Abstract
Background: The peritoneum is the most frequent site of disease recurrence in gastric cancer, and the prognosis remains poor. This study assessed the role of adjuvant intraperitoneal (IP) chemotherapy with whole abdominal hyperthermia using external radiofrequency in gastric cancer patients after D2 dissection.
Methods: Patients with gastric cancer who underwent gastrectomy with D2 regional lymph node dissection were enrolled in the study. Patients received IP chemotherapy with whole abdominal hyperthermia. Preheated normal saline containing 75 mg/m2 of cisplatin was delivered into the abdominal cavity through a Tenckhoff catheter at McBurney’s point. Regional hyperthermia was performed using two sets of orthogonal radiofrequency waves immediately after all saline was irrigated into the abdominal cavity. For each patient, recurrent or metastatic sites and adverse events were evaluated.
Results: A total of 22 patients were finally included. All patients tolerated hyperthermia well. Only two patients experienced grade 1 superficial thermal injury. The most frequent grade 3/4 adverse events were myelosuppression, nausea/vomiting, trichomadesis and liver dysfunction. We also found IP chemotherapy with whole abdominal hyperthermia could reduce the total recurrent/metastatic rate, especially peritoneal metastasis (4.5%).
Conclusions: This hypothesis-generating study indicated that IP chemotherapy with whole abdominal hyperthermia might be feasible for gastric cancer patients after D2 resection.
1. Introduction
Gastric cancer is the most common cancer and the leading cause of cancer death worldwide [Citation1]. It is estimated that approximately 679 million new gastric cancer cases occurred in China each year [Citation2]. Curative resection has been considered as the only way to cure gastric cancer. The peritoneum is the most frequent site of disease metastases or recurrences in gastric cancer patients [Citation3,Citation4]. Moreover, 20–50% of gastric cancer patients will suffer a peritoneal recurrence or failure even after complete resection [Citation5,Citation6]. Although various approaches have been attempted, such as extended resection, combination chemotherapy, heated intraperitoneal (IP) chemotherapy and immunotherapy, the prognosis of patients with peritoneal carcinomatosis (PM) remains unsatisfactory. Therefore, it is necessary to explore an appropriate treatment to avoid or delay peritoneal recurrence in high-risk patients.
Hyperthermia is thought to be the fifth most common medical treatment after surgery, chemotherapy, radiation and biological therapy, and plays an important role in comprehensive treatment for malignant tumors. Hyperthermic IP chemotherapy is a promising technique that involves irrigating the pleural space with a hyperthermic liquid combined with a chemotherapeutic agent, such as cisplatin using specially devised extracorporeal circuits. The supraphysiologic temperature can decrease malignant cell vitality and enhance the antitumor effects of local IP chemotherapy. The hyperthermic IP chemotherapy technique is increasingly used as a curative treatment in primary and digestive PM [Citation7,Citation8]. Many randomized controlled trials have confirmed the efficacy of hyperthermic IP chemotherapy in treating advanced gastric cancer [Citation9,Citation10]. In China and other Asian countries, radical subtotal or total gastrectomy with D2 lymph node dissection surgery is the first choice for curative treatment of gastric cancer. However, few studies have explored the use of adjuvant chemotherapeutic hyperthermic IP perfusion after radical gastrectomy with D2 dissection. Recently, an ongoing phase 3 study GASTRICHIP is evaluating the value of hyperthermic IP chemotherapy after D2 dissection, however, the results have not been reported yet [Citation11]. Hyperthermic IP chemotherapy is not only able to kill tumor cells but can also induce an efficient anticancer immune response. Zunino et al. found that hyperthermic IP chemotherapy could lead to an anticancer immune response via the exposure of cell surface heat shock protein 90 (Hsp90) [Citation12]. In addition, hyperthermia can elicit anti-tumor immune responses by several mechanisms, including direct effects on immune cells, tumor cell damage, molecular changes on the tumor surface, heat shock proteins, exosomes and changes in tumor vasculature [Citation13]. Dewhirst and his colleagues from Duke University proposed a new technique using an annular phased array of radiofrequency for whole abdominal hyperthermia. In this study, we used a similar system to evaluate the safety of the combination of IP cisplatin and whole abdominal hyperthermia in gastric cancer patients after D2 dissection.
2. Materials and methods
2.1. Patient population
This retrospective study was conducted in Hangzhou Cancer Hospital. Patients were treated between July 2010 and December 2015. Eligibility criteria were as follows: histologically proven, resectable gastric cancer patients; underwent gastrectomy with D2 regional lymph node dissection; stage II A to III C according to the seventh edition of the American Joint Committee on Cancer staging system; age >18 years; sufficient liver and renal function; and received ≥2 cycles of combination chemotherapy and IP perfusion cisplatin with whole abdominal hyperthermia after surgery. Patients were excluded if they had other active malignancies or other severe medical conditions, including central nervous system diseases and myocardial infarction before 6 months of the study beginning. Written informed consent was obtained from all patients. The study was approved by the Scientific Research Board of Hangzhou Cancer Hospital.
2.2. Treatment
All patients received chemotherapy 4–6 weeks after D2 resection. The regimen contained intravenous 5-fluorouracil 500 mg/m2 on days 1–5, intravenous leucovorin 200 mg/m2 on days 1–5 and simultaneously hyperthermia IP cisplatin 75 mg/m2 on day 1. It was repeated at 3-week intervals and administered for at least two cycles.
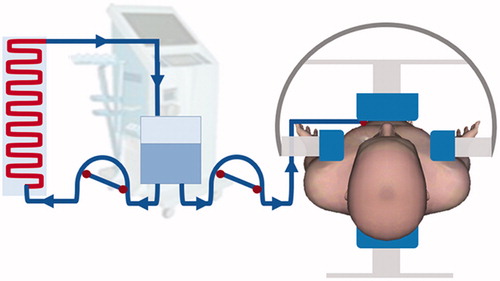
2.3. Intraperitoneal cisplatin perfusion with whole abdominal hyperthermia
After draining ascites, an indwelling peritoneal Tenckhoff catheter was inserted under local anesthetic control. A total of 2000 ml normal saline containing cisplatin 75 mg/m2 was preheated to 45 °C using the RHL2000A heat device (Jilin Maida Co., Jilin, China). The flow rate was set to 90–100 ml/min. As shown in , regional hyperthermia was delivered using two pairs of orthogonal radiofrequency waves NRL-002 type (Jilin Maida Co., Jilin, China) operating at 30.32 and 40.68 MHz immediately after the abdomen was irrigated with the total volume of saline. Electromagnetic power was applied between two external disk electrodes, 25 and 30 cm in diameter, and placed on opposite sides of the abdominal region. The power was incrementally increased until the IP temperature was 42 ± 0.5 °C. Power application continued, and was maintained for 45 min. Distribution of the perfusion fluid was evaluated using B-type ultrasound for each patient. The solution placed in the abdominal cavity was not drained to allow for self-absorption. The output power ranged from 600 to 800 W (Supplementary Table S1). Thermistor probes (Jilin Maida Co., Jilin, China) were placed in the abdominal cavity through the Tenckhoff catheter, the rectum and on the surface of the skin of the hyperthermia area. During thermotherapy, the temperature was monitored in real-time. The definition of intra-abdominal temperature was the temperature measured by the intra-abdominal thermometer probe. The monitored thermometry data for each patient were around 42 °C (Supplementary Table S1).
2.4. Toxicity
Adverse events were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. (Bethesda, MD).
2.5. Statistical analysis
Survival probabilities were calculated according to the Kaplan–Meier method using GraphPad Prism version 7.01 (GraphPad Software Inc., San Diego, CA).
3. Results
3.1. Patient characteristics
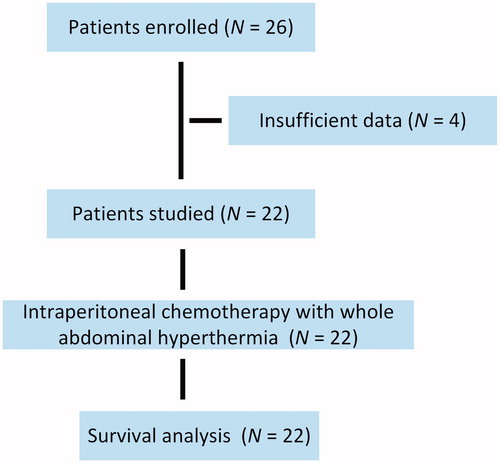
Twenty-six patients with gastric cancer were enrolled. After excluding four ineligible patients, a total of 22 patients were included in this study and their clinicopathological data were analyzed (). After a median follow-up of 58 months, 15 of the 22 patients (68.2%) had experienced disease recurrence or metastasis; 10 of the 22 patients (45.5%) had died. Patients underwent a median of 5.0 cycles (3–6 cycles) of treatment. The demographic and clinical characteristics are shown in . The median age was 51 years (range, 38–69 years) with 14 men and eight women. Adenocarcinoma was present in 14 patients (63.6%).
Table 1. Patient characteristics.
3.2. Safety and tolerability
All patients tolerated hyperthermia well. Grade 1 superficial thermal injury occurred in two patients; for these patients, ice bags wrapped in a towel were placed on any swollen skin areas. Toxicity profiles are listed in . The most frequent grade 3/4 adverse event was myelosuppression. Other grade 3/4 adverse events included nausea/vomiting, trichomadesis and liver dysfunction.
Table 2. Toxicities related to treatment.
3.3. Recurrence pattern
The most common recurrence or metastatic sites were remnant gastric carcinoma, retroperitoneal lymph node metastases, PM and hepatic metastasis (). Fourteen of 22 (63.6%) patients suffered recurrence or metastasis. Retroperitoneal lymph node (27.3%), remnant gastric carcinoma (22.7%) and hepatic metastases (9.1%) occurred most frequently.
Table 3. Recurrence or metastases after treatment.
4. Discussion
This study showed the safety of IP chemotherapy with whole abdominal hyperthermia in gastric cancer patients after D2 resection. IP chemotherapy with whole abdominal hyperthermia could reduce disease recurrence and metastases, especially peritoneal metastasis (4.5%).
Peritoneal dissemination is a life-threatening disease, and any strategy to control PM will significantly improve treatment outcomes. More than 50% of patients who have tumors that either extends beyond the serosa, or invade adjacent structures will develop PM. Currently, several studies have confirmed the efficacy of Hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with PM [Citation10,Citation14]. For those patients without PM, the role of IP chemotherapy and hyperthermia remains inconclusive. A study by Cui et al. found that neoadjuvant chemotherapy along with postoperative IP perfusion chemotherapy with hyperthermia achieved a better progression-free survival (33 vs. 26 months), and overall survival (OS) (36 vs. 27 months) than neoadjuvant chemotherapy alone [Citation15]. In contrast, a study by Huang et al. found that adjuvant IP chemotherapy with hyperthermia did not prolong OS (29.1 vs. 35.9 months) compared with chemotherapy alone [Citation16]. A recently published study showed that in ovarian cancer the addition of HIPEC to interval cytoreductive surgery resulted in longer recurrence-free survival and OS than surgery alone and had no higher rates of adverse effects [Citation17]. Our study showed that the addition of IP chemotherapy with whole abdominal hyperthermia after D2 resection reduced peritoneal metastasis and resulted in favorable disease-free survival and OS (data not shown). Given these exciting results, IP chemotherapy with whole abdominal hyperthermia might be actively pursued in the prevention of serous cavity carcinomatosis.
In this study, we used a single Tenckhoff type catheter to introduce cisplatin into the abdominal cavity and maintained the target temperature with an external heating device. HIPEC is commonly performed during surgery and it is challenging to use repeatedly in the same patient. However, the technique in our study makes repeated IP chemotherapy with hyperthermia possible. Conventional IP hyperthermia uses two catheters. The pre-heated liquid is imported with one catheter and drains away with another. The temperature of the liquid falls rapidly because heat is absorbed by the abdomen or it evaporates. In addition, a two-catheter hyperthermia system inevitably drains away some chemotherapeutic drugs; therefore, it is hard to precisely calculate the dose intensity. A one-catheter system combined with external whole abdominal hyperthermia improves the temperature homogeneity during treatment.
This study indicated the safety of adjuvant IP chemotherapy with whole abdominal hyperthermia in gastric cancer after D2 resection. However, there are some limitations to this study. This was a retrospective study with a small number of cases. Although the majority of patients achieved the observed endpoint, a few patients did not experience disease progression or death at the time of the last follow-up, which might have led to an underestimation of the efficacy of IP chemotherapy with hyperthermia.
In conclusion, we found that IP chemotherapy with whole abdominal hyperthermia offered better local recurrence control in gastric cancer patients after D2 resection. As data from this study were preliminary, a rigorous well-designed trial is urgently needed to confirm the clinical potential of IP chemotherapy with whole abdominal hyperthermia in gastric cancer patients after D2 resection.
Supplementary Table S1
Download PDF (377.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386.
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132.
- Nashimoto A, Akazawa K, Isobe Y, et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16:1–27.
- Coccolini F, Cotte E, Glehen O, et al. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur J Surg Oncol. 2014;40:12–26.
- Liu D, Lu M, Li J, et al. The patterns and timing of recurrence after curative resection for gastric cancer in China. World J Surg Oncol. 2016;14:305.
- D’Angelica M, Gonen M, Brennan MF, et al. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808–816.
- Elias D, Gilly F, Boutitie F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28:63–68.
- Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol. 2009;27:6237–6242.
- Kuramoto M, Shimada S, Ikeshima S, et al. Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann Surg. 2009;250:242–246.
- Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18:1575–1581.
- Glehen O, Passot G, Villeneuve L, et al. GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: a randomized and multicenter phase III study. BMC Cancer. 2014;14:183.
- Zunino B, Rubio-Patino C, Villa E, et al. Hyperthermic intraperitoneal chemotherapy leads to an anticancer immune response via exposure of cell surface heat shock protein 90. Oncogene. 2016;35:261–268.
- Toraya-Brown S, Fiering S. Local tumour hyperthermia as immunotherapy for metastatic cancer. Int J Hyperthermia. 2014;30:531–539.
- Ellison LM, Man Y, Stojadinovic A, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in treatment of gastric cancer with peritoneal carcinomatosis. Chin J Cancer Res. 2017;29:86–92.
- Cui HB, Ge HE, Bai XY, et al. Effect of neoadjuvant chemotherapy combined with hyperthermic intraperitoneal perfusion chemotherapy on advanced gastric cancer. Exp Ther Med. 2014;7:1083–1088.
- Huang O, Lu X, Xu X, et al. Fibrin-sealant-delivered cisplatin chemotherapy versus cisplatin hyperthermic intraperitoneal perfusion chemotherapy for locally advanced gastric cancer without peritoneal metastases: a randomized phase-II clinical trial with a 40-month follow-up. Cell Biochem Biophys. 2015;71:1171–1180.
- van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378:230–240.