Abstract
Purpose: This study aimed to elucidate the contribution of T cell-mediated antitumor immunity in the antitumor effect of local hyperthermia (LH).
Materials and methods: C57BL/6J mice were injected with the mouse lymphoma cell line, E.G7-OVA, in the right femur on day 0. LH was induced by immersing the right femur in a water bath at 42 °C for 60 min on day 7, followed by administration of anti-CD8 monoclonal antibodies (mAb) or anti-CTLA-4 mAb (days 8, 11, and 14). The effect of LH on tumor growth (TG) was assessed by measuring the duration until tumor volume reached 1000 mm3 and survival time. Tumor-specific T cell responses were measured using enzyme-linked immunospot (ELISpot) assay.
Results: TG with and without LH treatment was 9.0 ± 9.6 and 7.0 ± 1.6 days, respectively. TG was significantly slower with LH treatment (p = .01). The therapeutic effect of LH was mitigated by addition of anti-CD8 mAb (p < .05 for both TG and survival) compared with the untreated (control) group. Furthermore, addition of anti-CTLA-4 mAb did not significantly affect the therapeutic effect of LH. The ELISpot assay showed that the number of spots in the LH group (276.3 ± 14.5) was significantly greater than in the control group (59.0 ± 4.5, p < .001).
Conclusion: CD8-positive T cell-mediated antitumor immunity significantly contributes to the antitumor effect of LH.
Introduction
There is a recent increased interest in the significance of tumor immunity in the field of cancer treatment. Excellent outcomes have been reported by clinical trials for a variety of malignant tumors using immune checkpoint inhibitors (ICIs) such as anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) monoclonal antibodies (mAb), anti-programmed cell-death 1 (PD-1) mAb, and anti-programmed cell-death ligand 1 (PD-L1) mAb [Citation1–3]. Currently, ICIs play a significant role in cancer treatment. On the other hand, it was reported that conventional therapy, such as chemotherapy and radiotherapy, also activate the host’s antitumor immunity in certain conditions [Citation4–7]. Hyperthermia has long been used as a modality for cancer treatment, and a number of reports and reviews have suggested that hyperthermia may enhance antitumor immunity by inducing tumor immunogenicity or activating an immune response [Citation8–10]. We demonstrated that expression of major histocompatibility complex class I in tumor cells, which plays an important role in cytotoxic T lymphocyte (CTL) recognition of tumor cells, was enhanced in rectal cancer patients who underwent preoperative hyperthermo-chemoradiotherapy [Citation11]. The addition of hyperthermia to ICIs could further improve cancer treatment in the future; however, the interaction between hyperthermia and ICIs has not been well clarified. Clinical data have shown that tumor-specific CTLs have a central role in antitumor immunity [Citation12], and also synergistic effect of ICI can be observed when it is used in combination with other anti-cancer therapies, such as radiotherapy [Citation13,Citation14]. However, only few reports have described the mechanism of CTL induction by hyperthermia, and to the best of our knowledge no such report has been described in a clinical setting [Citation15,Citation16].
In this study, we used a mouse model of local hyperthermia (LH) to evaluate whether tumor-specific CTLs could be induced by LH and whether the antitumor effects of LH could be influenced by anti-CD8 mAb or anti-CTLA-4 mAb.
Materials and methods
Mice and tumor cell line
C57BL/6J female mice, aged 6–7 weeks were purchased from the Japan Shizuoka Laboratory Animal Center (Shizuoka, Japan). Mice were bred and maintained under specific pathogen-free conditions. The ovalbumin transfected EL-4 lymphoma cell line, E.G7-OVA, was purchased from American Type Culture Collection (Manassas, VA). Cells were cultured in RPMI-1640 medium (Life Technologies, Inc., Tokyo, Japan) containing 10% fetal bovine serum and 1% penicillin/streptomycin (Sigma, MO) at 37 °C in a humidified atmosphere of 5% CO2. C57BL/6J mice were injected with E.G7-OVA (2.0 × 106 cells/mouse) in the right femurs.
Local hyperthermia
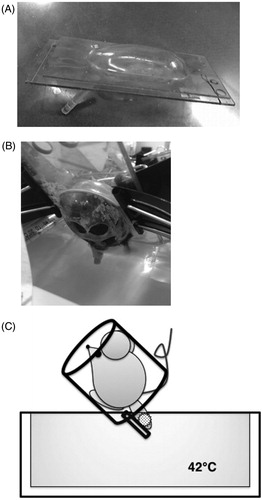
One week after tumor inoculation, mice were anesthetized via intraperitoneal injection of pentobarbital sodium and placed into acrylic capsules (Okamoto Seisakusyo Co Ltd., Osaka, Japan) (). The tumor-bearing legs were fixed to a plastic pole that was attached to the capsule. LH was performed by immersing the tumor-bearing leg in a constant-temperature circulating water bath at 42 °C for 60 min. Only the tumor-bearing legs were in hot water (). The control mice received only anesthesia and were not treated with LH.
Figure 1. Schema of LH in this study. (A) Acrylic capsule into which the mouse is placed after anesthesia. The tumor-bearing legs were fixed to a plastic pole that was attached to the capsule. (B) local hyperthermia (LH) was performed by immersing the tumor-bearing leg in a constant-temperature circulating water bath at 42 °C for 60 min. (C) Schematic diagram of LH.
Tumor growth assay
After LH treatment, tumor size was measured using callipers, three times per week for 2 weeks (n = 8 and n = 7 per group in the experiments with only LH and with LH and immunomodulatory antibodies, respectively). Tumor volume was calculated using the following formula: (a × b × c × π)/6, where a, b, and c represent the three orthogonal diameters of the tumor. The effect of LH on tumor growth (TG) was evaluated by “TG duration,” which was defined as the number of days taken until the tumor volume reached 1000 mm3 following LH treatment.
Immunomodulatory antibody treatment
To examine the antitumor effect of immunomodulatory antibodies on LH, anti-mouse CD8 mAb (66 mg/mouse, administered intravenously) or anti-mouse CTLA-4 mAb (200 mg/mouse, administered intraperitoneally) dissolved in 0.1 ml phosphate buffered saline (PBS) was injected on days 8, 11, and 14. In the control mice, 0.1 ml PBS was injected. Antibodies were purchased from eBioscience (San Diego, CA).
Enzyme-linked immunospot (ELISpot) assay
Activation of T cells is accompanied by an increased production of cytokines such as interferon-γ (IFN-γ). Tumor-specific CTL activation was detected using an ELISpot kit (R&D Systems, Minnesota) according to the manufacturer's instructions. Briefly, splenocytes (1 × 105 cells) obtained 21 days after tumour inoculation of mice from each group were incubated with/without 30 Gy-irradiated E.G7-OVA cells (1 × 105 cells) in a well of mouse anti-IFN-γ precoated microplates for 36 h [Citation17]. After incubation, the wells were washed and were subsequently incubated with biotinylated anti-mouse IFN-γ antibody and alkaline phosphatase-conjugated streptavidin. The reaction was stopped using distilled water and spots were counted using an automated ELISpot reader (KS ELISPOT Research, Zeiss, Germany). All assays were performed in triplicate.
Ethical statement
The animal experiments were carried out with permission and under the regulation of the Gunma University Animal Care and Use Committee (permit number 09–074).
Statistical analysis
All statistical analyses were performed using SPSS statistics 19.0 (SPSS Inc, Chicago, IL). Two-tailed, unpaired Student’s t tests were used for TG and ELISpot assays. Survival analyses were performed according to the Kaplan–Meier method with log-rank statistics. Values of p < .05 were considered statistically significant.
Results
LH suppressed TG of E.G7-OVA tumors
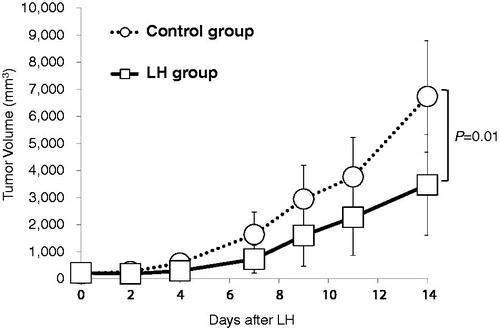
The therapeutic effect of LH against E.G7-OVA tumors was tested. TG of LH-treated (LH group) and untreated (control group) mice was 9.0 ± 9.6 and 7.0 ± 1.6 days, respectively (. LH significantly suppressed TG duration (p = .01).
Figure 2. LH suppressed TG. Local hyperthermia (LH) suppressed the growth of E.G7-OVA tumors in C57BL/6J mice. Tumor growth (TG) curves of EG.7-OVA tumors in the control group (open circles; ^) and LH-treated group (open squares; □). Data are showed as the mean tumor volume ± SE and n = 8 per group. LH: local hyperthermia.
LH-induced E.G7-OVA-specific IFN-γ-producing splenocytes
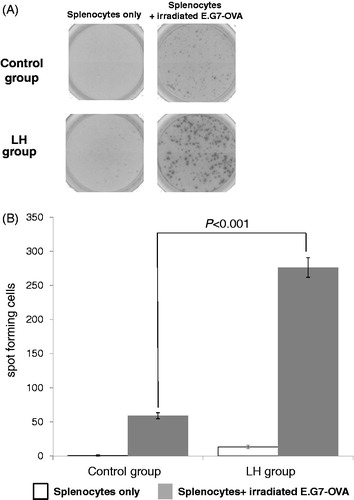
We investigated whether LH could induce a systemic antitumor response. ELISpot assays showed that the number of spots in the LH group incubated with irradiated E.G7-OVA (276.3 ± 14.5) was significantly higher compared with the control group (59.0 ± 4.4) (p < .001, . Although a certain population of IFN-γ-producing splenocytes showed specificity for E.G7-OVA in the control group, these results demonstrated that LH clearly induced E.G7-OVA-specific IFN-γ-producing splenocytes.
Figure 3. LH-induced tumor-specific splenocytes. To evaluate tumor-specific T cells induced by LH, ELISpot assays were performed using splenocytes obtained from mice from each group (n = 7 per group) as described in the Materials and Methods section. (A) Representative pictures of wells in the control and LH groups. Splenocytes were incubated with/without irradiated E.G.7-OVA. LH, local hyperthermia. (B) Data are reported as the mean number of spots ± SE of triplicate samples. Spot forming cells (SFC) were significantly increased in the LH group. Two-tailed, unpaired Student’s t-test was performed.
The therapeutic efficacy of LH was reduced by anti-CD8 mAb
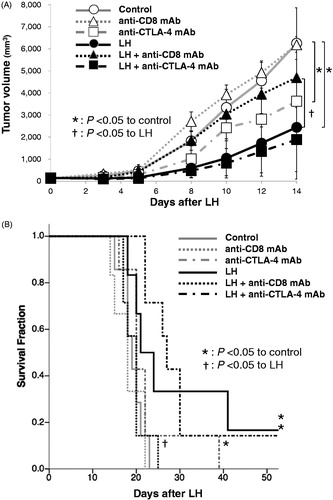
We next examined whether blockade of CD8-positive T cells affected the antitumor effect of LH. Compared with the control group, administration of anti-CD8 mAb alone affected neither TG duration (7.0 ± 0.8 versus 6.0 ± 0.4 days, p = .13) nor survival [median survival time (MST) 19.0 ± 2.5 versus 18.0 ± 3.4 days, p = .67] (). On the other hand, TG duration in the LH with anti-CD8 mAb group (6.0 ± 1.3 days) was significantly decreased compared with the LH alone group (9.0 ± 12.5 days) (p = .02 ). Furthermore, survival of the LH with anti-CD8 mAb group (20.0 ± 2.8 days) was significantly decreased compared with the LH alone group (22.5 ± 30.6 days) (p = .01, ).
Figure 4. CD8 depletion reduced the therapeutic efficacy of LH on TG and survival in EG.7-OVA-bearing C57BL/6 mice, and the addition of anti-CTLA-4 mAb to LH did not amplify the therapeutic efficacy of LH. (A) TG curves of EG.7-OVA tumors in the control group (open circles; ^), LH-treated group (closed circles; •), anti-CD8 mAb-treated control group (open triangles; △), LH with anti-CD8 mAb-treated group (closed triangles; ▲), anti-CTLA-4 mAb-treated control group (open squares; □), and LH with anti-CTLA-4 mAb-treated group (closed squares; ▪). Data are shown as the mean tumor volume ± SE of seven mice per group. *p < .05 between control and anti-CTLA-4 mAb-treated group, and control and LH-treated group. †p < .05 between LH group and LH with anti-CD8 mAb-treated groups. (B) Survival curves of E.G7-OVA-bearing C57BL/6J mice in the control group (gray solid line), LH-treated group (black solid line), anti-CD8 mAb-treated control group (gray dotted line), LH with anti-CD8 mAb-treated group (black dotted line), anti-CTLA-4 mAb-treated control group (gray dashed line), and LH with anti-CTLA-4 mAb-treated group (black dashed line). (n = 7 per group) *p < .05 for control, and †p < .05 for the LH-treated group.
Addition of anti-CTLA-4 mAb to LH did not enhance the therapeutic efficacy of LH
Finally, we examined whether addition of anti-CTLA-4 mAb to LH affected the antitumor effect of LH. Although administration of anti-CTLA-4 mAb significantly prolonged TG duration and survival compared with the control group, (9.0 ± 5.0 versus 7.0 ± 0.8 days; p = .02, 20.0 ± 7.4 versus 19.0 ± 2.5 days; p = .04, respectively), addition of anti-CTLA-4 mAb to LH slightly prolonged TG and survival compared with the LH group and this did not reach statistical significance (12.0 ± 32.4 versus 9.0 ± 12.5 days; p = .10, 27.0 ± 27.0 versus 22.5 ± 30.6 days; p = .92, respectively) ().
Discussion
Our study highlighted two main points. First, LH-induced tumor-specific IFN-γ-producing splenocytes in mice. Second, the antitumor effects of LH were reduced by administration of anti-CD8 mAb. These results suggest that CD8-positive T cell-mediated antitumor immunity is implicated in the antitumor effects of LH, at least in part.
Recently, there has been accumulating evidence supporting the concept that hyperthermia activates antitumor immunity, and tumor-specific immune responses induced by hyperthermia are required for effective cancer treatment [Citation9,Citation10]. Several reports have suggested that hyperthermia-induced antitumor immunity by releasing heat-shock protein (Hsp) from dying tumor cells [Citation18,Citation19]. Furthermore, it was also reported that Hsp helps antigen presentation in antigen presenting cells (APCs). Activated APCs may lead to CD8-positive T cell activation and subsequently induce tumor-specific CTLs [Citation20–22]. In this study, tumor-specific CTLs were induced by LH because the number of tumor-specific IFN-γ-secreting splenocytes was significantly greater in the LH group compared with the control group in the ELISpot assay.
Next, we determined to what extent LH-induced antitumor immunity activated was involved in antitumor effects. Blockade of CD8-positive T cells with anti-CD8 mAb significantly reduced the antitumor effects of LH in terms of TG duration as well as survival in E.G-7-OVA tumor-bearing mice. On the other hand, solo administration of anti-CD8 mAb did not affect TG duration and survival in the control group at all. These results, combined with the results of ELISpot assay, suggest that the antitumor effects of LH are not only via direct cytotoxicity by the high temperature but also antitumor immunity mainly involving tumor-specific CD8-positive T cells. Removal of CD8 using anti-CD8 mAb seemed to have no effect in the control group of this tumor model. We speculate that (1) the blockade of CD8-positive T cells with anti-CD8 mAb actually reduced the antitumor effects in the control group with regard to tumor volume on days 8, 10, and 12 as well as survival in E.G7-OVA tumor-bearing mice, although these results were not statistically significant and (2) that according to the ELISpot assay results, tumor-specific CTL was scarce in the control group. Hence, anti-CD8 mAb possibly has no effect on tumor suppression in the control group.
Accordingly, host antitumor immunity caused by LH is implicated in tumour-specific CD8-positive T cells. Therefore, we analysed the additional effects of anti-CTLA-4 mAb on antitumor effects of LH. CTLA-4 is a molecule that is expressed on the T cell surface and inhibits T cell activation. It has been shown that T cell activation is amplified by CTLA-4 blockade resulting in enhancement of antitumor effects [Citation23]. However, in the present study, although TG duration and survival were significantly prolonged by administration of anti-CTLA-4 mAb in control group, the antitumor effects of LH in terms of TG duration and survival were not significantly enhanced by addition of anti-CTLA-4 mAb (. Although there are several potential reasons why a synergistic effect was not observed with combined use of LH and CTLA-4 blockade, one explanation could be that LH and CTLA-4 blockade are both involved in cytotoxic T cell induction. Several studies showed that the effect of CTLA-4 blockade on T cell immunity is related to the inherent immunogenicity of the tumor cells [Citation24,Citation25]. Furthermore, recently a phase 3 trial showed that the efficacy of ipilimumab, an anti-CTLA-4 human mAb, on metastatic melanoma patients was not further improved by administration of a vaccination of tumor antigen gp100 which specifically targets melanoma cells [Citation1]. In this case, both gp100-peptide vaccine and anti-CTLA-4 mAb were involved in cytotoxic T cell induction. In fact, CTLA-4 blockade monotherapy resulted in significant improvement in TG duration and MST in the control group (. Taken together, this suggested that EG.7-OVA tumor cells could be so immunogenic that either LH or CTLA-4 was enough to fully activate innate T cell immunity in this mouse model and the antitumor effects of LH and anti-CTLA-4 mAb may have overlapped. In order to enhance the effect of LH, anti-PD-1/anti-PD-L1 mAbs, that enhance the effect of CTLs in tumor microenvironment, may be more suitable.
There are some advantages of this mouse model of LH. LH treatment of mouse femur tumors using 42 °C hot water brought about significant antitumor effects and induced tumor-specific CTLs. Compared with other hyperthermia methods, this LH treatment was much less invasive and the temperature of LH was relatively low, therefore, this can easily be applied in a clinical setting [Citation26,Citation27]. However, in our LH models () the actual temperature achieved in the tumor while inducing LH was not assessed due to tumor size, which reached approximately 150 mm3 after 1 week following tumor inoculation. In several reports, LH was performed in the same way as in our study; within few minutes after initiating LH, the tumor reached a slightly lower temperature than the bath temperature within a few minutes after LH was started [Citation28,Citation29]. Therefore, we speculated that tumor temperature was slightly lower than 42 °C in our model.
In conclusion, we demonstrated that LH-induced tumor-specific CTL activation in a mouse model and the antitumor effects of LH were mostly dependent on CD8-positive T cells. Since LH is a non-invasive treatment and can activate antitumor immunity, it is a clinically useful treatment and is worthy of further investigation to develop an optimal combination therapy using immunomodulating modalities.
Acknowledgments
The authors would like to thank Professor A. Takahashi, Gunma University Heavy Ion Medical Center for technical assistance with the experiments.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723.
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135.
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813.
- Nowak AK, Robinson BW, Lake RA. Gemcitabine exerts a selective effect on the humoral immune response: implications for combination chemo-immunotherapy. Cancer Res. 2002;62:2353–2358.
- Takeshima T, Chamoto K, Wakita D, et al. Local radiation therapy inhibits tumor growth through the generation of tumor-specific CTL: its potentiation by combination with Th1 cell therapy. Cancer Res. 2010;70:2697–2706.
- Fucikova J, Kralikova P, Fialova A, et al. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res. 2011;71:4821–4833.
- Park B, Yee C, Lee KM. The effect of radiation on the immune response to cancers. Int J Mol Sci. 2014;15:927–943.
- van der Zee J. Heating the patient: a promising approach?. Ann Oncol. 2002;13:1173–1184.
- Zhang HG, Mehta K, Cohen P, et al. Hyperthermia on immune regulation: a temperature's story. Cancer Lett. 2008;271:191–204.
- Frey B, Weiss EM, Rubner Y, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia. 2012;28:528–542.
- Sato H, Suzuki Y, Ide M, et al. HLA class I expression and its alteration by preoperative hyperthermo-chemoradiotherapy in patients with rectal cancer. PloS One. 2014;9:e108122.
- Aerts JG, Hegmans JP. Tumor-specific cytotoxic T cells are crucial for efficacy of immunomodulatory antibodies in patients with lung cancer. Cancer Res. 2013;73:2381–2388.
- Golden EB, Demaria S, Schiff PB, et al. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. 2013;1:365–372.
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:1919–1929.
- Mace TA, Zhong L, Kokolus KM, et al. Effector CD8+ T cell IFN-γ production and cytotoxicity are enhanced by mild hyperthermia . Int J Hyperthermia. 2012;28:9–18.
- Toraya-Brown S, Fiering S. Local tumour hyperthermia as immunotherapy for metastatic cancer. Int J Hyperthermia. 2014;30:531–539.
- Roy A, Krzykwa E, Lemieux R, et al. Increased efficiency of gamma-irradiated versus mitomycin C-treated feeder cells for the expansion of normal human cells in long-term cultures. J Hematother Stem Cell Res. 2001;10:873–880.
- Ren W, Strube R, Zhang X, et al. Potent tumor-specific immunity induced by an in vivo heat shock protein-suicide gene-based tumor vaccine. Cancer Res. 2004;64:6645–6651.
- Torigoe T, Tamura Y, Sato N. Heat shock proteins and immunity: application of hyperthermia for immunomodulation. Int J Hyperthermia. 2009;25:610–616.
- Jolesch A, Elmer K, Bendz H, et al. Hsp70, a messenger from hyperthermia for the immune system. Eur J Cell Biol. 2012;91:48–52.
- Noessner E, Gastpar R, Milani V, et al. Tumor-derived heat shock protein 70 peptide complexes are cross-presented by human dendritic cells. J Immunol. 2002;169:5424–5432.
- Binder RJ, Srivastava PK. Peptides chaperoned by heat-shock proteins are a necessary and sufficient source of antigen in the cross-priming of CD8+ T cells. Nature Immunol. 2005;6:593–599.
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736.
- Kwon ED, Hurwitz AA, Foster BA, et al. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci UsaU S A. 1997;94:8099–8103.
- van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366.
- Kobayashi T. Cancer hyperthermia using magnetic nanoparticles. Biotechnol J. 2011;6:1342–1347.
- Soares PI, Ferreira IM, Igreja RA, et al. Application of hyperthermia for cancer treatment: recent patents review. Recent Pat Anticancer Drug Discov. 2012;7:64–73.
- Masunaga S, Ono K, Takahashi A, et al. Usefulness of combined treatment with mild temperature hyperthermia and/or tirapazamine in the treatment of solid tumors: its independence of p53 status. Cancer Sci. 2003;94:125–133.
- Masunaga SI, Tano K, Nakamura J, et al. Adverse effect of mild temperature hyperthermia combined with hexamethylenetetramine compared to its effect combined with tirapazamine in the treatment of solid tumors. Exp Ther Med. 2010;1:169–174.

