Abstract
Purpose: Pancreatic cancer is a challenging malignancy with low treatment option and poor life expectancy. Thermal ablation techniques were proposed as alternative treatment options, especially in advanced stages and for unfit-for-surgery patients. This systematic review describes the thermal ablative techniques -i.e., Laser (LA), Radiofrequency (RFA), Microwave (MWA) Ablation, High-Intensity Focused Ultrasound (HIFU) and cryoablation- available for pancreatic cancer treatment. Additionally, an analysis of the efficacy, complication rate and overall survival for each technique is conducted.
Material and methods: This review collects the ex vivo, preclinical and clinical studies presenting the use of thermal techniques in the pancreatic cancer treatment, searched up to March 2018 in PubMed and Medline. Abstracts, letters-to-the-editor, expert opinions, reviews and non-English language manuscripts were excluded.
Results: Sixty-five papers were included. For the ex vivo and preclinical studies, there are: 12 records for LA, 8 for RFA, 0 for MWA, 6 for HIFU, 1 for cryoablation and 3 for hybrid techniques. For clinical studies, 1 paper for LA, 14 for RFA, 1 for MWA, 17 for HIFU, 1 for cryoablation and 1 for hybrid techniques.
Conclusions: Important technological advances are presented in ex vivo and preclinical studies, as the real-time thermometry, nanotechnology and hybrid techniques to enhance the thermal outcome. Conversely, a lack of standardization in the clinical employment of the procedures emerged, leading to contrasting results on the safety and feasibility of some analyzed techniques. Uniform conclusions on the safety and feasibility of these techniques for pancreatic cancer will require further structured investigation.
1. Introduction
The research about novel therapeutic paradigms for pancreatic cancer is an emerging field in biomedical science. Presently, the unique largely-accepted treatment to improve long-term survival is still the pancreatectomy. However, it remains one of the most operator-dependent and invasive surgical operation, with only 20% of the patients being eligible for surgery at the time of the diagnosis [Citation1,Citation2] and an extremely low survival rate in the most advanced stages (5-year survival of 2% in the unresectable stages) [Citation3,Citation4]. In Europe, about 80,000 new cases of pancreatic cancers have been registered in 2012, and almost the half of them (35,000) only in Germany, Italy and France [Citation5]. Among them, the 80% (about 64,000) could have benefitted from therapeutic approaches alternative to surgery in addiction to chemotherapy.
Despite the recent introduction of novel therapeutic schemes, chemotherapy treatment of advanced pancreatic cancers still relates with a poor long-term survival and significant ad interim systemic complications i.e. gastrointestinal adverse effects, neutropenia, leukopenia, anemia, lack of appetite and fatigue [Citation4,Citation6]. Additionally, only one third of patients are considered responsive to the systemic chemotherapeutical treatment [Citation2,Citation6,Citation7]. At the same time, the benefits of radiotherapy, alone or in combination with chemotherapy, are still uncertain, although the recent LAP07 trial [Citation8], did not show any advantage in term of overall survival as compared to chemotherapy alone.
To fill this gap, the growing interest in the development of minimally-invasive therapeutic strategies has opened the doors to several energy-based treatments. Basically, they rely on the introduction of one (or several) therapeutic needle(s) inside the tumor target, and aim at inducing a controlled change of tissue conditions and environment to achieve tumor necrosis and apoptosis.
Already well known for the treatment of other tumors (e.g., liver, prostate, kidney [Citation9–11]), ablative thermal therapies are emerging and largely studied as potential alternatives for the management of pancreatic cancer.
These include hyperthermal therapies, as laser ablation (LA), radiofrequency ablation (RFA), microwave ablation (MWA), and, in some extent, high intensity focused ultrasound (HIFU), and cryoablation as hypothermal treatment. Recent studies are also working on the integration of different techniques, with the goal of improving clinical outcome.
Literature is being increasingly updated with novel studies assessing the thermal outcome induced by the mentioned techniques used on the pancreatic tissue, in both clinical and preclinical terms; however, a poor standardization of the selected criteria has been observed, in contrast with a lively and promising research and with the investigation of cutting edge techniques.
Hence, this work presents an up-to-date literature review of the ex vivo, preclinical and clinical application of thermal ablation therapies in the management of pancreatic cancer. More specifically, a working principle presentation has been conducted for each ablative technique, followed by the analysis of the recent advances in their application in the ex vivo and preclinical field. Under the clinical point of view, this systematic review aims to present the current medical applications, the efficacy, the potential short- and long-term procedure-related complications and an evaluation of the overall survival (OS) and disease-free survival (DFS) according to the ablative technique used. In the mentioned scenario, the focus has been given on three main approaches of energy delivering from the ablation systems into the target: the percutaneous approach, the echo-endoscopic ultrasound (EUS)-guided approach, and the non-invasive one.
2. Materials and methods
Only ex vivo, preclinical and clinical studies assessing the use of ablation techniques in the treatment of pancreatic disease were included in this review. PubMed and Medline were searched up to March 2018. Only English language articles were selected. The following Mesh searching headings, as part of the title and/or abstracts and/or key words, were used: ‘pancreas’, ‘pancreatic’, ‘thermal’, ‘ablation’, ‘endoscopic’, ‘percutaneous’, ‘in vivo’, ‘ex vivo’, ‘radiofrequency’, ‘RFA’, ‘laser’, ‘LA’, ‘focused ultrasound’, ‘HIFU’, ‘microwave’, ‘MWA’ ‘cryoablation’. Combinations were performed with AND/OR. In case of doubt on abstract suitability, the full article was obtained.
2.1. Inclusion and exclusion criteria
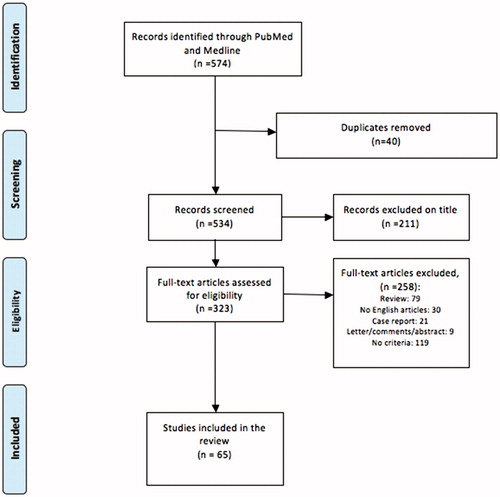
For inclusion in this review, only ex vivo, preclinical and clinical studies on pancreatic ablation (including open, percutaneous and endoscopic procedures) were considered. Abstracts, letters to the editor, expert opinions, reviews and non-English language manuscripts were excluded. In case of clinical application, efficacy of the ablation performed was evaluated when possible both immediately after the procedure (less than 30 days from the ablation) and at the last follow up. Post-procedural complications, OS and DSF were additionally analyzed when reported. The PRISMA flowchart is reported in .
3. Results
3.1. Laser ablation: working principle, clinical application and preclinical studies
3.1.1. Working principle
The LA phenomenon is related to the interaction between light and tissue, which entails the conversion of this light into heat. The light penetrating into the medium is absorbed and scattered inside the tissue, consequently resulting into tissue temperature rise [Citation12].
The heating of the tissue by means of light depends on the absorption capability of the tissue itself. Each tissue constituent and chromophore exhibit a wavelength-dependent absorption, entailing tissue-characteristic optical properties, hence specific tissue-laser interaction. In order to treat deep tumors, a high penetration depth of the light wavelength is desired. Wavelengths in the 940–1100 nm range are favored for LA purposes, because, within this optical window, the light shows a good balance between absorption and penetration into the tissue, with consequent development of large coagulation area (tens of millimeters). For tumor masses treatment, the continuous mode (no pulse) and low power values (<20 W, depending also on the emission modality of the applicator) are preferred [Citation13].
The most common laser sources are in the near infrared spectrum: Nd:YAG laser emitting at 1064 nm and the diode lasers, emitting in the range 800–970 nm. The laser light can be then guided into the target through two modalities: i) with contact, hence, by means of an energy delivering device, made up of an optical fiber (diameter <1 mm), conveniently designed to modulate light energy deposition into the tissue; ii) without contact, i.e., the laser beam is travelling through the air before reaching the tissue. For pancreas treatment, the contact applicator has been preferred, with low power delivered (1–10 W). The no-contact approach has been used only in preclinical studies, in combination with nanoparticles. It is worth evidencing that laser therapies can be easily guided with magnetic resonance (MR), computed tomography (CT) and US/EUS imaging [Citation14]. From the reviewed papers, the most used systems for LA in pancreas are produced by Elesta srl (Calenzano, Italy).
From the period of LA introduction in clinical practice [Citation15], only isolated tests involved LA for the treatment of pancreatic cancer; but nowadays, the pioneering application of EUS-guided LA [Citation16], and nanomedicine [Citation17,Citation18] are promoting a rebirth of this technique in the treatment of pancreatic cancer ().
Figure 2. Laser Ablation: (A) schematisation of the EUS-guided LA; (B) right side, during EUS-guided LA, the hyperechoic spot visible close to the needle tip, inside the tumour (arrow); left side, at the end of the procedure, EUS showed a hyperechoic area along the path of the probe surrounded by nonhomogeneous tissue with hyperechoic spots. Reprinted from [31] Copyright 2018, with permission from Elsevier. (C) H&E Histology of the lesion induced by Nd:YAG laser on in vivo healthy pig. (D) Schematization of nanoparticles-mediated LA.
![Figure 2. Laser Ablation: (A) schematisation of the EUS-guided LA; (B) right side, during EUS-guided LA, the hyperechoic spot visible close to the needle tip, inside the tumour (arrow); left side, at the end of the procedure, EUS showed a hyperechoic area along the path of the probe surrounded by nonhomogeneous tissue with hyperechoic spots. Reprinted from [31] Copyright 2018, with permission from Elsevier. (C) H&E Histology of the lesion induced by Nd:YAG laser on in vivo healthy pig. (D) Schematization of nanoparticles-mediated LA.](/cms/asset/718146e8-b1cb-4544-8079-d668fa610ee9/ihyt_a_1506165_f0002_c.jpg)
3.1.2. Preclinical and ex vivo studies
Regarding the use of LA in preclinical and ex vivo settings, 12 articles have been included according to the selection criteria [Citation18–29]. They present the use of laser light for therapy purpose, also in combination with nanotechnology ().
Table 1. Laser Ablation in preclinical and ex vivo settings.
One of the few preclinical studies on the LA on pancreas has been carried out by Stroszczynski et al. in 2001 [29]. They applied the radiation emitted by Nd:YAG laser on 15 in vivo healthy porcine pancreases, through percutaneous approach. MR imaging was used for both intraoperative temperature monitoring, and for the follow-up control. After 1 week from the treatment, the thermally induced lesions were solid in 12 out of 15 animals. No dilation of the common bile duct or pancreatic duct was observed. No signs of neither generalized or necrotizing pancreatitis nor peritonitis were visible on follow-up images. However, thrombosis of intrapancreatic vessels close to the treated area was a frequent finding.
In collaboration with Di Matteo [Citation19,Citation21,Citation24–28], our group deeply investigated the thermal response of pancreatic tissue undergoing LA. In these years, the procedure was always performed by guiding the laser light emitted by Nd:YAG system (1064 nm wavelength) though one or multiple bare optical quartz fibers (0.3 mm-diameter).
In 2010, the first LA study on 8 healthy pigs was performed by means of EUS guidance. All applications (at 2 W and 3 W, with 500 J and 1000 J energy delivery) were directed toward the body and tail of the pancreas, using a transgastric approach. After 24 h, the pigs were sacrificed and the ablated lesions excised for histopathologic evaluation [Citation21].
From 2011 to these days, several studies assessed the temperature gradient generated inside the organs during the procedure, and the resulting thermal damage. The two main approaches can be classified into: (1) the implementation of a mathematical model to predict the thermal response of the laser-irradiated pancreas, and (2) the investigation of thermometric techniques to monitor the temperature increase during the ex vivo LA on animal pancreases.
Different thermometric techniques have been investigated: contact approaches, by means of sensors, and no-contact methods, by using diagnostic imaging. Regarding the contact techniques, Fiber Bragg Gratings (FBGs) have been largely used. The main pros of optical fiber-based sensors as thermometers for LA procedures rely on: (1) the absence of metallic parts, and the consequent absence of artifacts due to self-heating, and lack of artifacts in CT imaging, (2) the MR compatibility, allowing for the use of FBGs as reference sensors in image-based thermometry. In fact, while it has been proved that thermocouples experience a self-heating during LA, resulting into a temperature overestimation related to the distance between sensors and laser source up to 20 °C [Citation27], FBGs do not. Additionally, FBGs served as reference temperature sensors to calibrate CT and MR images for thermometric purposes: during LA on ex vivo porcine pancreases, diagnostic images were scanned, and simultaneously temperature was measured through FBGs. This process allowed associating the change of images properties (intensity, density, etc.) to the actual temperature value [Citation19,Citation28]. Regarding CT-based thermometry, we found a thermal sensitivity of approximately 0.5 HU/°C for ex vivo pancreatic tissue [Citation28]. For this technique, an accuracy of 5 °C has been reported by other authors [Citation30]. Regarding the MR thermometry, two main sequences have been tested, which provided different sensitivity and accuracy (-1.4 ± 0.1 °C−1 for SRTF, −1.5 ± 0.1 °C−1 for IRTF) [Citation19].
Lastly, a numerical model has been developed, and further tested, to predict the thermal response (temperature and damaged volumes) of the pancreases undergoing different laser settings. It relies on Pennes equation and on the scattering and absorption properties of the tissues [Citation25]. FBGs have been used also to validate the predictions of the numerical model on the thermal response of ex vivo pancreas undergoing LA [Citation25]. Histology has been useful to estimate the damaged volumes induced in 60 ex vivo pancreases at different laser settings, and these values have been compared with the simulated results [Citation20,Citation26].
Albeit still in ex vivo and in vitro settings, nanotechnology offers interesting solutions to the application of laser light for pancreatic tumor treatment. The first attempt dates back to 2011, when the group of Mocan et al. [Citation18] started investigating the use of functionalized carbon nanotubes to mediate LA in pancreas. In their first study, the multiwalled carbon nanotubes were functionalized with human serum albumin. This protein was used as carrier of the nanotubes inside the tumor, as it is easily internalized by malignant cells, and pancreatic cancer is one of the most intrinsically drug-resistant tumors. The authors worked on both in vitro and ex vivo scenarios. In this latter case, human pancreases affected by adenocarcinoma were resected according to the clinical protocol, and then used to validate the purpose of the study. Few years later, in 2014, the same group demonstrated that a nano-biosystem based on multi-walled carbon nanotubes and polyethylene glycol (PEG) molecules induces apoptosis by triggering mitochondrial membrane depolarization mechanism [Citation23]. The nanoparticles were tested on a culture of human pancreatic adenocarcinoma cells undergoing no-contact 808 nm LA. Mitochondrial dehydrogenase activity was used to measure cell growth and viability. Authors showed that mitochondrial membrane permeabilization with consequent apoptosis represent the primary mode of death in this nanoparticles-mediated LA.
In 2013, Guo et al. used hybrid iron-oxide core gold-shell nanoparticles to induce selective laser ablation to human pancreatic cancer cells (PANC-1) [Citation22]. The authors compared the thermal effects induced by 808 nm-laser source, mediated by bare Fe3O4 nanoparticles, and by GoldMag nanoparticles, constituted by iron-oxide core inside a gold shell. These nanoparticles are visible and quantifiable by MR images. In absence of nanoparticles, temperature raised up to 13 °C at the end of laser treatment, while it overcame 80 °C with nanoparticles. After viability study, they showed that PANC-1 cell proliferation significantly decreased with exposure to increasing concentrations of the nanoparticles and increasing applied power densities.
3.1.3. Clinical application
Only one article was included in the analysis for a total of 9 patients [Citation31]. All patients had a diagnosis of unresectable adenocarcinoma. In most of the cases, the lesion was in the head of the pancreas (6 patients), followed by pancreatic body localization in 2 cases and pancreatic tail in 1 case. The median lesion size was 35.4 mm (range 21–45 mm). The patients were divided into three groups and treated with a total delivered energy of 800, 1000 and 1200 J, at a low-power setting (2, 3 and 4 W), respectively. All the procedures were performed under EUS guidance with color Doppler analysis to prevent injury of surrounding vessels and structures and no intraprocedural complication was detected. EUS was also used for real-time procedure monitoring. Additionally, in all cases, a contrast enhanced ultrosonography by means of a previous 5 ml of SonoVue injection followed by a 10-ml saline solution flush, was performed before and after LA. In all cases, the EUS revealed a hyperechoic area. Regarding the clinical short-term outcomes, no major events were recorded. Three patients reported a post-procedural fluid collection, while a 3-fold increase in the amylase serum level was evidenced in other 2 patients. All of them were successfully treated conservatively. Follow up was performed at 24 h, 7 and 30 days after the ablation by means of CT scans. In all the follow up periods, a clear margin ablation was evidenced with a gradual involution of the ablated areas. One patient died due to a not procedure-related myocardial infarction before the 30-day CT scan re-evaluation (mortality rate of 11%). Median OS was 7.4 months (range 29–662 days).
3.2. Radiofrequency ablation: working principle, clinical application and preclinical studies
3.2.1. Working principle
Biological tissues are conductive media, due to the abundant presence of ionic fluid. Consequently, the electrical conduction of an alternating current (for RF, frequency of ∼1 MHz) allows for the oscillatory movements of ions, with a velocity proportional to the magnitude of the electric field. The mechanism of tissue heating with RFA is based on ohmic energy loss associated with the ionic current. The control of tissue temperature during the treatment is a key factor for the therapy outcome, because high temperatures can cause tissue charring or desiccation, hence reducing energy penetration and increasing the impedance at the electrode [Citation32,Citation33].
The RF current can be applied in both monopolar and bipolar mode. The main difference between the two approaches is the use (monopolar mode) or not (bipolar mode) of grounding pads. In commonly used monopolar mode, RF current is guided inside the target tumor though an electrode, while a return electrode which is larger in surface area is fixed at distance on the patient's skin. This last is used to close the electrical circuit and to collect the current dispersed inside the body during RF treatment.
In bipolar mode, the ground pad is not necessary, because the used multiple interstitial electrodes embed both the anode and the cathode, hence, allow to close the electrical circuit. Consequently, the current is more concentrated between the probes than in monopolar mode, in which current travels outward toward a dispersive pad. However, the bipolar mode requires additional electrode insertions, and often requires saline infusion to improve results. In both cases, the electrodes are typically inserted inside the target tumor by means of percutaneous approach (i.e. for hepatic lesions) or through the GI tract (i.e. for pancreatic lesions), and the current is generated by an external RF generator. According to the reviewed papers, the most used systems for RF in pancreas are: for the EUS-guided RFA, 19 or 22 gauge Habib™ EUS-RFA catheter (Emcision Ltd., London), the 18-gauge electrode connected to the VIVA RF generator (STARmed, Koyang, Korea). For percutaneous approach: A RITA®System Generator 1500X (Mountain View, California, USA) was used, with the probe (StarBurstTM XL multiarray; StarBurst XL, Talon or UniBlate™ depending on tumour size and shape), and the 17 gauges Cool-tipTM RFAblation system (Radionics). Two different strategies can be chosen for RFA: the selection of the power suitable to the lesion size, the definition of the temperature threshold and the treatment time. In this last case, the RF electrode is equipped with one or multiple temperature sensors. US imaging is usually employed for guidance and real-time monitoring of percutaneous or endoscopic-guided RFA in pancreas. The main constraint is related to the heat-sink effect caused by heat dissipation due to the proximity of the target to blood vessels. RFA has been explored for the minimally invasive treatment of pancreatic cancer, as well as largely tested in clinical settings [Citation34] ().
Figure 3. Radiofrequency Ablation: A) Measurement of Hounsfield Unit value of the RFA induced lesion in pancreatic adenocarcinoma and B) of the necrotic area. Reprinted from [Citation43] Copyright 2018, with the permission of Pioneer Bioscience Publishing Company. C) Gross pathology of the porcine RF-ablated pancreas. D) Hystological image of the thermal outcome: coagulated necrosis, fibrous tissue and normal pancreatic parenchyma. (H&E, orig. mag. x40). Reprinted from [Citation39] Copyright 2018, with the permission of Elsevier.
![Figure 3. Radiofrequency Ablation: A) Measurement of Hounsfield Unit value of the RFA induced lesion in pancreatic adenocarcinoma and B) of the necrotic area. Reprinted from [Citation43] Copyright 2018, with the permission of Pioneer Bioscience Publishing Company. C) Gross pathology of the porcine RF-ablated pancreas. D) Hystological image of the thermal outcome: coagulated necrosis, fibrous tissue and normal pancreatic parenchyma. (H&E, orig. mag. x40). Reprinted from [Citation39] Copyright 2018, with the permission of Elsevier.](/cms/asset/d937d70e-1d33-46fc-b715-f5366aa40b28/ihyt_a_1506165_f0003_c.jpg)
3.2.2. Preclinical and ex vivo studies
Regarding the use of RFA ablation in pancreas in preclinical and ex vivo settings, 8 papers have been included according to the selection criteria [Citation35–42] (). Among them, three studies about the application of EUS-guided RFA on in vivo animal models have been found [Citation36,Citation38,Citation39]. The RFA electrode is clearly visualized extruding out of the working channel of the echoendoscope and can be inserted directly into the pancreatic parenchyma under real-time EUS imaging. During the ablation, an echogenic cloud forming around the tip of the RFA electrode is visible; it results into a round, hyperechoic lesion at the end of the procedure. The first EUS-guided preclinical study on the use of RF in pancreas was performed by Goldberg et al. in 1999 [Citation36]. The procedure was performed in 13 pigs, kept for survival up to 14 days, and intraprocedural temperature was measured with thermocouples. EUS imaging was also performed in the follow up period, together with contrast-enhanced CT and follow-up CT. All pigs well tolerated the RF ablation procedure, but in 3 out of 13 cases, transmural burns extending from the gastric mucosa through the serosa occurred. Forty-eight hours after ablation, one pig had a 36% elevation in serum amylase level with a normal serum lipase level. The only pig with a peripancreatic pseudocyst had a mild elevation in serum lipase.
Table 2. Radiofrequency Ablation in preclinical and ex vivo settings.
In 2012, Kim et al. [Citation39] and Gaidhane et al. [Citation38] published other two works.
In the first one, 10 pigs underwent the EUS-guided RFA procedure and were monitored for 7 days after the ablation. There were neither significant changes in the laboratory test results nor signs of major vessel injuries or adjacent organ damage. Fibrosis and adhesions were found in 3 pigs, none of which showed any behavioral distress.
In the second one, 3 days after the procedure, blood was withdrawn to evaluate total bilirubin, alkaline phosphatase, cell blood count, and amylase. The values were within normal range, and the pigs did not show any symptoms or abnormal behavior. No gross abnormalities were noted during the serial sectioning of the pancreas. Examination of the representative histologic sections showed focal areas of acute pancreatitis as evidenced by necrotic change of acinar pancreatic tissue. Only one pig showed moderate levels of pancreatitis, with involvement of 20% of the proximal pancreatic tissue.
In conclusion, RFA was well tolerated in all the animals, and with minimal amount of pancreatitis.
Other studies have been carried out by Date et al. in ex vivo models [Citation35,Citation37]. In these studies, temperature was measured during the procedure through thermocouples embedded in the RFA electrode. While setting different target temperature values to drive the ablation protocol, complete ablation of pancreatic parenchyma and thermal damage to portal vein and biliary structure was noted at a target temperature of 100 °C for 10 min. A temperature value of 90 °C for 5 min was associated with complete ablation in all 5 animals with no evidence of injury to peripancreatic structures. Complete ablation in all 20 pigs and absence of duodenal damage were observed. Bile duct was damaged in 1 pancreas treated at 90 °C and 100 °C; portal vein was damaged in 1 of the organs ablated at 80 °C and 100 °C; absence of damage to pancreatic tail was noted.
In 2014, two studies reported the in vivo application of RFA through a laparotomic approach. In the first study, Fegrachi et al. [Citation40] applied RFA on 6 pigs. Two weeks after the RFA, a total pancreatoduodenectomy was performed and the animal was sacrificed. Nicotinamide adenine dinucleotide (NADH), enzyme histochemistry, and haematoxylin and eosin staining (H&E) were used for histologic evaluation. An increase of the amylase more than threefold the preoperative value was considered an indication of pancreatitis. In 2 animals, amylase values more than threefold the preoperative value was observed. Clinically, these animals were in a good condition with maximal clinical scores. No major morbidity and no mortality were seen during a period of two weeks after RFA. In the second study, Quesada et al performed RFA on 39 rats, of which 7 were control [Citation41]. Also in this case, the animals well tolerated the procedure, no major intraoperative complications or deaths were reported. No histological changes were observed in endocrine or exocrine tissue of the control groups. During the histological study, no purulent inflammatory infiltrate was observed, but there were 3 microabscesses in 3 animals. The distal pancreas in 24 out of 32 animals treated with RFA showed a significant increase of small islets compared to control groups.
Raoof et al. [Citation42] studied the effects of nanoparticles-mediated external RF ablation on orthotopic pancreatic tumors in mice.
3.2.3. Clinical application
Regarding the use of RFA ablation of pancreatic lesions in clinical setting, 14 articles have been included according to the selection criteria [Citation43–56], with a total of 279 patients. In most cases (257 out of 279 – 92,1%) the ablative treatment was reserved to unresectable lesion (stage III-IV), while in the remaining 22 patients the unfit-for-surgery and refuse of surgery were the main indications. The 56,6% of lesions (158/279) was in the head of pancreas, 24,3% (68/279) in the body-tail and 0,7% (2) in the uncinate process, while in 51 patients, the location was not specified. Pancreatic adenocarcinoma was the most frequently ablated lesion (257 patients), followed by neuroendocrine tumors in 16 cases and cystic lesions in the remaining 6 patients. Patients’ clinical characteristics are reported in .
Table 3. Radiofrequency Ablation in clinical settings. PA: pancreatic adenocarcinoma; NT: neuroendocrine tumour.
Technically, the open surgery access was the preferred approach for ablation (212 patients), while the percutaneous and the EUS-guided treatments were performed in 25 and 19 cases, respectively. The percutaneous approach was successfully performed under US-guidance in 24 patients, while CT-guidance was necessary in 1 patient. Endoscopic fluoroscopy-guided approach was instead used in 23 patients. The power applied was reported in only 9 articles [Citation44,Citation46,Citation48–55], with a value ranging between 5 and 150 W, and the application time varying from 10 to 720 s.
The temperature reached during the procedure was reported in a total of 4 studies [Citation43,Citation47,Citation53,Citation56], with a value ranging between 90 and 100 °C. In those cases, temperature monitoring was performed by means of thermometers placed at the tip of the needle in 44 patients [Citation53,Citation56], while in the remaining patients the measurement method was not specified [Citation43,Citation47].
Intraprocedural local and surrounding tissues thermal damages monitoring were performed in a total of 265 patients (12 studies) [Citation43–45,Citation47–50,Citation52–56]. The US was the most frequently used (180 patients) [Citation43–45,Citation47,Citation54] especially during the open surgery procedures (162 out of 180) [Citation43,Citation45,Citation47,Citation54]. In 18 cases [Citation44] US was adopted during percutaneous ablations.
Of the remaining 85 patients, in the 41 of them [Citation48–50,Citation52,Citation55] who underwent EUS guided ablation, the thermal damage was monitored by means of the EUS itself; in the other 44 cases, thermocouples were used during laparotomic ablation [Citation53,Citation56].
An objective short-term evaluation of the ablation performed was reported in only 8 studies [Citation43,Citation44,Citation47,Citation50,Citation51,Citation54–56] after a mean time ranging between 1 and 180 days for a total of 124 patients. Except for one case [Citation55] in which US was used as post-procedural evaluation tool, in all the other studies CT scan was the most frequently used method. Among the 8 studies, only 6 reported a quantification of the tumor reduction for a total of 35 patients, with a complete response in 11 cases, a reduction between 50 and 75% in 21 cases, between 25 and 50% in 3 patients. In one study [Citation43] no quantification was reported.
In terms of post-operative course, a total 52 RFA-related complications were registered. Among the most frequent, pancreatic fistula was reported in 12 cases and portal thrombosis in 10. Pancreatitis occurred in a total of 8 patients. Regarding major complications, duodenal injury was evidenced in three cases [Citation45]. Intra-abdominal bleeding occurred in two patients leading to surgery. Two short-term deaths were registered [Citation45], due to hepatic failure after long-course chemotherapy (not RFA-related) in one case and to sepsis after duodenal perforation in the remaining patient.
Follow up was reported in 10 out of 17 studies for a total of 214 patients [Citation44–49,Citation51,Citation53–56]. Revaluation time ranged between 7 and 34 months and was mainly performed by means of CT-scan and MRI (7 studies) [Citation44–46,Citation49,Citation51,Citation53,Citation56]. In 27 patients (3 studies) [Citation48,Citation54,Citation55], follow up method was not reported. OS was available in 7 studies [Citation47,Citation49,Citation51,Citation53–56] with a value ranging between 0% [Citation54] and 100% [Citation47,Citation49,Citation51,Citation53,Citation55,Citation56], after a follow up ranging between 10 and 34 months. Tumor response at follow up was reported in a total of 5 studies [Citation44,Citation46,Citation49,Citation51,Citation56]. Only one study [Citation51] reported a complete tumor response to the ablation in a total of 10 patients affected by pancreatic neuroendocrine tumor after a median follow up of 34 months. A tumor growth was registered in 10 cases [Citation44,Citation46,Citation55,Citation56] after a follow up ranging between 10 and 18.2 months.
3.3. Microwave ablation: working principle, clinical application and preclinical studies
3.3.1. Working principle
Biological tissues are characterized by dielectric permittivity, magnetic permeability and conductivity allowing for the transmission of electromagnetic energy. When MW frequencies (915 MHz, 2.45 GHz) are applied to the tissue, the dielectric heating occurs. The interaction between polar water molecules of the biological medium and the electromagnetic field applied through an antenna, forces dipoles to continuously realign with such electromagnetic field, thereby producing frictional energy which is then converted into heat [Citation57]. Microwaves propagate through all types of tissues, including water vapor, and dehydrated tissues created during the ablative treatment; differently than RF ablation, it is not a self-limiting process due to the increasing tissue impedance to electrical current flow, and can hence produce larger lesions faster. MWA appeared to be less insensitive to blood perfusion with respect to other techniques [Citation58,Citation59], although blood perfusion can still limit outer boundary and maximum penetration. On the other hand, technical solutions for the detrimental backward heating that occurs along the coaxial feedline of the antenna during high power MWA, and for the weak shape control are under investigation [Citation60]. Lastly, MWA holds several theoretical advantages, and several studies are being carried out to evaluate its clinical efficacy [Citation61,Citation62]. A MW antenna is connected to a generator, and to a cooling water flow system. This last allows for the water to circulate within the antenna, aiming to reduce the temperature of the tissue in contact with the antenna. According to the unique reported paper, a 14 gauge straight MW antenna connected to the Evident MW Ablation System from Covidien (Mansfield, Massachusetts), working at 915 MHz was used to evaluate safety and efficacy in locally advanced and nonresectable pancreatic head cancer.
MWA was introduced for the treatment of prostate, kidney, lung, and liver tumor, and the following paragraph will present the results available for pancreatic cancer () [Citation63].
Figure 4. Microwave Ablation: A) Schematization of the MWA guided with the percutaneous. approach; B) US-image guided MWA (white arrow indicates the antenna); C) Sagittal CT view in a patient with internal/external biliary drainage (black arrow) showing the MWA antenna (white arrow) deployed a few millimetres away from the drain. Reprinted from [Citation65] Copyright 2018, with the permission of Elsevier.
![Figure 4. Microwave Ablation: A) Schematization of the MWA guided with the percutaneous. approach; B) US-image guided MWA (white arrow indicates the antenna); C) Sagittal CT view in a patient with internal/external biliary drainage (black arrow) showing the MWA antenna (white arrow) deployed a few millimetres away from the drain. Reprinted from [Citation65] Copyright 2018, with the permission of Elsevier.](/cms/asset/56540158-eecd-4fa8-889a-c3a64d825bf8/ihyt_a_1506165_f0004_c.jpg)
3.3.2. Preclinical and ex vivo studies
No studies regarding the use of MWA in preclinical settings has been found, in accordance to the criteria established for this review.
3.3.3. Clinical application
According to the inclusion criteria, only 1 article [Citation63] on the clinical microwave pancreatic ablation has been selected, including a total of 10 patients. In all cases, the ablative treatment was reserved to unresectable lesions (stage IV) with a mean size of 32 mm (range 20–43 mm). The 100% of lesions were adenocarcinomas located in the head of the pancreas.
Technically, the open approach and the percutaneous technique were respectively performed in 5 cases each. In all cases, the power used was 45 W (915 MHz) for an application time of 10 min. The antennas were continuously perfused with a saline solution at room temperature at a rate of 60 ml/min to avoid possible thermal damage along the proximal semiaxis of the antenna. Except for one patient in whom two antennas were used, in all the remaining cases only one antenna was sufficient for ablation.
Heat distribution was monitored by means of US in three cases, while in the remaining seven patients an US-guidance in combination with cone-beam CT was employed.
The mean follow-up was 9.2 months (range 3 –16 months), with duration less than 6 months in 2 cases, between 6 and 12 months for 4 patients and up to 12 months in the remaining 4. During the follow up period, the morbidity rate was 30% (3 patients). Two patients developed pancreatitis. One of them was successfully treated conservatively, while in the remaining patient a late drainage of a pancreatic pseudocyst was required. In the remaining case a pseudoaneurysm of the gastroduodenal artery was documented and treated by means of an endovascular approach.
A radiological follow up was performed up to 15 months after the procedure. None of the patients presented a complete response to the ablative treatment. According to RECIST’s criteria [Citation64], at 1 month follow up there were 1 partial response (PR), 8 stable diseases (SDs) and 1 progressive disease (PD); at 4 months, 1 PR, 7 SDs and one PR; at 9-month follow up: 1 PR, 4 SDs and 3 PDs. At 12-month evaluation 1 PR, 1 SD and 3 PRs were reported. At the last follow up, after 15 months from procedure, one patients had a PR while in another case a PD was documented.
3.4. HIFU ablation: working principle, clinical application and preclinical studies
3.4.1. Working principle
When a high-intensity ultrasound wave is focused on the tumor target, it can undergo HIFU ablation. Since the wave is produced through an oscillating piezoelectric crystal from a generator outside the body, the energy can be delivered into the human body transcutaneously and without any physical contact between the tissue and the delivery part. Before starting the ablation, the target organ and the surrounding tissues are identified by means of an US or CT evaluation [Citation65–67]. HIFU transducers deliver ultrasound to the focal region with power densities of 100–10 000 W·cm−2.
Mechanical and thermal mechanisms are simultaneously responsible of tissue damage: the convergence of multiple US beams into the focal zone entails increasing energy density, which is absorbed by the tissue and converted into heat. Additionally, ultrasound is a mechanical wave, thus it produces various mechanical effects within the biological tissue, i.e., cavitation, radiation forces and acoustic streaming. As a matter of fact, microbubbles are formed, and oscillate within the US field, with the risk of explosion. The cavitation phenomenon differentiates into stable harmonic and ultraharmonic cavitation, and inertial cavitation. In the first case, the steady oscillation of microbubbles when the pressure at focal point changes, can induces moderate effects at cellular level, e.g., the increasing of membrane permeability, microstreaming and consequent cell apoptosis; in case of inertial cavitation, explosion of the bubbles occurs.
Typical HIFU settings used in pancreatic therapy are: frequency of the US transducer of 0.8–1.1 MHz, total delivered power between 120 and 400 W, phased-array HIFU transducer of 200 to 250 elements, pulse length of 300–400 ms with a duty factor of 40–50%. The treatment time at each location of the tumor (focus of about 1.5 × 8 mm2) is usually established by using two criteria: the achievement of the temperature threshold (65 °C), or of the sonication time (30–50 s).
The HIFU treatment can be performed in two ways: (1) a point-by-point treatment of the tumor mass, in which each individual sonication produces a small ablative region with an elliptical shape (a few millimeters) and must be repeated to cover the whole tumor mass; (2) a volumetric plan, in which the transducer applies heat in a continuous manner to adjacent points in the target. Volume treatment versus point-by-point one limits the duration of the procedure and allows for complete target coverage and delivery of controlled thermal dose.
Phased-array technology allows controlling the focusing, amplitude and phase of each HIFU transducer, in order to scan the focus throughout the entire tumor volume according to the mentioned criteria. The US energy emitted by the transducer is required to traverse the boundary between the transducer and the patient, without getting reflected or scattered. Indeed, the presence of air between a transducer and a patient is a major problem: the attenuation of the US beam at the interface can entailing unwanted skin burn, while its reflection can create a secondary focal region close to the transducer face, which can potentially damage the transducer. For these reasons, coupling media based on aqueous solutions and mineral oils, e.g., water-filled tank and gel-pad, are needed to couple the transducers with the patient skin. The most used HIFU system in pancreas is the Chongqing Haifu (HIFU) Tech, (Chongqing, China). In case of deep organs such as the pancreas, multiple anatomical structures lay in the acoustic path. To avoid possible procedure-related damages, a strict bowel preparation is recommended. A specific protocol also investigated for the pancreas relies on the use of pulsed HIFU, in which short pulses of ultrasound waves (pulse duration of 1 ms, or less) are focused on the target. In this case, cavitation is the phenomenon leading to tissue destruction, while tissue temperature increase is avoided. The rationale is that mechanical tissue damage introduced by cavitating bubbles and/or acoustic radiation force can be exploited for drug-delivery [Citation68]. Pulsed HIFU has been demonstrated to enhance vascular permeability, disrupt tumor barriers and enhance drug penetration into tumor tissue through acoustic cavitation. The intra-procedural monitoring of HIFU can be performed through US and MR imaging, which can provide both the temperature and the elastography information of the treatment outcome (). The outpatient procedure takes usually a few hours, considering the patient preparation and the intra-procedural monitoring.
Figure 5. HIFU: (A) Schematization of HIFU procedure; (B) Volume ratio changes (tumour volume at day 28/tumour volume at day 0) with weekly treatment of PANC-1 xenografts in BALB/c nude mice with HIFU and/or gemcitabine (GEM). Reprinted from [Citation71] Copyright 2018, with the permission of Elsevier. (C) MR-guided HIFU treatment planning of the pancreatic cancer with the patient placed in the supine position over the US transducer (t): image shows liver (l), spleen (s), right kidney (k), and abdominal aorta (a), as well as the predicted ultrasound beam path (triangles) with the focal spot placed over the target lesions (rectangular box). (D) PRF shift-weighted image for MR thermometry demonstrate increase of tissue temperature in the focal spot area (up to about 80 °C) as well as heat distribution beyond the target zone (up to 95 °C), along the abdominal aorta and within the coeliac plexus. (E) Temperature chart shows increase of tissue temperature during sonication in the target area. Reprinted from [75] Copyright 2018, with the permission of Springer.
![Figure 5. HIFU: (A) Schematization of HIFU procedure; (B) Volume ratio changes (tumour volume at day 28/tumour volume at day 0) with weekly treatment of PANC-1 xenografts in BALB/c nude mice with HIFU and/or gemcitabine (GEM). Reprinted from [Citation71] Copyright 2018, with the permission of Elsevier. (C) MR-guided HIFU treatment planning of the pancreatic cancer with the patient placed in the supine position over the US transducer (t): image shows liver (l), spleen (s), right kidney (k), and abdominal aorta (a), as well as the predicted ultrasound beam path (triangles) with the focal spot placed over the target lesions (rectangular box). (D) PRF shift-weighted image for MR thermometry demonstrate increase of tissue temperature in the focal spot area (up to about 80 °C) as well as heat distribution beyond the target zone (up to 95 °C), along the abdominal aorta and within the coeliac plexus. (E) Temperature chart shows increase of tissue temperature during sonication in the target area. Reprinted from [75] Copyright 2018, with the permission of Springer.](/cms/asset/0a764a4e-eb7d-4d3e-9ca5-43d00e6fe692/ihyt_a_1506165_f0005_c.jpg)
3.4.2. Preclinical and ex vivo studies
Regarding the use of HIFU for pancreatic ablation in preclinical and ex vivo settings, 6 papers have been included according to the selection criteria [Citation69–74] (). The first use of HIFU in pancreatic animal models was performed in 2009 by Hwang et al. [Citation69]. Three different energy settings were used, and animal observed for 7 days before the sacrifice. At 750 J, no gross abnormalities were identified including evidence of injury to the target tissue. At 1000 J, one out of four animals had an obvious lesion in the targeted tissue, and one had a minor skin burn; pig treated at 1250 J had several significant findings on gross examination; two out of four pigs had minor skin burns, and three out of four animals had injury to the abdominal wall. No serious adverse events were observed in this study. Most importantly, there was no evidence of pancreatitis from HIFU treatment directed at normal pancreatic tissue.
Table 4. HIFU in preclinical and ex vivo settings.
In 2010 [Citation74] the pancreases of 12 mongrel pigs underwent both directly access HIFU (laparotomic approach) and extracorporeal HIFU through intact skin. All the animals survived at 7 days-control. The histopathologic and image-based controls at 24 h and 7 days provided absence of pancreatitis, and complete necrosis of the targeted region, but infiltration of inflammatory cells and fibrosis on the boundary were found in 7-days survival control group.
In 2014, Li et al. tested the effects of cavitation caused by pulsed HIFU at the range of peak-rarefactional pressures used of 5–11 MPa on phantoms, ex vivo bovine organs and transgenic mice [Citation72]. In the ex vivo tissue and phantoms, the cavitation occurs following the first few pulses of HIFU, whereas in vivo, cavitation events are distributed sporadically throughout the exposure. The same group studied endoscopic-guided HIFU in pancreas and liver of 5 porcine models [Citation73]. They developed an EUS-guided HIFU system, aiming at improving the targeting of pancreatic tumors with respect to the extracorporeal source, which is challenging due to the lack of an adequate acoustic window. After the treatment, the gastrointestinal tract was imaged by videoscope to identify any surface lesions, and histological analysis was performed. Immediately after the ablation, the endoscopic observations showed thermal injury of the gastric mucosa. The solid thermal lesions found in the excised liver and pancreas were larger in diameter than in the ex vivo case and involved a large area of the parenchymal surface.
A few studies have been performed while combining HIFU with the chemotherapeutic drug gemcitabine (GEM), aiming to enhance tumor apoptosis. In 2013, Lee performed pulsed HIFU with GEM in ex vivo models and in vivo mice bearing pancreatic tumor [Citation71]. Apoptosis rates were evaluated using the TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) assay and percentage of necrosis by using histopathological analysis. Overall rates of apoptosis were significantly higher in the group combining both HIFU and GEM than in the GEM group; also the tumor growth resulted to be the slowest.
Lastly, Kim et al. studied combined high power HIFU and GEM, combined low power HIFU and GEM, and the controls (only HIGU and only GEM) in 4 groups of mice [Citation70]. Pathology results showed that combined GEM and low-power HIFU treatment had a more effective response than other treatments.
3.4.3. Clinical application
Seventeen articles on pancreatic HIFU ablation have been included, for a total of 581 patients [Citation75–91]. In most cases (515 out of 581–88.6%) the ablative treatment was reserved to unresectable lesion (stage III-IV), while 38 lesions were classified as borderline. For the remaining 28 patients the tumor stage was not specified. The 28.4% of the lesions (165) were located in the head of pancreas, 52.4% (304) in the body-tail and 0.17% (1) in the uncinate process, while in 111 patients, location was not specified. Adenocarcinoma was the most frequently ablated lesion (455 patients-78.3%), followed by neuroendocrine tumor in 4 cases while in 122 patients the histotype was not specified. Patients clinical characteristic are reported in .
Table 5. HIFU in clinical settings.
Technically, HIFU can ablate the deep tissues from an external source using high-intensity focused ultrasound. The effects of HIFU can result in cell destruction and tissue necrosis. Thus, HIFU therapy is properly considered a minimally invasive or noninvasive therapy, and consequently promoted as a method to ablate the tumor and achieve pain relief in unresectable pancreatic carcinomas [Citation92].
The power applied was reported in 5 articles, with a value ranging between 200 and 580 W [Citation79,Citation81,Citation86,Citation87,Citation89]. The procedural monitoring was described only in one article and performed by means of MRI [Citation75].
In terms of post-operative course, a total 280 complications, divided into minor (235) and major (45) were evidenced. Only one paper did not document any HIFU-related complication [Citation80]. Among the major complications, vertebral necrosis was the most frequently registered one for a total of 35 cases with one associated case of duodenal injury and one third-degree skin burn. Among the minors, first and second-degree skin burns were the most frequently encountered ones for a total of 43 cases followed by transient pancreatitis in 32 cases. Other complications included fever in 3 cases, abdominal pain and dyspepsia in 50 patients and subcutaneous edema in 55 patients.
An objective short-term evaluation within 30 days from the ablation performed was reported in 14 studies for a total of 531 patients [Citation76–87,Citation89,Citation91]. In 9 studies was described a post-procedural evaluation using CT scan [Citation80,Citation81,Citation86,Citation87,Citation89,Citation90] and MRI [Citation75,Citation81,Citation83,Citation85,Citation86,Citation90] and only in one paper [Citation90] postoperative color Doppler US evaluation was used.
Among the 9 studies, only 6 reported a quantification of the tumor reduction for a total of 120 patients, with a complete response in 17 cases, a reduction more than 75% in 84 cases, between 50 and 75% in 13 cases and between 25 and 50% in 5 patients and 0 to 25% in 1 case.
In terms of tumor related pain, in 9 papers [Citation75,Citation79–81,Citation83,Citation84,Citation88,Citation90,Citation91] a post-procedural analysis using VAS scale was reported for a total of 148 patients. A complete or partial (75–100%) relief of cancer-related pain was achieved in 123 patients, while a reduction between 50% and 75% and between 25% and 50% was respectively achieved in 12 and 13 cases.
Tumor response at follow up was reported in a total of 6 studies [Citation78,Citation80,Citation81,Citation84,Citation87,Citation91]. Only two studies [Citation81,Citation91] reported a complete tumor response in 10% and 5.1% of patients respectively. A partial response was registered in 4 studies [Citation80,Citation81,Citation84,Citation91] with a range between 38.5% and 70%. Guojing Wang [Citation87] reported the best result in terms of minor progression rate of 10% after 12 months and a survival rate of 96.7% after one year. The worst outcome was reported by Jae Young Lee et al. [Citation78] with a progression tumor rate of 100% after 13 months, while Yu-Jiang Li [Citation80] and Hong Zhao [Citation91] reported a stable tumor disease after 6 and 12 months in 25% and 38.5% respectively.
3.5. Cryoablation: working principle, clinical application and preclinical studies
3.5.1. Working principle
While LA, RFA, MWA and HIFU rely on the severe increase of tissue temperature in the tumor masses, cryoablation uses cold to induce coagulative necrosis and tumor apoptosis. The procedure is performed by means of a cryoprobe and a cryogenic freezing unit: the unit allows for a high-pressure gas (e.g., Argon, at a temperature of −196 °C) to circulate within the lumen of the cryoprobe. The low pressure within the lumen causes a rapid expansion of gas, inducing the temperature decrease and the formation of an iceball around the probe tip [Citation93]. At the iceball boundary, the temperature is 0 °C, whereas lethal values between lower than −20 °C are produced inside the iceball. Cold temperature can induce cell damage and necrosis by means of a direct cell injury, and indirect mechanisms, identified as vascular injury and ischemia, apoptosis, and immunomodulation [Citation94–96]. The direct mechanism is responsible for tissue dehydration: the quick drop in temperature until −40 °C, and the absorption of heat by means of the probe results in the formation of ice crystals inside cells and in tonicity increase in the extracellular space. The cells surrounding undergo dehydration due to the osmotic pressure. Simultaneously, the accumulation of solutes in the extracellular space injures the cellular membranes and the enzymes. During thaw, the intracellular compartment becomes hypertonic, and fluid shift causes the cell to burst. The vascular injury is related to vasoconstriction and impairment to the microvasculature, contributing in causing micro-thrombosis and stasis. Such phenomena lead the targeted region to ischemic death and coagulative necrosis. Thus, cryonecrosis, cryoapoptosis, and anti-angiogenesis are some of the most important mechanisms of living tissue damage as a response to cryoablation.
Cryoablation is mainly known for the treatment of liver, prostate, and lung cancer. In the clinical application in the pancreas, has been reported the use of an argon gas-based cryosurgical unit (Endocare, Irvine, Calif) and 1 to 3 freeze/thaw cycles. The cryoprobe can be inserted percutaneously via the retroperitoneal, transhepatic, or transgastric approach, according to the position of the tumor. The procedure can be guided through CT and US imaging () [Citation97].
Figure 6. Cryoablation. Hyperechoic line along the path of the probe surrounded by nonhomogeneous tissue with hyperechoic spots. T, tumour; SMA, superior mesenteric artery. Reprinted from [Citation103] Copyright 2018, with the permission of Elsevier.
![Figure 6. Cryoablation. Hyperechoic line along the path of the probe surrounded by nonhomogeneous tissue with hyperechoic spots. T, tumour; SMA, superior mesenteric artery. Reprinted from [Citation103] Copyright 2018, with the permission of Elsevier.](/cms/asset/09a8e2b4-2c01-4bb8-8dcd-c047efe04ec1/ihyt_a_1506165_f0006_c.jpg)
3.5.2. Preclinical and ex vivo studies
Only one preclinical study about the test of cryoablation effects on pancreatic tissue has been performed. In 2007, Korpan et al. [Citation98] performed cryoablation on mongrel dogs, organized into 4 groups, each composed by 14 animals, to test two different ablation settings (with the respective control). A disk-shaped cryoprobe measuring about 20 mm in diameter was placed on the pancreas through laparotomy. Two different temperature values were reached in the two treated groups (−80 °C and −180 °C). Histopathology of ablated pancreas parenchyma and biopsy were collected up to 1 day after thawing. Damaged pancreatic cells in the postcryosurgical zone lead to cryonecrosis and cryoapoptosis. Vascular changes and circulatory stagnation indicate the anti-angiogenesis mechanism of biological tissue injury after low temperature exposure to −180 °C and −80 °C.
3.5.3. Clinical application
One article [Citation99] on the pancreatic cryoablation has been selected, including a total of 67 patients; of these, 36 underwent a pure cryoablative treatment. Conversely, 31 patients underwent a combined cryo-immunotherapy treatment. The ablative treatment was reserved to unresectable lesions. Adenocarcinoma was the only treated histotype (100%), but the pancreatic lesion location was not specified in any case.
Technically, the percutaneous approach under double-row CT or color ultrasonographic guidance was the only technique used.
In terms of post-operative course, no severe complications occurred. The authors described the increase of amylase serum level associated with the abdominal pain in 19 patients. Other early complications described were: increase of fasting blood glucose levels in 7 patients with a known diagnosis of diabetes, a mild decrease in platelet count in 12 patients, abdominal distension and nausea in 21, poor appetite in 17 cases. Self-limited abdominal bleeding occurred in 14 patients, while in 19 cases fever was encountered. All the complications were successfully treated conservatively.
In the first week after the cryoablation treatment, the pain score decreased by at least 50% in 54 patients, while analgesic consumption decreased by 50% in 57 patients and the Karnofsky Performance Score (KPS) increased by 20 in 43 cases.
The authors also analyzed the relation between number of sessions and the post-operative risk of complications. Of the total 114 cryoablation treatments performed in 67 patients, the 29% had a significant serum amylase level increase after 29 sessions against the 10% after 12 sessions.
In terms of long-term follow up, a median OS of 7 months was documented, with a better outcome in case of cryo-immunotherapy (13 months) as compared to the pure cryoablation treatment (7 months).
3.6. Hybrid and alternative techniques
3.6.1. Description
Often, different ablative techniques are combined to take advantage from the synergic effects of the single approaches.
3.6.2. Preclinical and ex vivo studies
In 2008, Carrara et al presented the EUS-guided application of a new hybrid cryotherm probe [Citation100]. The probe combines the cryotherapy with RFA, guided by linear EUS device. The rationale behind this dual modality strategy relies on their potential combined positive effects on the pancreas. Indeed, it has been demonstrated that RFA without additional cooling can be dangerous for this organ. Hence, this probe is used to reinforce the RF − induced interstitial devitalization with the effective cooling induced by the cryogenic gas. Consequently, less collateral damage is expected because a lower power input is needed compared to conventional RFA systems in order to obtain the same thermal outcome. All pigs well tolerated the procedure and the pancreatic body and tail were easily visualized from the stomach using the linear EUS probe; conversely, ultrasound imaging overestimates the lesion size. At histology, 2 pigs showed histological signs of pancreatitis meanwhile the rest showed well defined coagulative necrotic lesions at 1 week follow up. At two weeks follow up, there was no evidence of clinical distress. Regarding the safety, the mortality was zero; there was one major (7%), and 6 minor complications (43%), related to the dose of treatment. The same group evaluated the same device while treating neoplastic tissue of explanted pancreatic tumors of patients with pancreatic adenocarcinoma [Citation101]
As alternative techniques, Adams et al. designed an endoluminal US applicator for MR-guided thermal ablation of the pancreatic cancer, and tested the prototypes in porcine models [Citation102]. The advantage of this technique is the energy delivery from the gastrointestinal tract in the pancreas, without contact with the organ. A summary of these hybrid technologies is presented in .
Table 6. Hybrid and new ablation techniques in preclinical and ex vivo settings.
3.6.3. Clinical application
Only Arcidiacono et al. [Citation103] reported a combined ablative treatment in a clinical setting. The ablation comprised the cryothermal and the RFA techniques for a total of 22 patients. In all cases, only unresectable adenocarcinomas were included. The tumor was located in the pancreatic head/neck in 16 patients, uncinate process in 2 and in the body of the pancreas in the remaining 4. The mean lesion size was 35.7 mm (range 23–54 mm).
In all cases, the endoscopic ultrasonography (EUS) technique was performed. The RFA power was 18 W, delivered with an application time between 10 and 360 s (mean time 107 ± 86 s), varying on tumor size.
In all patients a radiological CT scan evaluation was performed 48 h after the ablation, with no evidence of pancreatitis, pancreatic necrosis or fluid collections.
An additional radiological objective tumor response was reported after a mean time of 14.6 ± 15.8 days (range 0–37 days). Only in 6 patients the CT scan was clear enough to interpret the images. In all these cases, no evidence of tumor growth for up to 78 days was documented.
In terms of OS, a median value of 6 months was documented for a total of 13 patients. Six patients died at 6 months, while 7 reported a progressive disease.
4. Discussion and conclusions
Despite the enormous advances in the oncological medical field, prognosis of pancreatic cancer remains poor [Citation3]. Surgical resection is currently the only potential curative treatment. However, due to the late onset of symptoms and to the disease aggressiveness, only the 20% of patients are eligible for surgery [Citation2,Citation104]. As consequence, most of the patients present at unresectable stages (III and IV) and palliative chemotherapy is employed to prolong survival and to control tumor growth. At this last regard, major advances have been made in combination therapies, here including multiple chemotherapeutical schemes, radiotherapy alone or in combination to chemotherapy as well as stereotactic body radiotherapy (SBRT) and stereotactic radiation therapy (SRT). However, despite the slight amelioration in long-term survival, the rate of patients responsive to these treatments remains low.
Since the introduction, in 2011, of FOLFIRINOX as chemotherapeutic scheme for locally advanced pancreatic cancer treatment, showing improvement of OS [Citation105], multiple therapeutic schemes have been proposed [Citation6]. Despite these advances, only one third of patients are responsive to the treatment, while complication related to chemotherapy significantly increased, here including gastrointestinal adverse event in almost 30% of patients, hematological side effects (namely anemia and leukopenia in up to 81% of patients [Citation106]), anorexia, fatigue and loss of appetite [Citation6].
With the aim to enhance chemotherapy efficacy, a combination therapy with radiation has been proposed. However, results are still controversial and unclear. Despite the promising results achieved in two large retrospective studies [Citation107,Citation108] as compared to chemotherapy alone, the recent LAP-07 trial [Citation8] failed in showing any benefit in terms of overall survival for the additional radiation treatment. Additionally, the combination of chemotherapy with radiation could enhance systemic toxicity, potentially precluding the administration of subsequent systemic therapies [Citation109].
As alternative, the stereotactic body radiotherapy (SBRT) treatment has been introduced. SBRT is an advanced technique able to precisely delivery a high dose of radiation to the target in 1–5 fractions (hypofractionated regimen), as complementary treatment to chemotherapy. However, particular care is required during planning and delivery, since any imprecise targeting could lead to surrounding normal structures toxicity [Citation110]. More specifically, cases of gastrointestinal perforation and/or hemorrhage, biliary or duodenal stricture as consequence of secondary fibrosis for pancreatic cancer treatment have been reported in the literature, with an incidence rate of Grade ≥2 late toxicity up to 20%. In terms of long-term outcomes, an improvement of the local disease control as compared to chemotherapy alone has been demonstrated, while OS remains poor.
Based on these premises, efforts have been recently made to investigate minimally invasive ablative techniques to provide a better local tumor control and to improve patients’ OS especially in the wide group of patients not eligible for surgery. RFA, HIFU, cryoablation, MWA, and LA have been described as valuable techniques both in preclinical and clinical settings. However, the lack of standardization in the employment of these different techniques and the different approaches used makes it difficult to draw homogenous conclusion on their real safety and feasibility.
With the aim of giving our contribution to better define the therapeutic role of these different ablative methods, we conducted an analysis of each of them, going through the methodological analysis, as well as the preclinical advances and the current clinical applications.
The largest part of the preclinical and ex vivo studies for ablative treatment of pancreas has been carried out using LA (12 papers), RFA (8 papers) and HIFU (6 papers). Only 1 work has been found describing the animal application of cryoablation, and no papers about MWA resulted from the search performed though the described selection criteria.
Preclinical and ex vivo studies are fundamental to drive and design successive clinical studies. The nature of the preclinical study allows performing an extensive histopathology analysis at different time lapse from the ablation procedure, allowing to better understand the temporal evolution of the treatment outcome. In addition, it is possible to adopt technological strategies that are not applicable in human studies, yet. For instance, in several preclinical and ex vivo studies, thermometry is used as a mean for intraprocedural control. These studies demonstrate the importance of having a real-time temperature control during the procedure, and how this information can be related with the induced thermal damage. The intraprocedural knowledge of this thermal damage could represent a helpful feedback for the clinician who can decide in real-time to tune the procedure (for instance, performing a second treatment in a close target region).
Additionally, the implementation of a numerical-based tool and its use in synergy with the real-time measured thermal information may be particularly useful to successfully plan the therapy settings (power and time, for LA, RFA, MWA and HIFU, pressure and time for cryoablation).
According to the evidences pointed out by our review, it emerges how the use of thermometric systems to monitor target temperature intraoperatively is still limited in the clinical practice, unlike in the preclinical and ex vivo studies, although several of them have been approved for medical use. This delay could rely on the lack of infrastructures needed for thermometry (e.g., MR system), or on the slow upgrade of approaches and techniques.
Regarding the emerging technologies, the assessment of nanoparticles to enhance the thermal effects of ablation therapies is on the stage since 2003 [Citation111]; however, they have been rarely used in human [Citation112] because their biocompatibility and long-term effects is still under investigation, and their use is still restrained to the preclinical experimentation.
Despite the wide experience reported in the preclinical setting, more limited applications have been documented in the clinical field for all the ablative techniques included in our review. Even if LA resulted the most frequently investigated in the preclinical setting, only one clinical study with limited cases is present in the literature meeting the inclusion criteria. Conversely, 17 and 14 papers on HIFU and RFA application respectively have been included, while only one article describing the cryoablation and microwave employment met the inclusion criteria.
HIFU has been investigated for the first time in the early 2000 for pain-related treatment of pancreatic cancer [Citation69]. Regarding the present market approval, one device has been already CE approved for the MR-guided clinical treatment of uterine fibroids and palliative pain treatment of bone tumors [Citation113], while another one is also FDA 510(k) cleared for the laparoscopic or intraoperative ablation of soft tissue [Citation114]. The use in pancreas is still under evaluation, and several studies have been performed to investigate whether its clear advantages can be effectively exploited for pancreatic cancer treatment. However, some limitations need to be outlined. It is an US-based technique, and, therefore, artifacts, such as acoustic shadowing, refraction and reverberation could interfere on its application especially for deep-site lesions. For pancreatic lesions, the interposition of bowel gas may significantly obstruct the acoustic window, leading to two main potential consequences: incomplete ablation of the target and also the thermal damage of the interposed bowel due to the heat deposition at the gas-tissue interface. Consequently, to evacuate gas into the stomach and colon (having the patients fast the night before the procedure) as well as applying a slight abdominal pressure to the target area are fundamental steps to achieve adequate results and to avoid procedure-related complications [Citation115]. Other limitations are also related to the potential interposition of fatty, fibrotic and highly vascularized tissues [Citation116], bones [Citation117], and major vessels [Citation118], that significantly attenuate the sound energy applied. This, inevitably, lead to the need of adequately ensure a careful pre-procedural planning and to precisely locate the target lesion [Citation116].
Conversely, RFA, cryoablation and MWA require a direct contact with the neoplastic tissue by means of an open, endoscopic or percutaneous approach. This main difference could bring to reflect into two significant consequences: the intra-tumoral heat distribution and post-procedural complication rate. As a matter of fact, it has been already demonstrated how HIFU allows an uniform intra-tumoral heating while heat distribution with the other techniques mainly depends on the right probe insertion [Citation119]. In addition, RFA strongly depends on the necrotic tissue impedance, inducing a self-limiting ablation [Citation120,Citation121] as compared to both HIFU and MWA. In terms of post-procedural complications, the mechanical insertion of electrodes, antennas or cryoprobes into the neoplastic tissue would relate with a higher risk of post-procedural complications, induced by both the ‘mechanical trauma’ and the local temperature reached. As expected, HIFU related with a significantly lower incidence of complications for a total of 117 in 565 patients (20.7%), as compared to 41.9% (52 complications in 124 patients) in the RFA clinical application, 30% (3 patients) post-procedural morbidity after microwave ablation, 28.5% (19 patients) after cryoablation and 33% after LA. However, a clinical distinction between major and minor complications is mandatory. Despite the lower overall complication rate after HIFU ablation, the 16.1% (45 complications) is considerable as major technique-related adverse events, while relevant complications occurred in only 9.6% (5 patients) of cases after RFA, while cryoablation, microwave and laser ablation induced minor complications. When compared to the conventional treatment strategies (including chemotherapy with or without radiation and SBRT), the ablative techniques have shown to be safe and feasible. Rising complications are mainly minor, local and easily manageable as compared to systemic complications (i.e. leukopenia) for chemotherapy. Similarly, advantages can be evidenced as compared to SBRT and radiation, causing similar adverse events rate in case of HIFU employment, but significantly lower in case of LA, RFA, cryoablation and microwave.
One of the major topics to be analyzed regarding the minimally invasive ablative techniques is the short- and long-term efficacy on the tumor dimension reduction and the related long-term survival. A short-term quantification can be drawn only for HIFU and RFA, with a complete response reached respectively in the 14.2% and 31.4% of cases. For both the techniques, CT scan was the most frequently employed evaluation tool. Despite the significant difference between HIFU and RFA, these results should be interpreted with caution, since different type of tumors and different lesion sizes have been reported for both the techniques.
Also regarding long-term outcomes, solid conclusions cannot be defined. The inclusion of both adenocarcinomas and neuroendocrine tumors does not allow valuable comparisons. For each of the techniques evaluated, tumor response and OS significantly varied and categorization according to the neoplastic disease was not applicable. The only valid data can be defined for cryoablation and laser ablation. In both cases, only adenocarcinomas were enrolled in the studies, with a median OS of 7 months for cryoablation and 7.4 months for laser ablation respectively. As a matter of fact, these last values do not significantly differ as compared to the mean OS in chemotherapy palliative treatment of stage III–IV pancreatic cancer [Citation1]. Of note, better results have been achieved in case of concomitant HIFU and gemcitabine therapy with a median survival of 12.6 months [Citation79]. However, the results reported by only one center cannot be considered sufficient to generalize the long-term efficacy for each of these techniques.
Acknowledgements
The authors would like to thank Ms. Agnese Perno for the support with some of the figures.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–1046.
- Kommalapati A, Tella SH, Goyal G, et al. Contemporary management of localized resectable pancreatic cancer. Cancers (Basel). 2018;10(1):1–15.
- American Cancer Society. Cancer facts and figures 2013. Atlanta: American Cancer Society; 2013.
- Sheahan AV, Biankin AV, Parish CR, et al. Targeted therapies in the management of locally advanced and metastatic pancreatic cancer: a systematic review. Oncotarget. 2018;9:21613–21627.
- International Agency for Research on Cancer (IARC). http://www.iarc.fr/.
- Chin V, Nagrial A, Sjoquist K, et al. Chemotherapy and radiotherapy for advanced pancreatic cancer. Cochrane Database Syst Rev. 2018;3:CD011044.
- Gillen S, Schuster T, Meyer Zum Buschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267.
- Hammel P, Huguet F, van Laethem JL, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315:1844–1853.
- Marien A, Gill I, Ukimura O, et al. Target ablation—Image-guided therapy in prostate cancer. Urol Oncol; 2014:32(6):912–923.
- Petre EN, Sofocleous C. Thermal ablation in the management of colorectal cancer patients with oligometastatic liver disease. Visc Med. 2017;33:62–68.
- O'Neal D, Cohen T, Peterson C, et al. Contrast-enhanced ultrasound-guided radiofrequency ablation of renal tumors. 2018;5:7–14.
- Welch AJ, Van Gemert MJ. Optical-thermal response of laser-irradiated tissue. Vol. 2. Dordrecht: Springer; 2011.
- Schena E, Saccomandi P, Fong Y. Laser ablation for cancer: past, present and future. J Funct Biomater. 2017;8:19.
- Saccomandi P, Schena E, Diana M, et al. Thermal treatments of tumors: principles and methods. In: Piemonte V, Basile A, Ito T, Marrelli L, editors. Biomedical engineering challenges: a chemical engineering insight. Chapter 10. 1st ed. Wiley; 2018. p. 199–228.
- Menovsky T, Beek J, Van Gemert M, et al. Interstitial laser thermotherapy in neurosurgery: a review. Acta Neurochir (Wien). 1996;138:1019–1026.
- Di Matteo F, Picconi F, Martino M, et al. Endoscopic ultrasound-guided Nd:YAG laser ablation of recurrent pancreatic neuroendocrine tumor: a promising revolution? Endoscopy. 2014;46: (Suppl 1 UCTN):E380–E381.
- Mooney R, Schena E, Saccomandi P, et al. Gold nanorod-mediated near-infrared laser ablation: in vivo experiments on mice and theoretical analysis at different settings. Int J Hyperther. 2017;33:150–159.
- Mocan L, Tabaran FA, Mocan T, et al. Selective ex-vivo photothermal ablation of human pancreatic cancer with albumin functionalized multiwalled carbon nanotubes. Int J Nanomed. 2011;6:915–928.
- Allegretti G, Saccomandi P, Giurazza F, et al. Magnetic resonance-based thermometry during laser ablation on ex-vivo swine pancreas and liver. Med Eng Phys. 2015;37:631–641.
- Di Matteo F, Martino M, Rea R, et al. US-guided application of Nd:YAG laser in porcine pancreatic tissue: an ex vivo study and numerical simulation. Gastrointest Endosc. 2013; 78:750–755. doi: 10.1016/j.gie.2013.04.178.
- Di Matteo F, Martino M, Rea R, et al. EUS-guided Nd:YAG laser ablation of normal pancreatic tissue: a pilot study in a pig model. Gastrointest Endosc. 2010;72:358–363.
- Guo Y, Zhang Z, Kim DH, et al. Photothermal ablation of pancreatic cancer cells with hybrid iron-oxide core gold-shell nanoparticles. Int J Nanomed. 2013;8:3437–3446.
- Mocan T, Matea CT, Cojocaru I, et al. Photothermal treatment of human pancreatic cancer using PEGylated multi-walled carbon nanotubes induces apoptosis by triggering mitochondrial membrane depolarization mechanism. J Cancer. 2014;5:679.
- Saccomandi P, Schena E, Caponero MA, et al. Theoretical analysis and experimental evaluation of laser-induced interstitial thermotherapy in ex vivo porcine pancreas. IEEE Trans Biomed Eng. 2012;59:2958–2964.
- Saccomandi P, Schena E, Di Matteo FM, et al. Theoretical assessment of principal factors influencing laser interstitial thermotherapy outcomes on pancreas. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:5687–5690.
- Saccomandi P, Schena E, Di Matteo FM, et al. Laser interstitial thermotherapy for pancreatic tumor ablation: theoretical model and experimental validation. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:5585–5588.
- Schena E, Majocchi L. Assessment of temperature measurement error and its correction during Nd: YAG laser ablation in porcine pancreas. Int J Hyperthermia. 2014;30:328–334.
- Schena E, Saccomandi P, Giurazza F, et al. Experimental assessment of CT-based thermometry during laser ablation of porcine pancreas. Phys Med Biol. 2013;58:5705–5716.
- Stroszczynski C, Hosten N, Puls R, et al. Histopathological correlation to MRI findings during and after laser-induced thermotherapy in a pig pancreas model. Invest Radiol. 2001;36:413–421.
- Bruners P, Levit E, Penzkofer T, et al. Multi-slice computed tomography: A tool for non-invasive temperature measurement? Int J Hyperthermia. 2010;26:359–365.
- Di Matteo FM, Saccomandi P, Martino M, et al. Feasibility of EUS-guided Nd:YAG laser ablation of unresectable pancreatic adenocarcinoma. Gastrointest Endosc. 2018;88(1):168–174.e1.
- Goldberg SN. Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound. 2001;13:129–147.
- Pereira PL, Trubenbach J, Schenk M, et al. Radiofrequency ablation: in vivo comparison of four commercially available devices in pig livers. Radiology. 2004;232:482–490.
- Fegrachi S, Besselink MG, van Santvoort HC, et al. Radiofrequency ablation for unresectable locally advanced pancreatic cancer: a systematic review. HPB (Oxford). 2014;16:119–123.
- Date RS, McMahon RF, Siriwardena AK. Radiofrequency ablation of the pancreas. I: Definition of optimal thermal kinetic parameters and the effect of simulated portal venous circulation in an ex-vivo porcine model. JOP. 2005;6:581–587.
- Goldberg SN, Mallery S, Gazelle GS, et al. EUS-guided radiofrequency ablation in the pancreas: results in a porcine model. Gastrointest Endosc. 1999;50:392–401
- Date RS, Biggins J, Paterson I, et al. Development and validation of an experimental model for the assessment of radiofrequency ablation of pancreatic parenchyma. Pancreas. 2005;30:266–271.
- Gaidhane M, Smith I, Ellen K, et al. Endoscopic Ultrasound-Guided Radiofrequency Ablation (EUS-RFA) of the Pancreas in a Porcine Model. Gastroenterol Res Pract. 2012;2012:1.
- Kim HJ, Seo DW, Hassanuddin A, et al. EUS-guided radiofrequency ablation of the porcine pancreas. Gastrointest Endosc. 2012;76:1039–1043.
- Fegrachi S, Molenaar IQ, Klaessens JH, et al. Radiofrequency ablation of the pancreas: two-week follow-up in a porcine model. Eur J Surg Oncol. 2014;40:1000–1007.
- Quesada R, Burdío F, Iglesias M, et al. Radiofrequency pancreatic ablation and section of the main pancreatic duct does not lead to necrotizing pancreatitis. Pancreas. 2014;43:931–937.
- Raoof M, Cisneros BT, Corr SJ, et al. Tumor selective hyperthermia induced by short-wave capacitively-coupled RF electric-fields. PLoS One. 2013;8:e68506.
- D'Onofrio M, Barbi E, Girelli R, et al. Variation of tumoral marker after radiofrequency ablation of pancreatic adenocarcinoma. J Gastrointest Oncol. 2016;7:213–220.
- D’Onofrio M, Crosara S, De Robertis R, et al. Percutaneous radiofrequency ablation of unresectable locally advanced pancreatic cancer: preliminary results. Technol Cancer Res Treat. 2017;16:285–294.
- Giardino A, Girelli R, Frigerio I, et al. Triple approach strategy for patients with locally advanced pancreatic carcinoma. HPB (Oxford). 2013;15:623–627.
- Hadjicostas P, Malakounides N, Varianos C, et al. Radiofrequency ablation in pancreatic cancer. HPB (Oxford). 2006;8:61–64.
- Ikuta S, Kurimoto A, Iida H, et al. Optimal combination of radiofrequency ablation with chemoradiotherapy for locally advanced pancreatic cancer. World J Clin Oncol. 2012;3:12–14.
- Kallis Y, Phillips N, Steel A, et al. Analysis of endoscopic radiofrequency ablation of biliary malignant strictures in pancreatic cancer suggests potential survival benefit. Dig Dis Sci. 2015;60:3449–3455.
- Lakhtakia S, Ramchandani M, Galasso D, et al. EUS-guided radiofrequency ablation for management of pancreatic insulinoma by using a novel needle electrode (with videos). Gastrointest Endosc. 2016;83:234–239.
- Pai M, Habib N, Senturk H, et al. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J Gastrointest Surg. 2015;7:52–59.
- Rossi S, Viera FT, Ghittoni G, et al. Radiofrequency ablation of pancreatic neuroendocrine tumors: a pilot study of feasibility, efficacy, and safety. Pancreas. 2014;43:938–945.
- Song TJ, Seo DW, Lakhtakia S, et al. Initial experience of EUS-guided radiofrequency ablation of unresectable pancreatic cancer. Gastrointest Endosc. 2016;83:440–443.
- Spiliotis JD, Datsis AC, Michalopoulos NV, et al. Radiofrequency ablation combined with palliative surgery may prolong survival of patients with advanced cancer of the pancreas. Langenbecks Arch Surg. 2007;392:55–60.
- Varshney S, Sewkani A, Sharma S, et al. Radiofrequency ablation of unresectable pancreatic carcinoma: feasibility, efficacy and safety. JOP. 2006;7:74–78.
- Waung JA, Todd JF, Keane MG, et al. Successful management of a sporadic pancreatic insulinoma by endoscopic ultrasound-guided radiofrequency ablation. Endoscopy. 2016;48:E144–E145.
- Zou YP, Li WM, Zheng F, et al. Intraoperative radiofrequency ablation combined with 125 iodine seed implantation for unresectable pancreatic cancer. World J Gastroenterol. 2010;16:5104–5110.
- Rossmann C, Haemmerich D. Review of temperature dependence of thermal properties, dielectric properties, and perfusion of biological tissues at hyperthermic and ablation temperatures. Crit Rev Biomed Eng. 2014;42:467.
- Dodd GD, Dodd NA, Lanctot AC, et al. Effect of variation of portal venous blood flow on radiofrequency and microwave ablations in a blood-perfused bovine liver model. Radiology. 2013;267:129–136.
- Wright AS, Sampson LA, Warner TF, et al. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology. 2005;236:132–139.
- Yang D, Bertram JM, Converse MC, et al. A floating sleeve antenna yields localized hepatic microwave ablation. IEEE Trans Biomed Eng. 2006;53:533–537.
- Vietti Violi N, Duran R, Guiu B, et al. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3:317–325.
- Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Int J Hyperthermia. 2016;32:339–344.
- Carrafiello G, Ierardi AM, Fontana F, et al. Microwave ablation of pancreatic head cancer: safety and efficacy. J Vasc Interv Radiol. 2013;24:1513–1520.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247.
- Al-Bataineh O, Jenne J, Huber P. Clinical and future applications of high intensity focused ultrasound in cancer. Cancer Treat Rev. 2012;38:346–353.
- Crouzet S, Murat FJ, Pasticier G, et al. High intensity focused ultrasound (HIFU) for prostate cancer: current clinical status, outcomes and future perspectives. Int J Hyperthermia. 2010;26:796–803.
- Illing RO, Kennedy JE, Wu F, et al. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br J Cancer. 2005;93:890–895.
- Mo S, Coussios C-C, Seymour L, et al. Ultrasound-enhanced drug delivery for cancer. Expert Opin Drug Deliv. 2012;9:1525–1538.
- Hwang JH, Wang YN, Warren C, et al. Preclinical in vivo evaluation of an extracorporeal HIFU device for ablation of pancreatic tumors. Ultrasound Med Biol. 2009;35:967–975.
- Kim JH, Kim H, Kim YJ, et al. Dynamic contrast–enhanced ultrasonographic (DCE-US) assessment of the early response after combined gemcitabine and HIFU with low-power treatment for the mouse xenograft model of human pancreatic cancer. Eur Radiol. 2014;24:2059–2068.
- Lee ES, Lee JY, Kim H, et al. Pulsed high-intensity focused ultrasound enhances apoptosis of pancreatic cancer xenograft with gemcitabine. Ultrasound Med Biol. 2013;39:1991–2000.
- Li T, Chen H, Khokhlova T, et al. Passive cavitation detection during pulsed HIFU exposures of ex vivo tissues and in vivo mouse pancreatic tumors. Ultrasound Med Biol. 2014;40:1523–1534.
- Li T, Khokhlova T, Maloney E, et al. Endoscopic high-intensity focused US: technical aspects and studies in an in vivo porcine model (with video). Gastrointestinal Endoscopy. 2015;81:1243–1250.
- Xie B, Li YY, Jia L, et al. Experimental ablation of the pancreas with high intensity focused ultrasound (HIFU) in a porcine model. Int J Med Sci. 2010;8:9–15.
- Anzidei M, Napoli A, Sandolo F, et al. Magnetic resonance-guided focused ultrasound ablation in abdominal moving organs: a feasibility study in selected cases of pancreatic and liver cancer. Cardiovasc Intervent Radiol. 2014;37:1611–1617.
- Ge H-Y, Miao L-Y, Xiong L-L, et al. High-intensity focused ultrasound treatment of late-stage pancreatic body carcinoma: optimal tumor depth for safe ablation. Ultrasound Med Biol. 2014;40:947–955.
- Jung SE, Cho SH, Jang JH, et al. High-intensity focused ultrasound ablation in hepatic and pancreatic cancer: complications. Abdom Imaging. 2011;36:185–195.
- Lee JY, Choi BI, Ryu JK, et al. Concurrent chemotherapy and pulsed high-intensity focused ultrasound therapy for the treatment of unresectable pancreatic cancer: initial experiences. Korean J Radiol. 2011;12:176–186.
- Li P-Z, Zhu S-H, He W, et al. High-intensity focused ultrasound treatment for patients with unresectable pancreatic cancer. Hepatobiliary Pancreatic Dis Int. 2012;11:655–660.
- Li Y-J, Huang G-L, Sun X-L, et al. The combination therapy of high-intensity focused ultrasound with radiotherapy in locally advanced pancreatic carcinoma. World J Surg Onc. 2016;14:60.
- Marinova M, Rauch M, Mücke M, et al. High-intensity focused ultrasound (HIFU) for pancreatic carcinoma: evaluation of feasibility, reduction of tumour volume and pain intensity. Eur Radiol. 2016;26:4047–4056.
- Orgera G, Monfardini L, Della Vigna P, et al. High-intensity focused ultrasound (HIFU) in patients with solid malignancies: evaluation of feasibility, local tumour response and clinical results. Radiol Med. 2011;116:734–748.
- Orsi F, Zhang L, Arnone P, et al. High-intensity focused ultrasound ablation: effective and safe therapy for solid tumors in difficult locations. Amer J Roentgenol. 2010;195:W245–W252.
- Strunk H, Henseler J, Rauch M, et al. editors. Clinical use of High-Intensity Focused Ultrasound (HIFU) for tumor and pain reduction in advanced pancreatic cancer. RöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren; 2016. Rofo. 2016;188(7):662–670.
- Sung HY, Jung SE, Cho SH, et al. Long-term outcome of high-intensity focused ultrasound in advanced pancreatic cancer. Pancreas. 2011;40:1080–1086.
- Vidal-Jove J, Perich E, del Castillo MA. Ultrasound guided high intensity focused ultrasound for malignant tumors: the Spanish experience of survival advantage in stage III and IV pancreatic cancer. Ultrasonics Sonochem. 2015;27:703–706.
- Wang G, Zhou D. Preoperative ultrasound ablation for borderline resectable pancreatic cancer: A report of 30 cases. Ultrasonics Sonochem. 2015;27:694–702.
- Wang K, Chen Z, Meng Z, et al. Analgesic effect of high intensity focused ultrasound therapy for unresectable pancreatic cancer. Int J Hyperther. 2011;27:101–107.
- Wang K, Zhu H, Meng Z, et al. Safety evaluation of high-intensity focused ultrasound in patients with pancreatic cancer. Oncology Research and Treatment. Onkologie. 2013;36:88–92.
- Wu F, Wang Z-B, Zhu H, et al. Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: initial experience. Radiology. 2005;236:1034–1040.
- Zhao H, Yang G, Wang D, et al. Concurrent gemcitabine and high-intensity focused ultrasound therapy in patients with locally advanced pancreatic cancer. Anti-Cancer Drugs. 2010;21:447–452.
- Sofuni A, Moriyasu F, Sano T, et al. The current potential of high‐intensity focused ultrasound for pancreatic carcinoma. J Hepato-Biliary-Pancreatic Sci. 2011;18:295–303.
- Tatli S, Acar M, Tuncali K, et al. Percutaneous cryoablation techniques and clinical applications. Diagn Interv Radiol. 2010;16:90–95.
- Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14:199–208.
- Erinjeri JP, Clark TW. Cryoablation: mechanism of action and devices. J Vasc Interv Radiol. 2010;21:S187–S191.
- Lovelock JE. The haemolysis of human red blood-cells by freezing and thawing. Biochim Biophys Acta. 1953;10:414–426.
- Luo XM, Niu LZ, Chen JB, et al. Advances in cryoablation for pancreatic cancer. World J Gastroenterol. 2016;22:790–800.
- Korpan NN. Cryosurgery: ultrastructural changes in pancreas tissue after low temperature exposure. Technol Cancer Res Treat. 2007;6:59–67.
- Niu L, Chen J, He L, et al. Combination treatment with comprehensive cryoablation and immunotherapy in metastatic pancreatic cancer. Pancreas. 2013;42:1143–1149.
- Carrara S, Arcidiacono P, Albarello L, et al. Endoscopic ultrasound-guided application of a new hybrid cryotherm probe in porcine pancreas: a preliminary study. Endoscopy. 2008;40:321–326.
- Petrone MC, Arcidiacono PG, Carrara S, et al. US-guided application of a new hybrid probe in human pancreatic adenocarcinoma: an ex vivo study. Gastrointest Endosc. 2010;71:1294–1297.
- Adams MS, Salgaonkar VA, Plata, ‐et al. Endoluminal ultrasound applicators for MR‐guided thermal ablation of pancreatic tumors: Preliminary design and evaluation in a porcine pancreas model. Med Phys. 2016;43:4184–4197.
- Arcidiacono PG, Carrara S, Reni M, et al. Feasibility and safety of EUS-guided cryothermal ablation in patients with locally advanced pancreatic cancer. Gastrointest Endosc. 2012;76:1142–1151.
- Zuckerman DS, Ryan DP. Adjuvant therapy for pancreatic cancer: a review. Cancer. 2008;112:243–249.
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825.
- Xinopoulos D, Dimitroulopoulos D, Karanikas I, et al. Gemcitabine as palliative treatment in patients with unresectable pancreatic cancer previously treated with placement of a covered metal stent. A randomized controlled trial. J Buon. 2008;13:341–347.
- Huguet F, Andre T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326–331.
- Krishnan S, Rana V, Janjan NA, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer. 2007;110:47–55.
- Grainne M, Knox JJ. Locally advanced pancreatic cancer: an emerging entity. Curr Probl Cancer. 2018;42(1):12–25.
- Ng SP, Herman JM. Stereotactic radiotherapy and particle therapy for pancreatic cancer. Cancers (Basel). 2018;10:75.
- Hirsch LR, Stafford RJ, Bankson J, et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Nat Acad Sci. 2003;100:13549–13554.
- Stern JM, Kibanov Solomonov VV, Sazykina E, et al. Initial evaluation of the safety of nanoshell-directed photothermal therapy in the treatment of prostate disease. Int J Toxicol. 2016;35:38–46.
- Sonalleve MR-HIFU System. http://www.profoundmedical.com/sonalleve/.
- Sonacare Medical HIFU system. https://sonacaremedical.com/
- Khokhlova TD, Hwang JH. HIFU for palliative treatment of pancreatic cancer. J Gastrointest Oncol. 2011;2:175–184.
- She WH, Cheung TT, Jenkins CR, et al. Clinical applications of high-intensity focused ultrasound. Hong Kong Med J. 2016;22:382–392.
- Zhu H, Zhou K, Zhang L, et al. High intensity focused ultrasound (HIFU) therapy for local treatment of hepatocellular carcinoma: role of partial rib resection. Eur J Radiol. 2009;72:160–166.
- Zhang L, Zhu H, Jin C, et al. High-intensity focused ultrasound (HIFU): effective and safe therapy for hepatocellular carcinoma adjacent to major hepatic veins. Eur Radiol. 2009;19:437–445.
- Marrocchio C, Dababou S, Catalano C, et al. Nonoperative Ablation of Pancreatic Neoplasms. Surg Clin North Am. 2018;98:127–140.
- D’Onofrio M, Ciaravino V, De Robertis R, et al. Percutaneous ablation of pancreatic cancer. World J Gastroenterol. 2016; 22:9661–9673.
- Iida H, Aihara T, Ikuta S, et al. Effectiveness of impedance monitoring during radiofrequency ablation for predicting popping. World J Gastroenterol. 2012;18:5870–5878.