Oncol Biol Phys. 1994;28:163–169.
- Paulides MM, Van Rhoon GC. Towards developing effective hyperthermia treatment for tumours in the nasopharyngeal region. Int J Hyperthermia. 2011;27:523–525.
- van den Tempel N, Odijk H, van Holthe N, et al. Heat-induced BRCA2 degradation in human tumours provides rationale for hyperthermia-PARP-inhibitor combination therapies. Int J Hyperthermia. 2017;1–8. doi:10.1080/02656736.2017.1355487.
- Welz S, Monnich D, Pfannenberg C, et al. Prognostic value of dynamic hypoxia PET in head and neck cancer: results from a planned interim analysis of a randomized phase II hypoxia-image guided dose escalation trial. Radiother Oncol. 2017;124:526–532.
- Pignon JP, Le Maitre A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14.