Abstract
Background: Ablative therapies have been used for the treatment of neurological disorders for many years. They have been used both for creating therapeutic lesions within dysfunctional brain circuits and to destroy intracranial tumors and space-occupying masses. Despite the introduction of new effective drugs and neuromodulative techniques, which became more popular and subsequently caused brain ablation techniques to fall out favor, recent technological advances have led to the resurgence of lesioning with an improved safety profile. Currently, the four main ablative techniques that are used for ablative brain surgery are radiofrequency thermoablation, stereotactic radiosurgery, laser interstitial thermal therapy and magnetic resonance-guided focused ultrasound thermal ablation.
Object: To review the physical principles underlying brain ablative therapies and to describe their use for neurological disorders.
Methods: The literature regarding the neurosurgical applications of brain ablative therapies has been reviewed.
Results: Ablative treatments have been used for several neurological disorders, including movement disorders, psychiatric disorders, chronic pain, drug-resistant epilepsy and brain tumors.
Conclusions: There are several ongoing efforts to use novel ablative therapies directed towards the brain. The recent development of techniques that allow for precise targeting, accurate delivery of thermal doses and real-time visualization of induced tissue damage during the procedure have resulted in novel techniques for cerebral ablation such as magnetic resonance-guided focused ultrasound or laser interstitial thermal therapy. However, older techniques such as radiofrequency thermal ablation or stereotactic radiosurgery still have a pivotal role in the management of a variety of neurological disorders.
Introduction
The aim of ablative therapies for neurological disorders is the selective destruction of a targeted volume of cerebral tissue [Citation1,Citation2]. Several ablative procedures, based on various physical principles, have been used to date in the field of neurosurgery. These techniques are used both to create therapeutic lesions in the brain to interrupt maladaptive cerebral networks and to destroy abnormal tissue such as in brain tumors. Ablative procedures fell out of favor in the 1950s and 1960s due to the introduction of more effective drugs for the treatment of neurological disorders, and again in the 1990s with the development of neuromodulative procedures. However, in the last decade, recent advances in imaging and lesioning technologies have rekindled interest in lesioning for the treatment of many neurological disorders [Citation3]. The aim of this article is to briefly describe the physical principles and techniques that have been adopted for cerebral ablation and to review their neurosurgical applications.
Ablative techniques for brain surgery
Physical principles used for brain ablation have included chemical agents, mechanical devices, ionizing radiation, induction of heat or cryogenics, high-intensity focused ultrasound (HIFU), electromagnetic waves and radiofrequency (RF) [Citation4]. Currently, the four main ablative techniques that are used for brain pathologies are RF thermoablation, stereotactic radiosurgery (SRS), laser interstitial thermal therapy (LITT), and HIFU thermal ablation [Citation3] ().
Table 1. Advantages and disadvantages of the ablative techniques currently used in brain surgery.
Radiofrequency thermal ablation
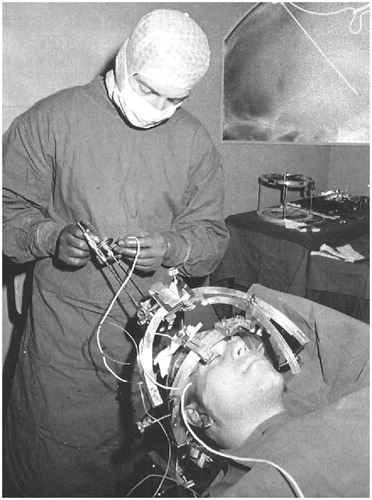
The interstitial RF technique consists of creating a lesion using heat through an intracranially placed electrode coupled to an RF generator (). This electrode is electrically insulated except at the tip, where the active electrode is located. When the generator is activated, the electric current flows in the circuit between the active and dispersive electrodes. The electric field between the two contacts oscillates with the RF frequency and causes the nearby charged ions in the electrolyte medium to move back and forth in space at the same high frequency, which is typically about 500,000 cycles per second for most modern generators. The frictional heating within the tissue resulting from the RF ionic oscillation, i.e., the current density, is the basic mechanism by which the tissue heats up and by which the RF heat lesion is made. The greatest heating takes place in the region of highest current density, which is near the tip of the active electrode [Citation5,Citation6].
Laser interstitial thermal therapy
Lasers are a form of nonionizing radiation that produces a coherent and collimated beam of light energy. Their effect on tissues is due to two principles: absorption and scatter. Absorption is the conversion of laser energy to heat after the laser’s photons collide with molecules in the target tissue called chromophores (oxyhemoglobin and deoxyhemoglobin are the key absorbers). This energy transfer to chromophores causes the release of heat and subsequent damage to adjacent cells and structures. Scatter occurs when a photon’s trajectory is deviated by interacting with particles in the tissue, resulting in an increased spatial distribution of light and heat [Citation7,Citation8].
After more than 50 years of technological development, lasers are used for stereotactic ablations in the form of magnetic resonance imaging (MRI)-guided LITT. In this technique, laser light is transmitted interstitially (an applicator is inserted into the target volume) through flexible fiberoptic wires coupling the generator to the patient’s tissue [Citation7,Citation8]. MRI-guidance and MR thermography combined with dedicated software allow precise, real-time monitoring of the procedure and direct visual feedback regarding the three-dimensional (3D) distribution of the induced thermal tissue damage, thus providing an opportunity to avoid injury to functionally important brain structures [Citation8,Citation9]. LITT also offers the ability to produce lesions of various volumes and shapes by modifying the laser probe position along the planned trajectory [Citation10].
Radiosurgery
SRS is an external ablative treatment modality that delivers a large single dose of radiation to a limited intracranial target volume while sparing surrounding tissue. Computerized dosimetry planning and highly accurate radiation delivery systems are necessary to achieve this effect [Citation11,Citation12]. Target location is defined by image-guided stereotaxy [Citation13]. In SRS, energy is delivered to the target in the form of ionizing radiation, which is any radiant entity that has enough energy to remove an electron from an atom, thus creating ions. In turn, these charged particles interact with the living tissue in the target and generate a biologic response such as cell death or halted mitosis [Citation12]. Currently, several devices are used to perform SRS procedures, with each of them based on a different source of ionizing radiation. For example, Gamma Knife radiosurgery (GKRS), developed by Lars Leksell, takes advantage of gamma rays originating from the excited nuclei of 60Co. Linear accelerators are a more cost-effective alternative for SRS and generate a single high-energy X-ray beam that is focused by special collimators with the intent to narrow, modulate or shape the beam to the tissue volume [Citation12,Citation13].
Focused ultrasound thermal ablation
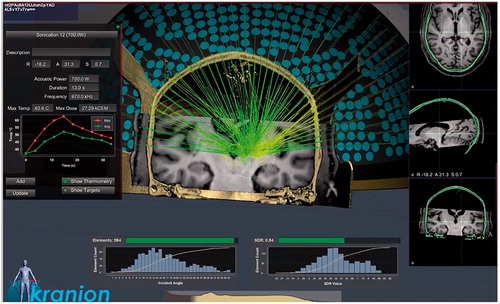
MR-guided focused ultrasound (MRgFUS) is a novel, noninvasive technique used for thermal ablation. In this procedure, HIFU beams are targeted to an intracranial region using a hemispheric phased-array of transducers that is fixed to the skull. This allows the passage of ultrasonic waves through the maximum available skull area, thus avoiding overheating and brain damage. The device is also coupled with a dedicated software that allows for the correction of phase distortions and aberrations of ultrasonic beams that are produced by the irregularities of the skull () [Citation14]. Ultrasonic mechanical energy is absorbed within the focal target volume and converted into heat that causes tissue destruction locally at the focus [Citation1]. At the same time, MRI-guidance and MR-thermography allow for accurate targeting and real-time monitoring of energy deposition [Citation15].
Movement disorders
Essential tremor (ET) is the most common movement disorder and is typically characterized by unilateral or bilateral postural and kinetic tremor of the upper extremities [Citation16]. Parkinson’s disease (PD) is a neurodegenerative disorder in which patients classically exhibit progressive tremor, rigidity, bradykinesia and postural instability [Citation17]. In the early twentieth century, open ablative procedures for PD and ET were carried out at the level of the precentral gyrus, cerebral peduncles and other parts of the pyramidal tract with considerable neurological side effects [Citation18–22]. It was the combination of work by Russel Meyers in the 1930s (by lesioning the extrapyramidal system) and Ernest Spiegel and Henry Wycis in the 1940s (by developing modern stereotactic methods) that allowed for reliable, safe and effective lesioning techniques for movement disorders [Citation23,Citation24]. Irving Cooper carried out the first successful thalamotomies and pallidotomies for the treatment of both tremor and PD in the 1950s. While cerebral lesioning fell out of favor with the discovery of levodopa replacement for PD in the 1960s and then again with the advent of deep brain stimulation (DBS) in 1990s, advanced imaging technologies have made thalamotomy, pallidotomy and subthalamotomy all viable options for patients with medically intractable tremor and PD who prefer not to or cannot safely undergo surgical implantation of hardware for neuromodulation () [Citation25,Citation26]. Dystonia is a movement disorder characterized by sustained or intermittent muscle contractions causing abnormal, often repetitive movements, postures or both [Citation27]. In the mid-20th century, it was treated more often with stereotactic thalamotomy than pallidotomy [Citation28]. This trend continued up to the 1990s. After then, the success of pallidotomy in relieving dyskinesias and dystonia in PD was demonstrated, and posteroventral pallidotomy was used with striking benefit also in patients affected by primary generalized dystonia [Citation29–32]. Thereafter, pallidal DBS rapidly replaced pallidotomy due to reversibility of stimulation [Citation33]. Today pallidotomy is still indicated, in particular for dystonic patients who present in emergency situations such as status distonicus, patients who experience severe DBS hardware-related adverse effects requiring the removal of their implant and in those who exhibit poor nutritional status with poor wound healing or very thin skin that would be incompatible with a DBS system [Citation34–37].
Figure 3. Axial (left) and coronal (right) T2-weighted MRIs demonstrating unilateral thalamotomy (A), pallidotomy (B), and subthalamotomy (C) using transcranial MRgFUS. MRI: magnetic resonance imaging; MRgFUS: magnetic resonance-guided focused ultrasound.
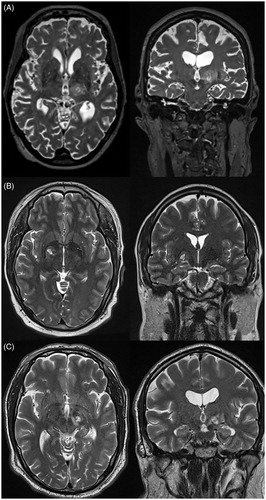
Thalamotomy
Surgical lesioning of the ventral intermediate (Vim) nucleus of the thalamus has been shown to provide significant rates of tremor control for patients with ET and tremor-dominant Parkinson’s disease (TDPD). In a retrospective study of 60 patients with medically refractory PD, ET or tremor from traumatic brain injury who underwent RF thalamotomy, Jankovic et al. demonstrated that over 80% of PD and ET patients had moderate to complete improvement in tremor at a mean follow-up of 53 months [Citation38]. These findings are supported by similar studies examining RF thalamotomy for PD and/or ET, showing moderate to complete tremor improvement in 60–100% of cases at last follow-up [Citation39–43]. Most adverse events with RF thalamotomy are transient as a result of perilesional edema that subsides over time; however, persistent ataxia, dysarthria and motor/sensory deficit may occur [Citation44]. The risk of dysarthria is significantly higher with bilateral lesions [Citation40]. Therefore, RF thalamotomy is generally only performed unilaterally.
Other means for thalamotomy include GKRS and transcranial MRgFUS, which obviate the risk of intracranial hemorrhage and infection seen in open surgical procedures [Citation45]. A prospective study by Ohye et al. utilizing GKRS thalamotomy for 72 patients with ET or PD demonstrated meaningful tremor improvement in 81% of the 53 patients who followed up at 24 months [Citation46], although other similar prospective studies were less promising [Citation47,Citation48]. There are several retrospective studies of GKRS for ET demonstrating meaningful tremor improvement in 69–96% of patients [Citation49–51]. MRgFUS allows for immediate lesioning, and its efficacy for treating tremor associated with ET and PD has been demonstrated in two randomized, sham-controlled clinical trials. In 76 patients who underwent this procedure for ET, Elias et al. demonstrated 47% improvement in mean tremor scores and 59% reduction in mean disability scores at three months postoperatively [Citation52]. This benefit was sustained at two-year follow-up [Citation53]. Bond et al. performed a similar study in 27 patients with TDPD, demonstrating 62% improvement in median tremor scores from baseline at 3 months postoperatively (22% in the sham group) [Citation54]. As a result of these trials, MRgFUS is currently approved by the FDA for the treatment of both ET and TDPD.
Pallidotomy
Ablation of the globus pallidus internus pars interna (GPi) has been shown to ameliorate the cardinal motor symptoms of PD, including tremor, rigidity and bradykinesia [Citation55]. Pallidotomy may also reduce levodopa-induced dyskinesias [Citation56]. While originally proposed by Leksell in the 1950s, Laitinen et al. reintroduced the posteroventral pallidotomy in the 1990s for the symptomatic treatment of PD. The posteroventral lesions performed by this surgeon were more effective on all symptoms of PD and resulted in a lesser degree of cognitive impairment than the lesions previously targeted to the anteromedial and dorsal regions of the GPi. Among the 32 patients in this study, 92% and 81% had almost complete resolution of rigidity/bradykinesia and tremor, respectively, at a mean follow-up of 28 months [Citation57]. Subsequent randomized controlled trials demonstrated improvements in the off-medication Unified Parkinson’s Disease Rating Scale (UPDRS) Part III by 31–65% at follow-up of 6–12 months [Citation56,Citation58,Citation59]. Pallidotomy carries similar motor and cognitive risks as thalamotomy, although there is also the risk of visual disturbance [Citation60]. MRgFUS has been explored as a noninvasive means of pallidotomy [Citation61,Citation62], and there is currently a large, multicenter trial underway to determine its efficacy. Finally, pallidotomy has been used for the treatment of dystonia. Considering studies that evaluated patients with standardized measures between 1996 and 2007, 12 patients with primary generalized dystonia experienced a 61% decrease in the Burke–Fahn–Marsden dystonia rating scale (BFMDRS), whereas there was only a 20% average decrease in patients with secondary dystonia [Citation31]. In contrast to patients with PD, the procedure for primary dystonic patients is usually bilateral, as most of these patients have axial and bilateral extremity symptoms and are better able to tolerate bilateral lesioning. Although most experience comes from RF lesioning, more recently, LITT has been used as a more controlled means for performing pallidotomy in the treatment of dystonia and has yielded good preliminary results [Citation63].
Subthalamotomy
Following research from the 1980s that revealed the overactive state of the subthalamic nucleus (STN) in PD, RF subthalamotomy was explored for the symptomatic treatment of the motor symptoms of PD [Citation64]. More recent uncontrolled and randomized controlled trials of RF subthalamotomy have yielded improvements in off-medication UPDRS Part III scores in the range of 43–52% at 12 months follow-up [Citation65,Citation66]. Contralateral dyskinesias and transient hemiballism have been reported following subthalamotomy, although there appears to be less risk of neurocognitive issues as compared to pallidotomy [Citation60,Citation65]. MRgFUS subthalamotomy was studied in an open-label trial of 10 patients by Martinez-Fernandez et al., demonstrating a total reduction of off-medication UPDRS Part III by 35% and mean levodopa equivalent dosage reduction of 24% [Citation67]. A randomized, multicenter trial is currently underway to further assess the efficacy of this procedure.
Psychiatric disorders
The study of functional neuroanatomy led to the belief that removing or destroying specific regions of the brain could alter behavior [Citation68]. Originally, psychosurgery was often carried out in an indiscriminate way, with frequent and severe side effects, lack of precision, no regulatory oversight and often bad outcomes, thus casting a shadow over the field. Thereafter, the adoption of stereotactic methodology and more strict patient selection criteria led to the improvement of safety and outcomes [Citation68–70].
Major depressive and obsessive–compulsive disorders
Obsessive–compulsive disorder (OCD) is characterized by repetitive and intrusive thoughts and behaviors that cause clinically significant distress or impairment [Citation71]. Major depressive disorder (MDD) includes depressive symptoms for a continuous period of at least two weeks that are unrelated to other causes, such as bereavement or other mood disorders [Citation72]. To address these symptoms, three main cerebral sites have been targeted and four distinct ablative operations have been performed, as shown in and [Citation68].
Table 2. Lesion procedures.
Anterior capsulotomy
The term anterior capsulotomy (AC) refers to lesioning of the anterior limb of the internal capsule (ALIC) just superior to the ventral striatum (). This procedure is intended to interrupt communication between the orbitofrontal cortex, cingulate cortex, ventral striatum and thalamus [Citation68,Citation71]. A recent literature review of observational studies involving AC via SRS or RF techniques for OCD reported a full response rate (>35% Y-BOCS reduction) of 54% with a transient and permanent adverse events rate of 56.2% and 21.4%, respectively [Citation71]. AC appeared more effective if performed bilaterally [Citation70,Citation73]. More recently, two large studies of GKRS capsulotomy confirmed AC as a safe, effective and long-lasting procedure for patients with OCD (in one of these studies, benefit persisted at five years) [Citation74,Citation75]. AC has also been successfully used for the treatment of MDD [Citation70]. Most recently, MRgFUS was used to perform AC in four patients with refractory OCD and in one patient with MDD, successfully controlling symptoms for both indications [Citation76,Citation77].
Subcaudate tractotomy
Subcaudate tractotomy (SCT) was introduced by Geoffrey Knight in 1964 and aims to selectively interrupt white matter tracts connecting the orbitofrontal cortex and subcortical limbic structures. The target is an area inferior to the head of the caudate nucleus overlying area 13 of the orbitofrontal cortex, which posteriorly includes the substantia innominata [Citation78,Citation79]. Originally, Knight adopted a freehand technique for implanting bilateral B-emitting yttrium 90 (90Y) seeds. Subsequently, the stereotactic technique was introduced and 90Y seeds were replaced with RF coagulation. Overall, 40–60% of the >660 patients who were operated on for affective disorders (MDD and bipolar disorder), OCD and other anxiety disorders at the Brook General Hospital in London led normal or near-normal lives at the one-year postsurgical assessment. The procedure was relatively devoid of complications (one death from >660 cases examined, with 1.6% experiencing epilepsy as the most frequent complication) [Citation68]. In 1975, Goktepe et al. found a 50% improvement after a long period of follow-up in 208 OCD patients treated with SCT [Citation80]. More recently, Kim reported positive results in a small series of patients undergoing SCT [Citation81], and one OCD patient was successfully treated with MRI-guided SCT, thus supporting the current value of this procedure [Citation82].
Anterior cingulotomy
Anterior cingulotomy (ACT) involves lesioning of the dorsal anterior cingulate cortex and anterior cingulate bundle. These fibers carry information from the cingulate cortex to the orbitofrontal cortex and limbic system [Citation68,Citation71]. ACT was introduced as an open technique, which was associated with significant mortality and morbidity [Citation73,Citation83]. However, after the adaptation of stereotactic techniques, it was demonstrated to be a safe and effective procedure in a large number of patients with psychiatric illness, to the point that it was the procedure of choice for OCD and MDD patients in North America for more than 30 years [Citation69,Citation73,Citation83]. A recent literature review of the SRS and RF ACT observational studies for OCD reported a full response rate (>35% Y-BOCS reduction) in 41% of patients, with transient and permanent adverse events rates of 14.3% and 5.2%, respectively [Citation71]. When patients with MDD undergoing ACT are considered, a literature review showed an improvement of standardized outcomes ranging from 39–65% [Citation70].
Limbic leucotomy
Kelly introduced stereotactic limbic leucotomy (SLL) in 1973 as a combination of bilateral ACT and SCT [Citation84]. The rationale was that the dual lesions in the lower medial quadrant of the frontal lobe would produce better results than either single lesion. The first lesion in the subcaudate area was thought to sever frontolimbic connections, whereas the cingulate lesion was intended to disconnect Papez circuit [Citation68,Citation69,Citation73]. Several techniques were used to carry out SLL, including wire loops, blunt instruments, cryoablation (Kelly’s original technique), radioactive materials and more recently MRI-guided RF thermoablation [Citation85]. Various studies showed encouraging results both in the treatment of MDD and OCD patients [Citation70,Citation85,Citation86].
Addiction
Addiction is defined as a behavior in which an individual has impaired control resulting in harmful consequences [Citation87]. Stereotactic lesioning surgery was widely adopted for its treatment. Leucotomy, hypothalamotomy, ACT and lesions in the nucleus accumbens (Nac) were consequently performed with varying degrees of success [Citation88]. More recently, 335 drug-addicted patients underwent bilateral cryocingulotomies in Russia. Despite a reported successful outcome in about 60% of patients, the Russian government stopped the study due to a lack of a clear evidence for efficacy and safety concerns [Citation89]. Likewise, in China, beginning in the year 2000, bilateral RF ablation of the Nac was performed in 28 patients to treat heroin addiction. Eleven patients did not relapse during the 15 months follow-up period, and they showed an improvement in several psychological domains without cognitive impairment. The therapeutic effect was considered to be excellent (7 patients) or good (10 patients) in 65.4% of patients. However, also in this case, the Chinese government halted the study in 2004 due to several limitations, including the nonblinded nature of the study, the absence of a control group and the small number of patients [Citation90].
Eating disorders
Anorexia nervosa (AN) is characterized by an extremely low body mass index (BMI) (<18.5 kg/m2) and concomitant anxiety and preoccupations related to weight and body image. To date, six studies have examined the role of ablative procedures in patients with severe AN [Citation91]. The targets for ablation were the frontal lobe white matter [Citation86,Citation92], the dorsomedial thalamus [Citation93] and more recently the ALIC [Citation94,Citation95] and Nac [Citation96]. The first five of these papers are case reports or small series and show variable results in terms of weight gain and psychiatric improvement. More recently, Liu et al. have reported on a large group of 76 patients who underwent RF AC and have been followed for a relatively long period (3 years). In this study, the authors reported excellent results in terms of weight gain normalization and comorbid psychiatric symptomatic improvement (BMI had increased from 13.6 ± 1.6 to 19.3 ± 3.6 kg/m2 with 46 patients reaching a healthy BMI >18.5 kg/m2, and there was a significant improvement in validated scores of anxiety, depression and social function). However, the study was criticized due to the lack of disease-specific scales to analyze the important cognitive and behavioral aspects of AN, as well as the lack of a control group [Citation95].
Morbid obesity (mOB) is defined by a BMI >40 or >35 kg/m2 in the presence of a significant obesity-related comorbid condition [Citation91]. To date, one study explored brain ablation for this disorder. In 1974, Quaade et al. reported on three patients undergoing stereotactic RF ablation of the lateral hypothalamus in an area where electrical stimulation was able to evoke hunger-related responses, which were not further specified. These patients showed a statistically significant, but transient, decrease from preoperative to postoperative spontaneous calorie intake, while body weight decreased slightly and temporarily, not being significantly affected [Citation91,Citation97].
Aggressive and disruptive behavior
Aggressive behavior was defined by Sano as ‘behavior that leads to damage or destruction of some objective and is not necessarily considered to be provoked in the usual sense’ [Citation98]. This behavior is thought to result from a disturbance of the delicate balance between various neuronal circuits in the hypothalamic–limbic system. In the past century, stereotactic lesioning of the amygdala and the hypothalamus were the main procedures performed to address this condition. Other operations, such as ACT and AC, were carried out in smaller groups of patients [Citation99,Citation100].
Patients selected for hypothalamotomy or amygdalotomy were usually cognitively impaired and had low IQ, even though patients without cognitive deficits were treated as well [Citation100]. The procedures were performed bilaterally in staged or single sessions, and they could be combined in refractory patients [Citation100]. Ramamurthi et al. reported on a series of 603 patients with aggressive behavior disorder who were treated with stereotactic amygdalotomies and hypothalamotomies. Success was sustained in 70% of patients at three years, with no deterioration in IQ or behavior [Citation100]. Likewise, Sano published a series of 37 patients undergoing hypothalamotomy for aggressive behavior. The results were considered to be satisfactory in 29 cases (78%) and were stable after a 10-year follow-up period. Several other smaller series of patients have been treated with hypothalamotomy and amygdalotomy, with satisfactory clinical improvement in 80% or more of patients [Citation98]. However, all these series lack detailed information on their measuring instruments, reporting of side effects, and stringent monitoring by an ethics committee. These requirements were met by a recent study on bilateral combined AC and ACT, which reported a significant reduction of aggressiveness and improvement in social and family relationships in 10 patients [Citation101].
Tourette syndrome
Tourette syndrome (TS) is a chronic neurodevelopmental disorder characterized by motor and phonic tics that, by definition, occurs with a childhood onset [Citation102].
Many ablative procedures were performed to treat intractable TS. Initially, frontal lobotomies and leucotomies were predominantly carried out [Citation103]. Subsequently, these procedures were abandoned in favor of less destructive and more accurate stereotactic operations, such as chemothalamectomy by Cooper et al. in 1962 [Citation104], or lesioning of the intralaminar and medial thalamic nuclei by Hassler and Dieckmann in 1970 [Citation105]. Thereafter, numerous cerebral sites were lesioned alone or in a combined manner, with mixed results. Ablated structures included thalamic nuclei, structures of the limbic system, Forel’s field, the zona incerta and the dentate nucleus of the cerebellum [Citation106]. However, it is unclear how the authors were certain of their target localization, and in some articles, the target is not even mentioned. In addition, there was a lack of criteria according to which the diagnosis of TS was made, and, finally, the tic evaluation methods were frequently not reported [Citation106]. Today, DBS has replaced lesioning surgery for TS, although the ideal target has not been defined as of yet [Citation107].
Chronic pain
Pain is regarded as chronic when it lasts or recurs for at least three months [Citation108]. Since the beginning of the last century, many ablative procedures have been performed for the treatment of chronic pain. Thalamotomy, mesencephalotomy and cingulotomy have been the most frequent (), whereas lesions in the pulvinar, the hypothalamus, the pituitary, the frontal lobe, the primary motor cortex and the primary sensory cortex (SI) have been much less common. In the last two decades, the use of these techniques has been significantly reduced due to the advent of neuromodulatory techniques and intrathecal opiate pumps [Citation109]. Nevertheless, contemporary interest in stereotactic destructive lesions to control pain is again growing as new and safer technologies have been developed and the limits of neurostimulation have emerged [Citation110].
Table 3. Most common brain ablative procedures performed for chronic pain.
Thalamotomy
Stereotactic thalamotomy was performed extensively in the early years of human stereotaxis [Citation111] as it provided significantly less surgical and neurological morbidity than procedures performed at the pontine or mesencephalic level [Citation112]. At first, lesions were made in the lateral sensory thalamic nuclei (ventroposterolateral and ventroposteromedial nuclei). Subsequently, due to an excessively high rate of somatosensory sequalae associated with these lesions, many surgeons targeted the medial thalamic nuclei (centralis lateral nucleus, centromedian/parafascicular complex, posterior complex, the medial pulvinar [Citation112–115] and the posterior central lateral nucleus (CLp)) [Citation114], which relieved pain without inducing any clinically detectable sensory loss or central pain [Citation111,Citation115]. Nonspecific medial thalamic nuclei are interconnected with associative and paralimbic areas of the brain, and the stimulation or lesioning of these areas is thought to address predominantly the affective-motivational component of chronic pain. Conversely, surgeries directed in the lateral thalamus directly interrupt the somatotopically arranged ascending pain pathways as they enter the thalamus. Accordingly, the effect of these surgeries is thought to result from an influence on the sensory-discriminative component of pain [Citation116]. Finally, the posterior complex and CLp nuclei are considered to have an intermediate functional role between diffuse and nondiffuse nuclei and project to large cortical domains, including areas mediating discriminative (SI), affective-motivational (anterior cingulate cortex insula), cognitive (prefrontal cortex) and motor (premotor cortex) aspects of pain [Citation114]. Their surgical lesioning is therefore thought to have a multimodal effect. The most recent study targeting the mesial thalamus with SRS showed that >50% pain relief was achieved in 67% of patients, with 20% of patients achieving complete relief [Citation117]. Similar results were observed in the largest trial of centrolateral thalamotomy, which reported that more than 50% of patients had >50% pain relief and 20% had complete pain relief [Citation118]. Despite variable results in terms of pain relief and side effect profiles (), a high rate of pain recurrence following ablative thalamic lesions has been commonly reported by authors, especially in the management of pain of benign origin [Citation112].
Cingulotomy
The rationale for performing ACT in the treatment of pain is to interrupt the afferent fibers connecting the midline thalamic nuclei to limbic and anterior cingulate cortex structures, and, therefore, influence the affective component of pain [Citation119,Citation120]. The earliest study of stereotactic ACT for pain was Foltz and White’s report on the treatment of 16 patients with pain and strong emotional factors augmenting their symptoms [Citation121]. These authors observed excellent, good or fair pain relief in 14 out of 16 patients. Thereafter, the good safety and efficacy profile of the procedure was confirmed by several series of, usually bilateral, ACT. Subjects of these procedures were preferentially terminally ill patients with intractable cancer pain [Citation122]. Particularly, those with malignant diseases of the head and neck were included, in which a psychogenic element arising from intolerable suffering caused by aspiration, choking, dysarthria or respiratory problems was thought to be consistent and intertwined with pain [Citation120]. In 2002, Abdelaziz et al. reviewed all 394 patients undergoing ACT reported up to that point. In patients with pain of benign origin, ACT was useful in 121 (53%) and not useful in 109 patients (47%). In patients with pain of malignant origin, the procedure was useful in 80 (52%) and not useful in 73 patients (48%). Overall, 53% of patients experienced benefit from the procedure [Citation120]. However, most of the data were derived from small clinical series with heterogeneous patient populations and limited follow-up, and the initial good response to ACT was shown to wane over time [Citation120]. The most frequent side effects of the procedure were cognitive, including decreased attention, decreased activity and apathy, although cases with no adverse effects have been documented. Most ACTs have been performed with SRS and RF, and more recently, Patel et al. reported on three cases of ACT for chronic pain that were successfully carried out with LITT, thus widening the surgeon’s armamentarium for creating these lesions [Citation123].
Mesencephalotomy
Mesencephalotomy was originally intended as an open lesion of the trigeminothalamic or spinothalamic tracts at a level superior enough to treat unilateral pain involving the upper extremity or even the head and neck [Citation111,Citation124]. Due to the excessive neurological morbidity, this initial open procedure was refined with the stereotactic technique, which resulted in improved safety. As demonstrated by many surgeons, successful short-term pain relief was obtained in a significant proportion of patients (up to 85% of patients with malignancies causing pain of the head, neck and upper extremities [Citation125], and 67% of patients with chronic pain of benign origin [Citation126]). However, despite successful pain relief, stereotactic mesencephalotomy was still burdened by high rates of pain recurrence and severe neurological side effects. Dysesthesias and gaze disturbances were the most common, with the latter occurring in virtually every patient in whom a mesencephalic tractotomy was performed [Citation124]. Several targeting modifications were attempted to reduce the occurrence of those deficits [Citation111,Citation127,Citation128]; however, the procedure remained affected by significant morbidity [Citation111,Citation124]. Today, when dealing with treatment-refractory head and neck pain, most neurosurgeons have come to prefer nondestructive techniques such as DBS or lesioning supratentorial targets, such as the medial thalamus [Citation117] or the anterior cingulate bundle [Citation123,Citation129]. However, recent technological advancements, such as high-resolution imaging techniques and new stereotactic techniques such as MRgFUS, could make this procedure safer and therefore could favor its return in the current armamentarium of the neurosurgeon [Citation130]. Recently, a successful case of pain relief following MRI-guided RF mesencephalotomy was reported [Citation131].
Drug-resistant epilepsy
Drug-resistant epilepsy may be defined as failure of adequate trials of two tolerated and appropriately chosen antiepileptic drugs (whether as monotherapies or in combination) to achieve sustained control of seizures [Citation132].
Ablative techniques comprising stereotactic RF coagulation, LITT and SRS have been used for quite some time in epilepsy surgery [Citation133]. The main limitation of these techniques is that their selectivity in creating lesions often works against seizure remission, as many patients have epileptic zones that exceed volumes that can be reasonably treated with a ‘minimally invasive’ approach [Citation134]. On the contrary, their advantages over open surgery are more favorable neurocognitive outcomes, short recovery times, greater acceptability by patients, improved cosmetic outcomes and minimal pain [Citation134]. Recently, MRgFUS has emerged as a potential transcranial ablative technique for epilepsy surgery. This therapeutic modality has the benefit that treatment can be staged, as there is no dose-accumulation effect. However, technological limitations prevent its application to traditional limbic and neocortical epilepsies, as target areas are close to the skull and heating of the bone could cause damage to nearby neural structures [Citation134,Citation135]. In contrast, lesions from tuberous sclerosis and hypothalamic hamartoma (HH), which are centrally located, may be amenable to ultrasound ablation, although no clinical studies have been conducted as of yet [Citation135].
Since the first ‘golden age’ of stereotaxy in the 1950s and 1960s, a large spectrum of stereotactic ablative procedures has been performed to halt seizure propagation or ablate epileptogenic lesions. These procedures have included dentatolysis, fornicotomy, thalamotomy, hypothalamotomy, amygdalotomy and many others [Citation136–138]. Today, stereotactic ablative techniques are used for limited conditions: arteriovenous and cavernous malformations, HHs, mesial temporal lobe sclerosis and, to a lesser extent, complex and deep focal cortical dysplasias, nodular periventricular heterotopias, tuberous sclerosis [Citation139] and subependymal giant cell astrocytomas [Citation140]. Favorable reports exist also for stereoelectroencephalography-guided surgery of neocortical nonlesional epilepsy [Citation10].
Mesial temporal lobe epilepsy
Mesial temporal lobe epilepsy (MTLE) is generally caused by atrophy, gliosis and selective neuronal loss within the hippocampus and associated limbic system [Citation134]. Anterior temporal lobectomy (ATL) has become the most commonly performed surgery for this syndrome, as several studies have demonstrated its efficacy [Citation141,Citation142]. However, many of these studies have also documented variable postoperative cognitive decline in some patients [Citation142]. Therefore, less-invasive surgical interventions such as selective amygdalohippocampectomy or stereotactic ablative procedures were introduced. One of these approaches is SRS. Regis et al. were the first to use SRS for MTLE. They found that 81% of 16 treated patients were seizure-free at 24 months. Further series, including a multicenter controlled trial (ROSE trial), confirmed these results [Citation143]. The ROSE trial showed that ATL has an advantage over SRS in terms of the extent of seizure remission, whereas 78% of ATL patients achieved seizure remission versus 52% in the SRS arm. On the contrary, cognitive outcome was more favorable for SRS [Citation143]. Despite the fact that SRS avoids a craniotomy and damage to the temporal neocortex, it still has shortcomings. First, SRS causes delayed cerebral edema that often requires corticosteroids; second, the benefits of seizure control often do not appear until 12 months after treatment and are not maximal until 24 months; finally, radiation effects may cause damage to the adjacent temporal structures [Citation142]. LITT is another emerging minimally invasive technique for MTLE. The operative procedure, termed as selective laser amygdalohippocampectomy (SLAH), is achieved by inserting the laser catheter along a slightly lateralized occipital trajectory that transverses the length of the hippocampus to the amygdala (). In this way, the amygdala, hippocampus and uncinate gyrus are ablated, while adjacent temporal structures are spared. Since 2012, SLAH has had promising results for controlling epilepsy, although preliminary results raise the possibility that it may be somewhat less effective for seizure control than ATL (54–65% seizure freedom rate of SLAH, compared with 60–80% in ATL patients [Citation140,Citation144]). SLAH carries the advantages inherent to a minimally invasive approach and spares the temporal neocortex and white matter tracts, thus reducing cognitive impairment (preliminary reports suggest that it may have less of a negative impact on memory and language function [Citation145,Citation146]). Moreover, repeat LITT or open surgery can still be performed if the first procedure is unsuccessful [Citation142,Citation147]. Up until now, evidence for the efficacy of this procedure comes from case series, which have confirmed that there is reduced morbidity using SLAH. A large prospective cohort study (SLATE) is currently being conducted and should clarify the role of SLAH in MTLE [Citation142].
Hypothalamic hamartoma
HHs are developmental malformations centered around the tuber cinereum that are associated with medically refractory epilepsy, gelastic seizures, developmental delay and often precocious puberty [Citation142]. The deep location of these lesions, which is close to several critical neural and vascular structures, has discouraged direct surgical resective approaches, while favoring the adoption of minimally invasive ablative stereotactic procedures or disconnecting surgery. Stereotactic RF coagulation is one of the alternatives to surgical resection; however, multiple probe passes are often necessary due to the irregular conformations of HHs, and the extension of the RF lesion cannot be accurately predicted or monitored. This entails a risk of damaging the hypothalamus, optic pathways and perforating arteries [Citation142,Citation148]. Despite this, Kaneyama et al. recently reported a 71% seizure-free rate with little morbidity in a large series of 100 patients who were treated with a novel disconnecting MRI-guided RF technique. These results suggest that modern imaging technologies could make the traditional RF technique safer [Citation149]. SRS is another safe and effective alternative for HH ablation. Two prospective trials and several case series showed good results in terms of seizure freedom and morbidity [Citation133,Citation148,Citation150]. SRS is particularly suitable to HHs as it allows the surgeon to accurately conform the radiation dose to the lesion shape [Citation142,Citation148]. More recently, LITT has emerged as a promising treatment modality for HHs. The largest case series published thus far included 71 patients. Freedom from gelastic seizures after single or multiple laser ablations was achieved in 93% of these patients [Citation151]. Another case series of 18 patients reported rates of gelastic and nongelastic seizure freedom of 81% and 56%, respectively [Citation75]. The postoperative complication rate for LITT is lower than that of ATL, but disabling complications may still happen [Citation152]. However, MRI-guidance and thermal energy monitoring keep the risk of injuring nearby critical structures to a minimum [Citation142].
Cavernous and arteriovenous malformations
The anticonvulsant effects of ablative SRS have been well described for arteriovenous malformations (AVMs) and cavernous malformations (CMs). Regarding AVMs, the across-study mean seizure remission rate is 70% [Citation134], while for CMs it is around 50%, as demonstrated by retrospective series. The inclusion of the hemosiderin-stained tissue surrounding the CM in the treatment volume seems to be associated with a better outcome; however, the higher doses of radiation needed raise concerns in terms of toxicity [Citation134]. More recently, CMs have been treated with LITT with 4 out of 5 patients becoming seizure-free after at least 12 months of follow-up. Perioperative hemorrhage was not observed, despite the risk of bleeding inherent to the insertion of a probe into a bed of blood vessels [Citation153].
Brain tumors
The standard of care therapy for most primary and secondary brain tumors consists of a combination of resective surgery, chemotherapy and/or radiation therapy. Over time, these standard treatments have been complemented by several thermal and nonthermal ablative procedures [Citation9,Citation154]. These have included cryodestruction [Citation155], SRS and several other techniques that increase the temperature within tumors, such as thermal ablation attained with RF current, microwaves [Citation156], HIFU [Citation157] and LITT [Citation7].
Radiofrequency/microwaves
Among electromagnetic wave energies, both RF and microwaves have been delivered through interstitial applicators for the thermal ablation of brain tumors [Citation154,Citation156]. These techniques were used for the management of deep-seated and/or critically located lesions, and they were coupled with MRI guidance so as to safely and accurately reach the planned intracranial targets [Citation158]. Anzai et al. treated 14 lesions in 12 patients with primary and metastatic brain tumors. They were able to achieve local control, documented with MRI, of all treated lesions for up to 10 months of follow-up [Citation2,Citation158]. However, the progression of RF tumor ablation into clinical practice was halted by several shortcomings, such as the limited volume of the thermal ablation, which was unsuitable for treating large tumors, and the impossibility of monitoring intracranial temperature changes without invasive measures.
Laser interstitial thermotherapy
LITT for brain tumors has been used since the late 1970s [Citation7,Citation159]. However, the first lasers did not enter mainstream clinical practice at that time due to serious technological limitations and the inability to control thermal damage to the tissue [Citation7,Citation160]. Subsequently, probes that were able to be cooled to prevent charring and MRI guidance with thermometry were introduced. These technological advances allowed for careful control of the extent of the ablation and to minimize the thermal damage to normal surrounding parenchyma [Citation160]. Today, LITT is being increasingly used for treating several types of tumors and tumor-like masses, both as a first-line therapy and as a secondary or salvage therapy. Focally contained, surgically inaccessible lesions are primary targets, although LITT is being more frequently used in cases of surgically accessible lesions. Several trials showed that LITT is a safe and well-tolerated technique for ablating gliomas. Overall, LITT was demonstrated to provide prolonged survival in patients with unresectable recurrent glioblastomas when compared to best palliative care [Citation161] or brachytherapy [Citation9]. The main limitation of LITT in brain tumor ablation is with the ablation of large tumors, as the ablation of larger neoplasms carries a high risk of cerebral edema and intracranial hypertension [Citation9,Citation159,Citation161].
Two recent retrospective studies, although limited in patient numbers, have demonstrated that LITT is a viable treatment option for recurrent meningiomas, particularly those deemed unresectable or harbored by patients whose comorbidities prevent an invasive open resection [Citation162,Citation163]. Preliminary successful data regarding LITT were reported for pediatric brain tumors, such as primitive neuroectodermal tumor [Citation164] and subependymal giant cell astrocytoma [Citation165]. Finally, LITT was proven to be a low-risk and safe surgical procedure for the ablation of radiographic lesions growing after SRS in patients with brain metastases (either recurrent tumor or radionecrosis – labeled as progressive in-field recurrence). A recent large multicenter prospective trial showed that LITT minimizes cognitive decline, preserves quality of life and functional status and permits the cessation of steroids in some of these patients [Citation166]. Another study showed that progression-free survival and overall survival were similar between patients undergoing LITT or craniotomy for recurrent metastases or radionecrosis [Citation167]. Finally, LITT was also considered as a first strategy in patients with metastases who are surgically ineligible for resection. The main complications of LITT in brain tumor cases were neurological deficits (13% transient and 3% permanent), seizures, hemorrhage (2.5%), edema, infection or technical issues (most commonly regarding the cooling mechanism) [Citation7].
Ultrasound
The initial use of ultrasound energy to thermally ablate cerebral tumors dates back to the 1980s and the 1990s. These studies adopted computed tomography and ultrasound-guidance to deliver focused ultrasound beams to intracranial targets through a surgically created cranial defect. However, due to the lack of an accurate method to monitor intraoperative temperature, this technique did not have a sustained clinical use [Citation14,Citation157]. In 2006, Ram et al. performed HIFU tumor ablation through a craniectomy in three high-grade gliomas. All of them had relatively long-term survival after treatment, and histological analysis confirmed the ablation, thus providing evidence of feasibility for this modality in the treatment of brain malignancies [Citation14,Citation157,Citation168]. Subsequently, technical advancements, such as MR-thermometry and hemispheric phased-array transducers, allowed for the delivery of focused ultrasound beams through the intact skull. Park et al. described the effective ablation of an anaplastic astrocytoma through the intact skull, as demonstrated by reduced tumor volume on follow-up MRI [Citation169]. Thereafter, McDannold et al. began targeting deep-seated brain tumors with the Exablate 3000 (Insightec, Tirat Carmel, Israel) focused ultrasound system. However, this trial was halted as one patient expired following a procedure-related hemorrhage, which was likely due to nonlinear effects of ultrasound propagation into the brain [Citation170]. More importantly, these studies failed to obtain thermal coagulation in the focus due to the technical constraints of the instrumentation [Citation157]. In 2014, the first successful transcranial MRgFUS ablation of a recurrent glioblastoma was reported [Citation171], and no adverse effects were seen with this trial. Three trials are currently ongoing to determine the safety and feasibility of transcranial MRgFUS tumor ablation. Finally, ultrasound contrast agents are being tested in preclinical settings to enhance the effects of ultrasound locally within the tumor. This may also reduce the amount of acoustic energy required for tumor ablation, thus lowering the possibility of complications [Citation157].
Radiosurgery
SRS is used for several types of intracranial neoplasms, mainly metastases, meningiomas, pituitary adenomas, recurrent gliomas and vestibular schwannomas (VS). Regarding gliomas, a recent meta-analysis showed that SRS is a safe and slightly effective treatment for recurrent high-grade gliomas, whereas it does not provide survival benefits for those that are newly diagnosed. However, this data still need to be validated by large prospective randomized trials [Citation172]. The role of SRS for brain metastases has been clarified by recent guidelines: SRS represents a valid alternative to surgical resection of solitary metastases when the latter is likely to induce new neurological deficits and when tumor volume and location are not likely to be associated with radiation-induced injury to surrounding structures. SRS is also widely used to deliver radiation to the surgical cavity of solitary brain metastases, as this was proven to remarkably decrease the risk of local recurrence. Finally, SRS alone is recommended in place of whole brain radiotherapy in patients with more than two metastases having a cumulative volume <7 mL, as this approach provides the patients with a longer overall survival [Citation173]. SRS is an accepted treatment for VS, with reported 5- to 10-year tumor control rates of over 93%, good quality of life and preservation of function following irradiation [Citation174]. It has also emerged as the perfect complementary adjunct for residual or recurrent tumor after subtotal microsurgical resection. In these situations, several studies have demonstrated local tumor control rates between 94% and 96%, associated with a low rate of neurological morbidity [Citation175,Citation176].
Finally, SRS is recommended either as an upfront or adjuvant treatment for primary and recurrent meningiomas of different brain regions. In fact, it allows for high rates of tumor control with a low incidence of neurological deficits [Citation177]. In particular, SRS is offered for the treatment of small tumors in elderly patients, or for tumors that are not safely accessible by surgery [Citation178].
Future directions
There are several ongoing efforts to use novel ablative therapies directed toward the brain. The recent development of techniques that allow for precise targeting, accurate delivery of thermal doses and real-time visualization of induced tissue damage during the procedure have resulted in novel procedures for cerebral ablation such as MRgFUS and LITT. MRgFUS has been shown to be a valid, less-invasive method of creating functional therapeutic lesions within dysfunctional brain circuits, thus improving the condition of patients affected by several diseases, including ET, PD and chronic pain. This range of indications will undoubtedly widen, as many clinical and preclinical trials are currently ongoing. Moreover, this technique is becoming more popular in brain tumor therapy. LITT is still in its infancy for the treatment of many functional disorders, but the initial results are promising, and its role in treating certain types of tumors and MTLE or HH epilepsy is being established. Older techniques such as RF or SRS ablation still have a pivotal role in the management of a variety of diseases, as their safety and efficacy has been demonstrated by long-term clinical experience. Their widespread availability still makes them valid means in the physician’s armamentarium.
Acknowledgments
The authors would like to thank the Focused Ultrasound Foundation for the support provided for both manuscript and figures. The authors would also like to acknowledge John Snell, PhD, for having developed and shared the images for using the Kranion software (open source interactive transcranial focused ultrasound visualization system software).
Disclosure statement
The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Haar GT, Coussios C. High intensity focused ultrasound: physical principles and devices. Int J Hyperthermia. 2007;23:89–104.
- Gazelle GS, Goldberg SN, Solbiati L, et al. Tumor ablation with radio-frequency energy. J Radiol. 2000;217:633–646.
- Elias WJ, Khaled M, Hilliard JD, et al. A magnetic resonance imaging, histological, and dose modeling comparison of focused ultrasound, radiofrequency, and Gamma Knife radiosurgery lesions in swine thalamus. J Neurosurg. 2013;119:307–317.
- Voges J, Büntjen L, Schmitt F. Radiofrequency-thermoablation: General principle, historical overview and modern applications for epilepsy. Epil Res. 2018;142:113–116.
- Cosman E, Nashold B, Bedenbaugh P. Stereotactic radiofrequency lesion making. Stereotact Funct Neurosurg. 1983;46:160–166.
- Cosman E, Cosman E. Radiofrequency lesions. In: Lozano A, Gildenberg P, Tasker R, editors. Textbook of stereotactic and functional neurosurgery. Berlin, Heidelberg: Springer; 2009. p. 1359–1382.
- Ashraf O, Patel NV, Hanft S, et al. Laser-induced thermal therapy in neuro-oncology: a review. World Neurosurg. 2018;112:166–177.
- Patel NV, Mian M, Stafford RJ, et al. Laser interstitial thermal therapy technology, physics of magnetic resonance imaging thermometry, and technical considerations for proper catheter placement during magnetic resonance imaging–guided laser interstitial thermal therapy. Neurosurgery. 2016;79:Suppl_1:S8–S16.
- Karampelas I, Sloan AE. Laser-induced interstitial thermotherapy of gliomas. Prog Neurol Surg. 2018;32:14–26.
- Ross L, Naduvil AM, Bulacio JC, et al. Stereoelectroencephalography-guided laser ablations in patients with neocortical pharmacoresistant focal epilepsy: concept and operative technique. Oper Neurosurg. 2018;15:656–663.
- Friedman W, Bova F. Linac radiosurgery. In: Lozano A, Gildenberg P, Tasker R, editors. Textbook of stereotactic and functional neurosurgery. Berlin, Heidelberg: Springer; 2009.
- De Salles AA, Gorgulho AA, Pereira JL, et al. Intracranial stereotactic radiosurgery: concepts and techniques. Neurosurg Clin N Am. 2013;24:491–498.
- Luxton G, Petrovich Z, Jozsef G, et al. Stereotactic radiosurgery: principles and comparison of treatment methods. Neurosurgery. 1993;32:241–259.
- Colen RR, Sahnoune I, Weinberg JS. Neurosurgical applications of high-intensity focused ultrasound with magnetic resonance thermometry. Neurosurg Clin N Am. 2017;28:559–567.
- Eames MD, Farnum M, Khaled M, et al. Head phantoms for transcranial focused ultrasound. Med Phys. 2015;42:1518–1527.
- Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:534–541.
- Dorsey ER, Elbaz A, Nichols E, et al. Global, regional, and national burden of Parkinson's disease, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2018;17:939–953.
- Horsley V. The Linacre Lecture on the function of the so-called motor area of the brain: Delivered to the Master and Fellows of St. John's College, Cambridge, May 6th, 1909. Br Med J. 1909;2:121.
- Bucy PC. Surgical relief of tremor at rest. Ann Surg. 1945;122:933
- Bucy PC, Keplinger JE, Siqueira EB. Destruction of the “pyramidal tract” in man. J Neurosurg. 1964;21:385–398.
- Walker AE. Cerebral pedrinculotonig for the relief of involuntary movements: I. Hemiballisnius. Acta Psychiatr Neurol. 1949;24:723–729.
- Walker AE. Cerebral pedunculotomy for the relief of involuntary movements: II. Parkinsonian tremor. J Nerv Ment Disease. 1952;116:766–775.
- Spiegel E, Wycis H, Thur C. The stereoencephalotome (model III of our stereotaxic apparatus for operations on the human brain). J Neurosurg. 1951;8:452–453.
- Abel TJ, Walch T, Howard MA. Russell Meyers (1905-1999): pioneer of functional and ultrasonic neurosurgery. J Neurosurg. 2016;125:1589–1595.
- Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA. 2014;311:1670–1683.
- Hariz MI, Hariz GM. Therapeutic stimulation versus ablation. Handb Clin Neurol. 2013;116:63–71.
- Sanger TD, Chen D, Fehlings DL, et al. Definition and classification of hyperkinetic movements in childhood. Mov Disord. 2010;25:1538–1549.
- Krack P, Vercueil L. Review of the functional surgical treatment of dystonia. Eur J Neurol. 2001;8:389–399.
- Ondo WG, Desaloms JM, Jankovic J, et al. Pallidotomy for generalized dystonia. Mov Disord. 1998;13:693–698.
- Lin JJ, Lin SZ, Chang DC. Pallidotomy and generalized dystonia. Mov Disord. 1999;14:1057–1059.
- Gross RE. What happened to posteroventral pallidotomy for Parkinson’s disease and dystonia? Neurotherapeutics. 2008;5:281–293.
- Eltahawy HA, Saint-Cyr J, Giladi N, et al. Primary dystonia is more responsive than secondary dystonia to pallidal interventions: outcome after pallidotomy or pallidal deep brain stimulation. Neurosurgery. 2004;54:613–621.
- Ostrem JL, Starr PA. Treatment of dystonia with deep brain stimulation. Neurotherapeutics. 2008;5:320–330.
- Ben-Haim S, Flatow V, Cheung T, et al. Deep brain stimulation for status dystonicus: a case series and review of the literature. Stereotact Funct Neurosurg. 2016;94:207–215.
- Franzini A, Franzini A, Levi V, et al. An unusual surgical indication for cerebral tuberculosis: status dystonicus. Case report. Acta Neurochir. 2018;160:1355–1358.
- Franzini A, Levi V, Franzini A, et al. Staged pallidotomy: MRI and clinical follow-up in status dystonicus. Br J Neurosurg. 2019;33(2):184–187.
- Marras CE, Rizzi M, Cantonetti L, et al. Pallidotomy for medically refractory status dystonicus in childhood. Dev Med Child Neurol. 2014;56:649–656.
- Jankovic J, Cardoso F, Grossman RG, et al. Outcome after stereotactic thalamotomy for parkinsonian, essential, and other types of tremor. Neurosurgery. 1995;37:680–687.
- Shahzadi S, Tasker R, Lozano A. Thalamotomy for essential and cerebellar tremor. Stereotact Funct Neurosurg. 1995;65:11–17.
- Nagaseki Y, Shibazaki T, Hirai T, et al. Long-term follow-up results of selective VIM-thalamotomy. J Neurosurg. 1986;65:296–302.
- Mohadjer M, Goerke H, Milios E, et al. Long-term results of stereotaxy in the treatment of essential tremor. Stereotact Funct Neurosurg. 1990;54:125–129.
- Fox MW, Ahlskog JE, Kelly PJ. Stereotactic ventrolateralis thalamotomy for medically refractory tremor in post-levodopa era Parkinson's disease patients. J Neurosurg. 1991;75:723–730.
- Akbostanci MC, Slavin KV, Burchiel KJ. Stereotactic ventral intermedial thalamotomy for the treatment of essential tremor: results of a series of 37 patients. Stereotact Funct Neurosurg. 1999;72:174–177.
- Dallapiazza RF, Lee DJ, De Vloo P, et al. Outcomes from stereotactic surgery for essential tremor. J Neurol Neurosurg Psychiatry. 2018;90(4):474–482.
- Okun MS, Stover NP, Subramanian T, et al. Complications of gamma knife surgery for Parkinson disease. Arch Neurol. 2001;58:1995–2002.
- Ohye C, Higuchi Y, Shibazaki T, et al. Gamma knife thalamotomy for Parkinson disease and essential tremor: a prospective multicenter study. Neurosurgery. 2012;70:526–536.
- Witjas T, Carron R, Krack P, et al. A prospective single-blind study of Gamma Knife thalamotomy for tremor. Neurology. 2015;85:1562–1568.
- Lim S-Y, Hodaie M, Fallis M, et al. Gamma knife thalamotomy for disabling tremor: a blinded evaluation. Arch Neurol. 2010;67:584–588.
- Kondziolka D, Ong JG, Lee JY, et al. Gamma Knife thalamotomy for essential tremor. J Neurosurg. 2008;108:111–117.
- Niranjan A, Raju SS, Kooshkabadi A, et al. Stereotactic radiosurgery for essential tremor: Retrospective analysis of a 19‐year experience. Mov Disord. 2017;32:769–777.
- Young RF, Shumway-Cook A, Vermeulen SS, et al. Gamma knife radiosurgery as a lesioning technique in movement disorder surgery. J Neurosurg. 1998;89:183–193.
- Elias WJ, Lipsman N, Ondo WG, et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2016;375:730–739.
- Chang JW, Park CK, Lipsman N, et al. A prospective trial of magnetic resonance–guided focused ultrasound thalamotomy for essential tremor: Results at the 2‐year follow‐up. Ann Neurol. 2018;83:107–114.
- Bond AE, Shah BB, Huss DS, et al. Safety and efficacy of focused ultrasound thalamotomy for patients with medication-refractory, tremor-dominant Parkinson disease: a randomized clinical trial. JAMA Neurol. 2017;74:1412–1418.
- Alkhani A, Lozano AM. Pallidotomy for Parkinson disease: a review of contemporary literature. J Neurosurg. 2001;94:43–49.
- de Bie RM, de Haan RJ, Nijssen PC, et al. Unilateral pallidotomy in Parkinson's disease: a randomised, single-blind, multicentre trial. Lancet. 1999;354:1665–1669.
- Laitinen LV, Bergenheim AT, Hariz MI. Leksell's posteroventral pallidotomy in the treatment of Parkinson's disease. J Neurosurg. 1992;76:53–61.
- Dogali M, Fazzini E, Kolodny E, et al. Stereotactic ventral pallidotomy for Parkinson's disease. Neurology. 1995;45:753–761.
- Vitek JL, Bakay RA, Freeman A, et al. Randomized trial of pallidotomy versus medical therapy for Parkinson's disease. Ann Neurol. 2003;53:558–569.
- Coban A, Hanagasi H, Karamursel S, et al. Comparison of unilateral pallidotomy and subthalamotomy findings in advanced idiopathic Parkinson's disease. Br J Neurosurg. 2009;23:23–29.
- Na YC, Chang WS, Jung HH, et al. Unilateral magnetic resonance–guided focused ultrasound pallidotomy for Parkinson disease. Neurology. 2015;85:549–551.
- Jung NY, Park CK, Kim M, et al. The efficacy and limits of magnetic resonance–guided focused ultrasound pallidotomy for Parkinson’s disease: a Phase I clinical trial. J Neurosurg. 2018 [Aug 1];[1–9]. doi:10.3171/2018.2.JNS172514.
- Gross RE, Stern MA. Magnetic resonance–guided stereotactic laser pallidotomy for dystonia. Mov Disord. 2018;33:1502–1503.
- Mitchell I, Clarke C, Boyce S, et al. Neural mechanisms underlying parkinsonian symptoms based upon regional uptake of 2-deoxyglucose in monkeys exposed to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neuroscience. 1989;32:213–226.
- Alvarez L, Macias R, Lopez G, et al. Bilateral subthalamotomy in Parkinson's disease: initial and long-term response. Brain. 2005;128:570–583.
- Merello M, Tenca E, Lloret SP, et al. Prospective randomized 1-year follow-up comparison of bilateral subthalamotomy versus bilateral subthalamic stimulation and the combination of both in Parkinson's disease patients: a pilot study. Br J Neurosurg. 2008;22:415–422.
- Martínez-Fernández R, Rodríguez-Rojas R, del Álamo M, et al. Focused ultrasound subthalamotomy in patients with asymmetric Parkinson's disease: a pilot study. Lancet Neurol. 2018;17:54–63.
- Patel SR, Aronson JP, Sheth SA, et al. Lesion procedures in psychiatric neurosurgery. World Neurosurg. 2013;80:S31. e9–S31. e16.
- Coenen VA, Honey CR. Ablative procedures for depression. In: Lozano AM, Gildenberg PL, Tasker RR, editors. Textbook of stereotactic and functional neurosurgery. Berlin, Heidelberg: Springer; 2009.
- Volpini M, Giacobbe P, Cosgrove GR, et al. The history and future of ablative neurosurgery for major depressive disorder. Stereotact Funct Neurosurg. 2017;95:216–228.
- Brown LT, Mikell CB, Youngerman BE, et al. Dorsal anterior cingulotomy and anterior capsulotomy for severe, refractory obsessive-compulsive disorder: a systematic review of observational studies. J Neurosurg. 2016;124:77–89.
- Association AP. Diagnostic and statistical manual of mental disorders (DSM-5®). Arlington (VA): American Psychiatric Pub; 2013.
- Cosgrove GR, Rauch SL. Stereotactic cingulotomy. Neurosurg Clin N Am. 2003;14:225–235.
- Lopes AC, Greenberg BD, Canteras MM, et al. Gamma ventral capsulotomy for obsessive-compulsive disorder: a randomized clinical trial. JAMA Psychiatry. 2014;71:1066–1076.
- Gupta A, Shepard MJ, Xu Z, et al. An international radiosurgery research foundation multicenter retrospective study of gamma ventral capsulotomy for obsessive compulsive disorder. Neurosurgery. 2018;
- Jung H, Kim S, Roh D, et al. Bilateral thermal capsulotomy with MR-guided focused ultrasound for patients with treatment-refractory obsessive-compulsive disorder: a proof-of-concept study. Mol Psychiatry. 2015;20:1205.
- Kim M, Kim C-H, Jung HH, et al. Treatment of major depressive disorder via magnetic resonance–guided focused ultrasound surgery. Biol Psychiatry. 2018;83:e17–e8.
- Knight G. Stereotactic tractotomy in the surgical treatment of mental illness. J Neurol, Neurosurg Psychiatry. 1965;28:304.
- Knight G. Further observations from an experience of 660 cases of stereotactic tractotomy. Postgrad Med J. 1973;49:845–854.
- Göktepe E, Young LB, Bridges P. A further review of the results of stereotactic subcaudate tractotomy. Br J Psychiatry. 1975;126:270–280.
- Kim M-C, Lee T-K, Choi C-R. Review of long-term results of stereotactic psychosurgery. Neurol Med Chir. 2002;42:365–371.
- Woerdeman P, Willems P, Noordmans H, et al. Frameless stereotactic subcaudate tractotomy for intractable obsessive–compulsive disorder. Acta Neurochir. 2006;148:633–637.
- Le Beau J. Anterior cingulectomy in man. J Neurosurg. 1954;11:268–276.
- Kelly D, Richardson A, Mitchell-Heggs N, et al. Stereotactic limbic leucotomy: a preliminary report on forty patients. Br J Psychiatry. 1973;123:141–148.
- Montoya A, Weiss AP, Price BH, et al. Magnetic resonance imaging-guided stereotactic limbic leukotomy for treatment of intractable psychiatric disease. Neurosurgery. 2002;50:1043–1052.
- Mitchell-Heggs N, Kelly D, Richardson A. Stereotactic limbic leucotomy- a follow-up at 16 months. Br J Psychiatry. 1976;128:226–240.
- Zou Z, Wang H, d'Oleire Uquillas F, et al. Definition of substance and non-substance addiction. Adv Exp Med Biol. 2017;1010:21–41.
- Lu L, Wang X, Kosten TR. Stereotactic neurosurgical treatment of drug addiction. The Am J Drug Alcohol Abuse. 2009;35:391–393.
- Orellana C. Controversy over brain surgery for heroin addiction in Russia. Lancet Neurol. 2002;1:333
- Gao G, Wang X, He S, et al. Clinical study for alleviating opiate drug psychological dependence by a method of ablating the nucleus accumbens with stereotactic surgery. Stereotact Funct Neurosurg. 2003;81:96–104.
- Lee DJ, Elias GJ, Lozano AM. Neuromodulation for the treatment of eating disorders and obesity. Ther Adv Psychopharmacol. 2018;8:73–92.
- Kelly D, Mitchell-Heggs N. Stereotactic limbic leucotomy- a follow-up study of thirty patients. Postgrad Med J. 1973;49:865–882.
- Zamboni R, Larach V, Poblete M, et al. Dorsomedial thalamotomy as a treatment for terminal anorexia: a report of two cases. Acta Neurochir Suppl (Wien). 1993;58:34–35.
- Barbier J, Gabriëls L, Van Laere K, et al. Successful anterior capsulotomy in comorbid anorexia nervosa and obsessive-compulsive disorder: case report. Neurosurgery. 2011;69:E745–E751.
- Liu W, Li D, Sun F, et al. Long-term follow-up study of MRI-guided bilateral anterior capsulotomy in patients with refractory anorexia nervosa. Neurosurgery. 2018;83:86–92.
- Wang J, Chang C, Geng N, et al. Treatment of intractable anorexia nervosa with inactivation of the nucleus accumbens using stereotactic surgery. Stereotact Funct Neurosurg. 2013;91:364–372.
- Quaade F, Vaernet K, Larsson S. Stereotaxic stimulation and electrocoagulation of the lateral hypothalamus in obese humans. Acta Neurochir. 1974;30:111–117.
- Sano K, Mayanagi Y. Posteromedial hypothalamotomy in the treatment of violent, aggressive behaviour. Acta Neurochir Suppl (Wien). 1988;44:145–151.
- Broggi G, Franzini A. Treatment of aggressive behavior. In: Lozano AM, Gildenberg PL, Tasker RR, editors. Textbook of stereotactic and functional neurosurgery. Berlin, Heidelberg: Springer; 2009.
- Ramamurthi B. Stereotactic operation in behaviour disorders. Amygdalotomy and hypothalamotomy. Acta Neurochir Suppl (Wien). 1988;44:152–157.
- Jiménez F, Soto JE, Velasco F, et al. Bilateral cingulotomy and anterior capsulotomy applied to patients with aggressiveness. Stereotact Funct Neurosurg. 2012;90:151–160.
- Schrock LE, Mink JW, Woods DW, et al. Tourette syndrome deep brain stimulation: a review and updated recommendations. Mov Disord. 2015;30:448–471.
- Baker E. Gilles de la Tourette syndrome treated by bimedial frontal leucotomy. Can Med Assoc J. 1962;86:746.
- Cooper IS. Dystonia reversal by operation on basal ganglia. Arch Neurol. 1962;7:132–145.
- Hassler R, Dieckmann G. Stereotaxic treatment of tics and inarticulate cries or coprolalia considered as motor obsessional phenomena in Gilles de la Tourette's disease. Rev Neurol (Paris). 1970;123:89.
- Temel Y, Visser-Vandewalle V. Surgery in Tourette syndrome. Mov Disord. 2004;19:3–14.
- Porta M, Saleh C, Zekaj E, et al. Why so many deep brain stimulation targets in Tourette’s syndrome? Toward a broadening of the definition of the syndrome. J Neural Transm. 2016;123:785–790.
- Treede R-D, Rief W, Barke A, et al. A classification of chronic pain for ICD-11. Pain. 2015;156:1003
- Tasker R. Stereotactic medial thalamotomy for chronic pain: is it an effective procedure? In: Burchiel K, editor. Surgical management of pain. New York (NY): Thieme Medical Publishers Inc.; 2002. p. 805–811.
- Jeanmonod D, Werner B, Morel A, et al. Transcranial magnetic resonance imaging–guided focused ultrasound: noninvasive central lateral thalamotomy for chronic neuropathic pain. FOC. 2012;32:E1.
- Laitinen LV. Mesencephalotomy and thalamotomy for chronic pain. In: Lunsford LD, editor. Modern stereotactic neurosurgery. Topics in neurological surgery, vol 1. Boston (MA): Springer; 1988.
- Hariz MI, Bergenheim AT. Thalamic stereotaxis for chronic pain: ablative lesion or stimulation? Stereotact Funct Neurosurg. 1995;64:47–55.
- Hitchcock ER, Teixeira MJ. A comparison of results from center-median and basal thalamotomies for pain. Surg Neurol. 1981;15:341–351.
- Jeanmonod D, Morel A. The central lateral thalamotomy for neuropathic pain. In: Lozano AM, Gildenberg PL, Tasker RR, editors. Textbook of stereotactic and functional neurosurgery. Berlin, Heidelberg: Springer; 2009.
- Tasker RR. Thalamotomy. Neurosurg Clin N Am. 1990;1:841–864.
- Menon JP. Intracranial ablative procedures for the treatment of chronic pain. Neurosurg Clin N Am. 2014;25:663–670.
- Urgosik D, Liscak R. Medial Gamma Knife thalamotomy for intractable pain. J Neurosurg. 2018;129:72–76.
- Jeanmonod D, Magnin M, Morel A, et al. Surgical control of the human thalamocortical dysrhythmia: I. Central lateral thalamotomy in neurogenic pain. THL. 2001;1:(1):71–9.
- Yen C-P, Kuan C-Y, Sheehan J, et al. Impact of bilateral anterior cingulotomy on neurocognitive function in patients with intractable pain. J Clin Neurosci. 2009;16:214–219.
- Abdelaziz OS, Cosgrove GR. Stereotactic cingulotomy for the treatment of chronic pain. In: Burchiel KJ, editor. Surgical management of pain. New York (NY): Thieme; 2002. p. 812–820.
- Foltz EL, White LE. Pain “relief” by frontal cingulumotomy. J Neurosurg. 1962;19:89–100.
- Steel TR, Burchiel KJ. Ablative neurosurgical techniques in the treatment of chronic pain: overview. In: Burchiel KJ, editor. Surgical management of pain. New York (NY): Thieme Medical Publishers Inc.; 2002. p. 633–646.
- Patel NV, Agarwal N, Mammis A, et al. Frameless stereotactic magnetic resonance imaging-guided laser interstitial thermal therapy to perform bilateral anterior cingulotomy for intractable pain: feasibility, technical aspects, and initial experience in 3 patients. Oper Neurosurg. 2015;11:17–25.
- Gildenberg PL. Mesencephalotomy. In: Burchiel KJ, editor. Surgical management of pain. New York (NY): Thieme; 2002. p. 786–794.
- Gybels JM, Sweet WH. Neurosurgical treatment of persistent pain. Physiological and pathological mechanisms of human pain. Pain Headache. 1989;11:1–402.
- Shieff C, Nashold BS. Stereotactic mesencephalotomy. Neurosurg Clin N Am. 1990;1:825–839.
- Nashold B, Jr Wilson WS, Neurosurgery F. Central pain. Observations in man with chronic implanted electrodes in the midbrain tegmentum. Stereotact Funct Neurosurg. 1966;27:30–44.
- Amano K, Kawamura H, Tanikawa T, et al. Long-term follow-up study of rostral mesencephalic reticulotomy for pain relief–report of 34 cases. Stereotact Funct Neurosurg. 1986;49:105–111.
- Strauss I, Berger A, Moshe SB, et al. Double anterior stereotactic cingulotomy for intractable oncological pain. Stereotact Funct Neurosurg. 2017;95:400–408.
- Kim D-r, Lee S-w, Son B-c. Stereotactic mesencephalotomy for cancer-related facial pain. J Korean Neurosurg Soc. 2014;56:71.
- Fountas KN, Lane FJ, Jenkins PD, et al. MR-based stereotactic mesencephalic tractotomy. Stereotact Funct Neurosurg. 2004;82:230–234.
- Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–1077.
- McGonigal A, Sahgal A, De Salles A, et al. Radiosurgery for epilepsy: Systematic review and International Stereotactic Radiosurgery Society (ISRS) practice guideline. Epil Res. 2017;137:123–131.
- Quigg M, Harden C. Minimally invasive techniques for epilepsy surgery: stereotactic radiosurgery and other technologies: a review. J Neurosurg. 2014;121:232–240.
- Monteith S, Snell J, Eames M, et al. Transcranial magnetic resonance–guided focused ultrasound for temporal lobe epilepsy: a laboratory feasibility study. J Neurosurg. 2016;125:1557–1564.
- Kitchen N. Experimental and clinical studies on the putative therapeutic efficacy of cerebral irradiation (radiotherapy) in epilepsy. Epil Res. 1995;20:1–10.
- Leiphart JW, Young RM, Shields DC. A historical perspective: stereotactic lesions for the treatment of epilepsy. Seizure. 2014;23:1–5.
- Hullay J, Gombi R, Velok G. Effects of stereotactic lesions in intractable epilepsy. Stereotactic treatment of epilepsy. Acta Neurochir. 1976;23:Suppl:205–209.
- Tovar-Spinoza Z, Ziechmann R, Zyck S. Single and staged laser interstitial thermal therapy ablation for cortical tubers causing refractory epilepsy in pediatric patients. Neurosurg Focus. 2018;45:E9
- Hoppe C, Witt J-A, Helmstaedter C, et al. Laser interstitial thermotherapy (LiTT) in epilepsy surgery. Seizure. 2017;48:45–52.
- Wiebe S, Blume WT, Girvin JP, et al. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318.
- Prince E, Hakimian S, Ko AL, et al. Laser interstitial thermal therapy for epilepsy. Curr Neurol Neurosci Rep. 2017;17:63
- Barbaro NM, Quigg M, Broshek DK, et al. A multicenter, prospective pilot study of gamma knife radiosurgery for mesial temporal lobe epilepsy: seizure response, adverse events, and verbal memory. Ann Neurol. 2009;65:167–175.
- Youngerman BE, Oh JY, Anbarasan D, et al. Laser ablation is effective for temporal lobe epilepsy with and without mesial temporal sclerosis if hippocampal seizure onsets are localized by stereoelectroencephalography. Epilepsia. 2018;59:595–606.
- Kang JY, Wu C, Tracy J, et al. Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia. 2016;57:325–334.
- Drane DL, Loring DW, Voets NL, et al. Better object recognition and naming outcome with MRI‐guided stereotactic laser amygdalohippocampotomy for temporal lobe epilepsy. Epilepsia. 2015;56:101–113.
- Vakharia VN, Sparks R, Li K, et al. Automated trajectory planning for laser interstitial thermal therapy in mesial temporal lobe epilepsy. Epilepsia. 2018;59:814–824.
- Régis J, Scavarda D, Tamura M, et al. Epilepsy related to hypothalamic hamartomas: surgical management with special reference to gamma knife surgery. Childs Nerv Syst. 2006;22:881–895.
- Kameyama S, Shirozu H, Masuda H, et al. MRI-guided stereotactic radiofrequency thermocoagulation for 100 hypothalamic hamartomas. J Neurosurg. 2016;124:1503–1512.
- Mathieu D, Deacon C, Pinard C-A, et al. Gamma Knife surgery for hypothalamic hamartomas causing refractory epilepsy: preliminary results from a prospective observational study. J Neurosurg. 2010;113:215–221.
- Curry DJ, Raskin J, Ali I, et al. MR-guided laser ablation for the treatment of hypothalamic hamartomas. Epil Res. 2018;142:131–134.
- Shimamoto S, Wu C, Sperling MR. Laser interstitial thermal therapy in drug-resistant epilepsy. Curr Opin Neurol. 2019;32:237–245.
- McCracken DJ, Willie JT, Fernald BA, et al. Magnetic resonance thermometry-guided stereotactic laser ablation of cavernous malformations in drug-resistant epilepsy: imaging and clinical results. Oper Neurosurg. 2016;12:39–48.
- Bredlau A-L, McCrackin M, Motamarry A, et al. Thermal therapy approaches for treatment of brain tumors in animals and humans. Crit Rev Biomed Eng. 2016;44:443–457.
- Maroon JC, Onik G, Quigley MR, et al. Cryosurgery re-visited for the removal and destruction of brain, spinal and orbital tumours. Neurol Res. 1992;14:294–302.
- Winter A, Laing J, Paglione R, et al. Microwave hyperthermia for brain tumors. Neurosurgery. 1985;17:387–399.
- Prada F, Kalani MYS, Yagmurlu K, et al. Applications of focused ultrasound in cerebrovascular diseases and brain tumors. Neurotherapeutics. 2018;16:1–21.
- Anzai Y, Lufkin R, DeSalles A, et al. Preliminary experience with MR-guided thermal ablation of brain tumors. AJNR Am J Neuroradiol. 1995;16:39–48.
- Missios S, Bekelis K, Barnett GH. Renaissance of laser interstitial thermal ablation. FOC. 2015;38:E13.
- Sharma M, Balasubramanian S, Silva D, et al. Laser interstitial thermal therapy in the management of brain metastasis and radiation necrosis after radiosurgery: an overview. Expert Rev Neurother. 2016;16:223–232.
- Kamath AA, Friedman DD, Akbari SHA, et al. Glioblastoma treated with magnetic resonance imaging-guided laser interstitial thermal therapy: safety, efficacy, and outcomes. Neurosurgery. 2019;84(4):836–843.
- Rammo R, Scarpace L, Nagaraja T, et al. MR‐guided laser interstitial thermal therapy in the treatment of recurrent intracranial meningiomas. Lasers Surg Med. 2019;51(3):245–250.
- Ivan ME, Diaz RJ, Berger MH, et al. Magnetic resonance–guided laser ablation for the treatment of recurrent dural-based lesions: a series of five cases. World Neurosurg. 2017;98:162–170.
- Jethwa PR, Lee JH, Assina R, et al. Treatment of a supratentorial primitive neuroectodermal tumor using magnetic resonance–guided laser-induced thermal therapy. J Neurosurg Pediatr. 2011;8:468–475.
- Dadey DY, Kamath AA, Leuthardt EC, et al. Laser interstitial thermal therapy for subependymal giant cell astrocytoma: technical case report. FOC. 2016;41:E9.
- Ahluwalia M, Barnett GH, Deng D, et al. Laser ablation after stereotactic radiosurgery: a multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J Neurosurg. 2018;130:804–811.
- Hong CS, Deng D, Vera A, et al. Laser-interstitial thermal therapy compared to craniotomy for treatment of radiation necrosis or recurrent tumor in brain metastases failing radiosurgery. J Neurooncol. 2019;142:309–317.
- Ram Z, Cohen ZR, Harnof S, et al. Magnetic resonance imaging-guided, high-intensity focused ultrasound forbrain tumortherapy. Neurosurgery. 2006;59:949–956.
- Park JW, Jung S, Jung TY, et al. editors. Focused ultrasound surgery for the treatment of recurrent anaplastic astrocytoma: A preliminary report. AIP Conference Proceedings; 2006: AIP.
- McDannold N, Clement GT, Black P, et al. Transcranial magnetic resonance imaging- guided focused ultrasound surgery of brain tumors: initial findings in 3 patients. Neurosurgery. 2010;66:323–332.
- Coluccia D, Fandino J, Schwyzer L, et al. First noninvasive thermal ablation of a brain tumor with MR-guided focused ultrasound. J Ther Ultrasound. 2014;2:17.
- Fetcko K, Lukas RV, Watson GA, et al. Survival and complications of stereotactic radiosurgery: A systematic review of stereotactic radiosurgery for newly diagnosed and recurrent high-grade gliomas. Medicine. 2017;96:e8293.
- Graber JJ, Cobbs CS, Olson JJ. Congress of neurological surgeons systematic review and evidence-based guidelines on the use of stereotactic radiosurgery in the treatment of adults with metastatic brain tumors. Neurosurgery. 2019;84:E168–E170.
- Fu VX, Verheul JB, Beute GN, et al. Retreatment of vestibular schwannoma with Gamma Knife radiosurgery: clinical outcome, tumor control, and review of literature. J Neurosurg. 2018;129:137–145.
- Molina ES, van Eck AT, Sauerland C, et al. Local tumor control and clinical symptoms after gamma knife radiosurgery for residual and recurrent vestibular schwannomas. World Neurosurg. 2019;122:e1240–e6.
- Germano IM, Sheehan J, Parish J, et al. Congress of neurological surgeons systematic review and evidence-based guidelines on the role of radiosurgery and radiation therapy in the management of patients with vestibular schwannomas. Neurosurgery. 2018;82:E49–E51.
- Yang I, Udawatta M, Prashant GN, et al. Stereotactic radiosurgery for neurosurgical patients: a historical review and current perspectives. World Neurosurg. 2019;122:522–531.
- Goldbrunner R, Minniti G, Preusser M, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17:e383–e91.