Abstract
Objective: To compare the efficacy and safety of a novel thermochemotherapy scheme and the instillation of pirarubicin (THP) without hyperthermia in patients with intermediate- and high-risk nonmuscle-invasive bladder cancer (NMIBC).
Materials and methods: Between June 2012 and December 2016, 300 patients with urothelial carcinoma of the bladder undergoing intravesical adjuvant therapy with THP after transurethral resection of bladder tumors (TURBT) were enrolled in the study. These patients were divided into the CTHC group (thermochemotherapy composed of three consecutive sessions in which only the second hyperthermia was combined with THP, followed by intravesical instillation with THP without using hyperthermia) and the THP group (instillation of THP without hyperthermia). Cystoscopy and urinary cytology were repeated every 3 months. The primary endpoint was 24-month recurrence-free survival (RFS). Secondary endpoints included 24-month progression-free survival (PFS) and adverse event (AE) rates.
Results: Baseline characteristics of the CTHC (n = 76) and THP (n = 85) groups were well-balanced. The 24-month RFS was 82.9% in the CTHC group and 63.5% in the THP group (log-rank p = .008). A significantly higher percentage of patients in the CTHC group achieved PFS than in the THP group (97.4% versus 87.1%; log-rank p = .011). There was no significant difference in AEs between the two groups (p > .05). Based on Cox proportional hazards models, CTHC was the only factor that contributed independently to improved RFS (hazard ratio, 0.422; 95% confidence interval, 0.214–0.835; p = .013).
Conclusion: The CTHC scheme is a safe and effective adjuvant treatment option after TURBT for patients with intermediate- and high-risk NMIBC.
1. Introduction
On a global scale, bladder cancer is the eleventh most commonly diagnosed cancer and the seventh most common cancer in men [Citation1]. Nonmuscle-invasive bladder cancer (NMIBC), the most prevalent type of bladder cancer, accounts for approximately 75% of bladder cancer diagnoses [Citation2]. Strategies for intravesical treatment of NMIBC have not changed significantly over the past three decades. Transurethral resection of bladder tumors (TURBT) remains the foundation of treatment for patients with NMIBC. Despite the addition of adjuvant therapies, up to 52% of patients with high-risk NMIBC will experience disease recurrence, with up to 20% progressing to muscle-invasive bladder cancer (MIBC) within 5 years [Citation3], especially in T1 patients [Citation4].
For intermediate- and high-risk NMIBC, the standard treatment is TURBT followed by intravesical adjuvant therapy. However, an optimal intravesical adjuvant therapy strategy for preventing the recurrence of tumors after TURBT has not yet been elucidated. To date, intravesical adjuvant therapy focused primarily on immunotherapy such as Bacillus Calmette-Guérin (BCG) immunotherapy, chemotherapy (e.g., Mitomycin C (MMC), epirubicin, pirarubicin, gemcitabine and EO9) and chemotherapy combined with hyperthermia. In the United Kingdom, intermediate-risk NMIBC is treated with adjuvant chemotherapy, whereas high-risk NMIBC is treated with adjuvant immunotherapy [Citation5]. Regrettably, because of the worldwide shortage of BCG [Citation6], it is unavailable to treat high-risk bladder cancer in many countries. The same is true in China where the majority of hospitals cannot use BCG as a routine agent for high-risk NMIBC. Although a new immunotherapeutic agent named Pseudomonas aeruginosa-mannose-sensitive hemagglutinin is available in China [Citation7], its efficacy and safety have not been verified; therefore, adjuvant chemotherapy is the only option for bladder cancer in China.
Pirarubicin (THP), an analog of doxorubicin, contains tetrahydropyrans which enhance its anticancer ability [Citation8]. THP prevents DNA replication and transcription in tumor cells [Citation9,Citation10]; thus, tumor cells are prevented from dividing which reduces the risk of tumor recurrence. Adverse events (AEs) following intravesical delivery of THP predominantly involve local irritative voiding symptoms which are reported in 25.0–63.7% of patients [Citation11–13].
The goal of intravesical adjuvant therapy is to maximize the efficacy and reduce the toxicity of the treatment to preserve the bladder to the furthest extent. Hyperthermia alone has antitumoral effects through multiple mechanisms, including direct and immune-based cytotoxicity [Citation14,Citation15], which are further strengthened by the addition of chemotherapy [Citation16,Citation17]. Cancer cells are more susceptible to hyperthermia than nonmalignant cells, and hyperthermia is usually delivered at 42 °C to 45 °C in clinical practice [Citation18–20]. This approach of hyperthermia combined with chemotherapy has been used successfully for melanoma, cervical cancer, rectal cancer, breast cancer, gliomas, etc. [Citation21].
Over the past decade, we have used the BR-TRG-I Urinary Bladder Hyperthermia Treatment System (Bright Medical Technology Co., Ltd., Guangzhou, China) in a variety of schemes such as hyperthermia alone, hyperthermia combined with chemotherapy and sequential hyperthermia and chemotherapy. We describe a novel thermochemotherapy scheme in an attempt to achieve improved efficacy with lower healthcare costs. In this study, we compared the safety and efficacy of this novel scheme for the treatment of intermediate- and high-risk NMIBC.
2. Materials and methods
The treatment choice was based on patient–doctor discussions, and there was no randomization. The study was conducted with informed consent from patients and approval from the ethics committee of Henan Provincial People’s Hospital. Patients’ clinical data were derived from electronic or paper medical records as well as telephonic follow-up.
2.1. Patient selection
From June 2012 to December 2016, 300 cases of pathologically confirmed bladder cancer were enrolled in the study; all cases underwent intravesical instillation therapy with THP after TURBT in Henan Provincial People's Hospital (Zhengzhou, China). Inclusion criteria included age >18 years, Eastern Cooperative Oncology Group performance status <2, life expectancy >24 months and intermediate- and/or high-risk NMIBC as defined by the European Association of Urology NMIBC guidelines [Citation22]. Exclusion criteria included perioperative death, intention to conceive, pregnant/lactating women, THP allergy, the use of other intravesical treatment agents during treatment, end-stage cachexia, severe bleeding disorder, bladder diverticulum >1 cm, residual urine >100 ml, bladder volume <150 ml, active urinary tract infection, urinary incontinence, urethral stricture impeding 20 F catheterization, nonurothelial histology, coexisting upper urinary tract (renal pelvis, ureter) tumors, previous history of upper urinary tract tumors, clinical stage T2, partial cystectomy, previous pelvic radiotherapy/systemic chemotherapy and previous intravesical chemotherapy. The TNM classification for bladder cancer was used to define the stage of NMIBC [Citation23] while histological grade was defined by the World Health Organization 1973 grading system.
2.2.Transurethral resection of bladder tumors
Both groups underwent TURBT under general anesthesia. Bladder tumors were detected by cystoscopy after cystolithotomy. At the same time, continuous lavage-type resection (cutting control using 100–115 W, electro-coagulation control using 70–80 W) was performed. Saline (0.9%) was used as a flushing solution. An electro-resection endoscope was placed in the urethral position of patients to observe the intravesical tumors. The tumor body and pedicle were resected by lateral, anterograde and retrograde resection. The resection range included 2 cm of normal carrier tissue near the tumor deep into the muscle layer. All patient had routine indwelling of the three-way Foley catheter and then continuous bladder irrigation using a large amount of 0.9% saline at room temperature was performed until the color of urine became clear. Within 3 days after TURBT, treatment was implemented if there was no existence of gross hematuria.
2.3. Procedures of the BR-TRG-I urinary bladder hyperthermia treatment system
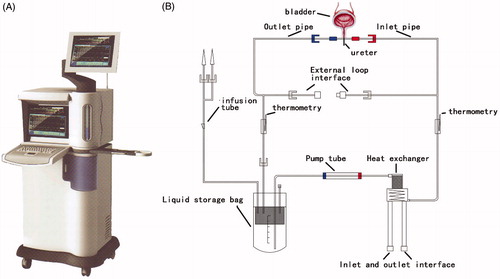
The BR-TRG-I urinary bladder hyperthermia treatment system, also known as the BR-TRG-I-type high-precision intraperitoneal thermal perfusion treatment system, which is a body cavity thermal perfusion system developed independently in China, has been described in detail previously [Citation24]. The BR-TRG-I urinary bladder hyperthermia treatment system () consists of a continuously circulated, closed circuit through a three-way Foley catheter and bladder. The heated THP is continuously instilled at a constant temperature into the bladder. The instillation temperature is 45 °C for a duration of 45 min. There is a dedicated disposable treatment tube assembly () which includes a dual ultrafiltration system to flush the bladder mechanically. The cancer cells can be filtered effectively without the possibility of siphoning back into the bladder. The inlet pipe is connected to one chamber using a 20 Fr three-way Foley catheter, and the outlet pipe is connected to another chamber. Each of the two pipelines is equipped with temperature probes. At the same time, the product uses a computerized numerical heating control system, an active automatic cooling system, an automatic safety guarantee system and a noninterference temperature measuring system. The system makes use of large-capacity intelligent water bath temperature controls and high-efficiency heat exchange technologies to accurately control the temperature at an accuracy of ±0.1 °C to maximize its safety and effectiveness. Intelligent treatment software uses a fuzzy control algorithm to precisely control system operations; parameters, including treatment time, temperature, flow and power, can be displayed in real time. This software can be adjusted accordingly and has a user-friendly interface. Professional technicians control the treatment process to ensure the safety of the treatment, and a doctor records the clinical data of patients on a specialized system.
2.4. Treatment schedules
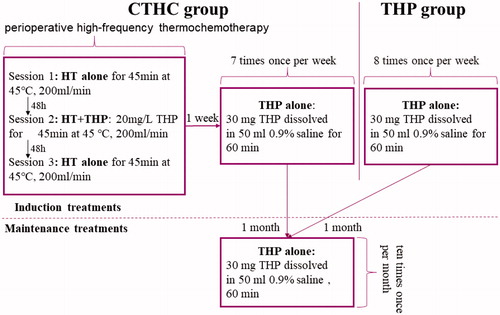
Instillation was performed after bladder emptying within 3 days after TURBT. In the THP group, 30 mg THP (Wanle Pharmaceutical Co. Ltd., Shenzhen, China) was dissolved in 50 ml 0.9% saline and maintained in the bladder for 60 min at room temperature. During the treatment, patients were held in the supine position, left supine position, right supine position and prone position for 15 min each. The induction treatment scheme was once-weekly THP instillation (8 times), followed by once-monthly THP instillation (10 times). In the CTHC group, the first treatment (perioperative high-frequency thermochemotherapy) comprised three 45-min sessions. The first and third sessions consisted of 45-min hyperthermia only using 0.9% saline; however, the second session consisted of a combination of hyperthermia and THP (20 mg/L THP for 45 min at 45 °C, 200 ml/min). These three sessions were conducted 48 h apart, and the subsequent treatment regimen was the same as for the THP group. shows the schematic diagram of treatment of both CTHC group and THP group.
2.5. Follow-up and evaluation of therapy
After completing TURBT, cystoscopy, urinary cytology and imaging examinations of the urinary tract were repeated every 3 months for 2 years [Citation25]. Recurrence and progression were pathologically confirmed. The study included patients with recurrent bladder cancer at the start of the study, but patients who relapsed during the course of the study were excluded.
2.6. Side-effects
Using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0, AEs were recorded during every treatment or follow-up. In the case of side effects, dose modifications were not allowed; instead, treatment was delayed.
2.7. Statistical analysis
The Mann–Whitney U test was used to compare parametric variables with skewed distribution; Pearson’s chi-square or Fisher’s exact test was used to compare categorical variables. Intention to treat analysis was performed in patients treated ≥1. Recurrence-free survival (RFS) and progression-free survival (PFS) after TURBT were estimated using the Kaplan–Meier method. The log-rank test was used to compare RFS between the two groups. Covariates included in the analyses comprised treatment (CTHC or THP alone), gender, age, the presence or absence of hypertension/diabetes, tumor size, tumor number, pathologic T stage combined with tumor grade, pathology type (papillary tumor only, carcinoma in situ [Cis] only/concurrent Cis), whether induction treatments were completed or not, and primary/recurrent tumor. Univariate and multivariate analyses using Cox proportional hazards models were performed to identify independent predictors of recurrence after TURBT. We present hazard ratios (HR) with 95% confidence intervals (CI). The reported p values were two-sided, and statistical significance was set at ≤.05. Statistical analyses were performed using GraphPad Prism software (version 7.04; GraphPad Software, Inc., San Diego, CA) and SPSS Statistical software (version 24; IBM Corporation, Armonk, NY). This study was conducted to conform to the TREND guidelines.
3. Results
3.1. Patients
Three hundred patients underwent intravesical instillation therapy with THP after completing TURBT. Of these 300 patients, one did not undergo postoperative cystoscopy due to perioperative death, and 72 patients were diagnosed with MIBC. Fifty-three patients with low-risk NMIBC and 10 patients with high-risk NMIBC received treatment prior to the availability of the pathological results. The 10 patients with high-risk NMIBC underwent radical cystectomy after the pathological results were confirmed. Three patients changed to other intravesical adjuvant agents of which each one voluntarily requested BCG and MMC; one patient changed to hydroxycamptothecin because of intolerance to THP. Finally, a total of 161 patients (75 CTHC versus 86 THP) were enrolled. Three patients moved, and one died of other diseases in the CTHC group, while four patients moved, and two died of other diseases in the THP group (). Follow-up ended in January 2019. Characteristics of patients were well balanced between the groups ().
Figure 3. Diagram of this study. BCG: Bacillus Calmette-Guérin; Cis: carcinoma in situ; CTHC: three consecutive hyperthermia treatments combined with single instillations; MIBC: muscle invasive bladder cancer; NMIBC: nonmuscle invasive bladder cancer; MMC: mitomycin C; ITT: intention to treat; RC: radical cystectomy; TURBT: transurethral resection of bladder tumors; THP: pirarubicin.
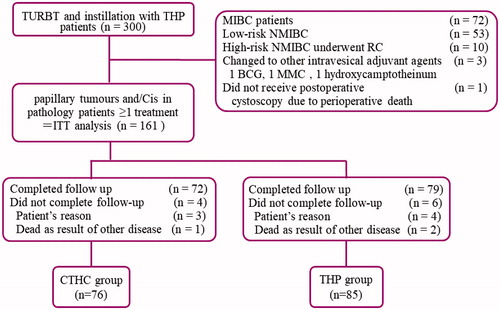
Table 1. Demographics and baseline characteristics (n = 161).
3.2. Efficacy
3.2.1. Recurrence-free survival
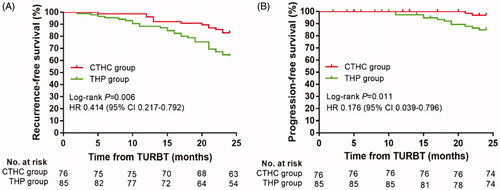
shows RFS curves for patients in the CTHC and THP groups. There was a significant difference between the CTHC and THP groups (log-rank p = .006) with a median follow-up period of 38.5 months (range 5–78 months) and 30 months (range 2–76 months), respectively. The 24-month RFS in the CTHC group was 82.9% (95% CI 74.4–91.4%) compared to 63.5% (95% CI 53.3–73.8%) in the THP group.
Figure 4. Bladder recurrence-free survival rates (A) and progression-free survival rates (B) of CTHC group and THP group estimated using the Kaplan–Meier method. The log-rank test was used for comparing recurrence-free survival rates and progression-free survival rates between the two groups. CTHC: three consecutive hyperthermia treatments combined with single instillations; THP: pirarubicin; TURBT: transurethral resection of bladder tumors.
3.2.2. Factors affecting recurrence
Variables significantly associated with recurrence in univariate analyses and clinical expertise were included in the multivariate analysis. In univariate analyses, treatment (CTHT versus THP), tumor number s, treatmecurrent tumors were significantly associated with recurrence. Although T-stage combined with grade was not statistically significant in the univariate analysis, it was included in the multivariate analysis because of its relevance to clinical knowledge. The multivariate analysis revealed that CTHC treatment (HR, 0.422; 95% CI 0.214–0.835; p = .013) was the only factor to independently predict improved RFS, while tumor number (HR, 2.247; 95% CI 1.079–4.679; p = .031) was the only factor to independently affect RFS. Univariate and multivariate analyses of factors associated with recurrence are described in . The results of the univariate analysis showed that the recurrence rate of patients with Cis disease did not increase significantly (HR 1.112; 95% CI 0.496–2.493, p = .798).
Table 2. Univariate and multivariate analysis of associated with recurrence.
3.2.3. Progression to muscle-invasive disease
shows PFS curves for patients in the CTHC and THP groups. Twenty-four-month PFS was 97.4% (95% CI 93.8–100%) and 87.1% (95% CI 79.9–94.2%) in the CTHC and THP groups, respectively. The CTHC group had a higher 24-month PFS, and there was a significant difference between the two groups (log-rank p = .011). Cox regression revealed that CTHC treatment predicted improved PFS (HR 0.176; 95% CI 0.039–0.796; p = .024).
3.3. Safety
No serious complications, such as bladder contracture and myelosuppression, were observed in this study. AEs associated with the instillation of THP were grades 1–2. The main AEs of the CTHC and THP groups were acute cystitis, hematuria, fever and gastrointestinal reactions. The acute cystitis rate was 25% (19/76) in the CTHC group compared to 20% (17/85) in the THP group. In contrast to the 9.21% (7/76) incidence of hematuria in the experimental group (CTHC), the control group (THP) had an incidence of 7.05% (6/85). The incidence of fever was higher in the CTHC group than in the THP group (6.57% [5/76] versus 4.71% [4/85]), whereas the CTHC group had a lower incidence of gastrointestinal reactions than the THP group (2.63% [2/76] versus 3.53% [3/85]). However, regarding the incidence of all AEs, there was no significant difference between the two groups ().
Table 3. Comparison of adverse events between the two groups.
4. Discussion
The shortage of BCG [Citation6] initiated research into the development of new therapeutic agents or methods for intermediate- or high-risk NMIBC. Since its discovery, hyperthermia has been an important therapy; however, the optimal scheme remains controversial. Currently, intravesical hyperthermia consists mainly of radiofrequency-induced hyperthermia (RITE) and conductive hyperthermic chemotherapy which are performed using the Combat Bladder Recirculating system and the Unithermia system [Citation26]. The BR-TRG-I urinary bladder hyperthermia treatment system used in this study is similar to the combat bladder recirculating system.
THP has been shown to be absorbed more rapidly into tumor cells than doxorubicin [Citation27]. A meta-analysis of 13 randomized controlled trials showed that THP was the most effective drug for reducing tumor recurrence [Citation28]. In the standard bladder instillation protocol, 30 mg THP is administered in 40 ml saline and maintained in the bladder for 1–2 h during each session; this procedure is repeated 6–8 times once per week or every other week [Citation13,Citation29,Citation30]. The scheme has been proven to be effective in preventing 50–70% of NMIBC recurrences in the first 2 years post-TURBT [Citation13,Citation30].
Several studies have been conducted regarding intravesical chemotherapy with THP. To reduce recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma, Ito et al. demonstrated the advantage of early intravesical instillation of THP (recurrence at 2 years: 16.9% in the THP group versus 42.2% in the control group) [Citation5]. A prospective randomized study with a 40.8-month follow-up performed by Okamura et al. compared single intravesical instillation of THP immediately after TURBT with TURBT alone for patients with a solitary <3-cm superficial bladder tumor. Multivariate analysis showed that THP instillation significantly decreased the risk of tumor recurrence (HR 0.31; 95% CI 0.17–0.56; p < .0001) [Citation11]. The laser scanning cytometer assay demonstrated a higher sub-G1 peak after double treatment with THP after a single treatment indicating that double treatment with THP could induce a higher rate of apoptosis of cancer cells [Citation31]. However, based on a prospective randomized study comparing RFS of single (n = 70) and two-time instillation (n = 73) of THP for solitary NMIBC (not including Cis), Tanimoto et al. found that the 5-year RFS rates were 58.6 and 68.6%, respectively, and double intravesical instillation of THP was not superior to single instillation in the prevention of bladder cancer recurrence (p = .3722) [Citation12].
Data from previous randomized controlled trials suggested that RITE had an advantage over BCG and MMC in BCG-naive patients [Citation32–34]. Even in patients with NMIBC who failed BCG immunotherapy, RITE with MMC had similar disease-free survival to standard second-line therapy [Citation35]. Previous studies about conductive hyperthermic chemotherapy mainly used MMC, but the durations, temperatures and concentrations differed [Citation36–40]. Most of these studies demonstrated the advantage of conductive hyperthermic chemotherapy over MMC [Citation36–39], and only one retrospective study showed similar RFS between conductive hyperthermic chemotherapy and MMC alone [Citation40]. There is no difference in disease progression to MIBC [Citation32,Citation36–40]. Regardless of RITE or conductive hyperthermic chemotherapy, most AEs are local and transient, and patients tolerate the treatment well [Citation32,Citation35,Citation41]. Studies of conductive hyperthermic chemotherapy reported RFS rates of 65–87.5% at 24-month to 41-month follow-ups [Citation36,Citation38–40], while in this study, the 24-month RFS was 82.9% (95% CI 74.4–91.4%) in the CTHC group, similar to or better than that reported previously. Notably, the CTHC group had significantly better 24-month PFS in this study: 97.4 and 87.1% in the CTHC and THP groups, respectively.
Nowadays, most studies about intravesical thermochemotherapy adopt a scheme that combines chemotherapy and hyperthermia at each treatment session. Regardless of the treatment device, patients are liable for the costs of special consumable materials at each treatment session. The CTHC protocol advocates early high-frequency implementation of hyperthermia followed by regular instillation of THP which can be performed in the outpatient department. Therefore, this protocol can greatly reduce the financial burden on patients without compromising the oncologic outcome.
Regrettably, this was a nonrandomized controlled trial; therefore, it has an inherent potential for bias. Moreover, it was a single-centre study with a limited sample size; we did not conduct the current study using propensity score matching because baseline characteristics were well-balanced. Our findings need to be validated by prospective randomized multicentre trials. However, this study is the first controlled trial to compare the efficacy of intravesical instillation of THP and intravesical hyperthermia combined with THP instillation under a novel scheme (CTHC) for intermediate- and high-risk NMIBC after TURBT. Our findings demonstrated the advantages of CTHC over THP alone and provide important insights into the potential effects of this treatment approach.
5. Conclusions
Our results suggest that the BR-TRG-I urinary bladder hyperthermia treatment system facilitates the smooth implementation of CTHC, and CTHC, with perioperative high-frequency thermochemotherapy as the core, reduces the recurrence and progression of intermediate- or high-risk NMIBC after TURBT.
Supplemental Material
Download PDF (489.2 KB)Acknowledgements
We are grateful to all the patients who participated in this study and to other urologists and doctors from the medical record room of Henan People's Hospital for their support in data collection and management. Further, we thank the professional technicians of Bright Medical Technology Co., Ltd for their efforts to keep patients safe while using the BR-TRG-I Urinary Bladder Hyperthermia Treatment System.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0: Estimated cancer incidence, mortality and prevalence worldwide in 2012, 2013, 2015. http://globocan.iarc.fr/Default.aspx.
- Compérat E, Larré S, Roupret M, et al. Clinicopathological characteristics of urothelial bladder cancer in patients less than 40 years old. Virchows Arch. 2015;466:589–594.
- Cambier S, Sylvester RJ, Collette L, et al. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non–muscle-invasive stage Ta–T1 urothelial bladder cancer patients treated with 1–3 years of maintenance bacillus Calmette-Guérin. Eur Urol. 2016;69:60–69.
- Sylvester RJ, van der Meijden APM, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–477.
- Tan WS, Rodney S, Lamb B, et al. Management of non-muscle invasive bladder cancer: a comprehensive analysis of guidelines from the United States. Europe and Asia. Cancer Treatment Rev. 2016;47:22–31.
- Bandari J, Maganty A, MacLeod LC, et al. Manufacturing and the market: rationalizing the shortage of bacillus Calmette-Guérin. Eur Urol Focus. 2018;4:481–484.
- Chang L, Xiao W, Yang Y, et al. Pseudomonas aeruginosa-mannose–sensitive hemagglutinin inhibits epidermal growth factor receptor signaling pathway activation and induces apoptosis in bladder cancer cells in vitro and in vivo. Urol Oncol Semin Original Invest. 2014;32:36. e11–36. e18.
- Umezawa H, Takahashi Y, Takeuchi T, et al. The absolute structures of THP-adriamycins. J Antibiot. 1984;37:1094–1097.
- Tsuruo T, Iida H, Tsukagoshi S, et al. 4'-O-tetrahydropyranyladriamycin as a potential new antitumor agent. Cancer Res. 1982;42:1462–1467.
- Zheng S, Xiong S, Lin F, et al. Pirarubicin inhibits multidrug-resistant osteosarcoma cell proliferation through induction of G2/M phase cell cycle arrest. Acta Pharmacol Sin. 2012;33:832–838.
- Okamura K, Ono Y, Kinukawa T, et al. Randomized study of single early instillation of (2r)-4-o-tetrahydropyranyl-doxorubicin for a single superficial bladder carcinoma. Cancer. 2002;94:2363–2368.
- Tanimoto R, Saika T, Ebara S, et al. Prospective randomized controlled trial of postoperative early intravesical chemotherapy with pirarubicin (THP) for solitary non-muscle invasive bladder cancer comparing single and two-time instillation. World J Urol. 2018;36:889–895.
- Miki T, Nonomura N, Kojima Y, et al. A randomized study on intravesical pirarubicin (THP) chemoprophylaxis of recurrence after transurethral resection of superficial bladder cancer. Hinyokika Kiyo Acta Urologica Japonica. 1997;43:907.
- Frey B, Weiss E-M, Rubner Y, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia. 2012;28:528–542.
- Toraya-Brown S, Fiering S. Local tumour hyperthermia as immunotherapy for metastatic cancer. Int J Hyperthermia. 2014;30:531–539.
- Matzkin H, Rangel MC, Soloway MS. In vitro study of the effect of hyperthermia on normal bladder cell line and on five different transitional cell carcinoma cell lines. J Urol. 1992;147:1671–1674.
- van der HA, Verhaegh G, Jansen CFJ, et al. Effect of hyperthermia on the cytotoxicity of 4 chemotherapeutic agents currently used for the treatment of transitional cell carcinoma of the bladder: an in vitro study. J Urol. 2005;173:1375–1380.
- Cavaliere R, Ciocatto EC, Giovanella BC, et al. Selective heat sensitivity of cancer cells. Biochemical and clinical studies. Cancer. 1967;20:1351–1381.
- Streffer C. Review: metabolic changes during and after hyperthermia. Int J Hyperthermia. 1985;1:305–319.
- Oleson JR. Eugene Robertson Special Lecture Hyperthermia from the clinic to the laboratory: a hypothesis. Int J Hyperthermia. 1995;11:315–322.
- Datta NR, Ordóñez SG, Gaipl US, et al. Local hyperthermia combined with radiotherapy and-/or chemotherapy: recent advances and promises for the future. Cancer Treat Rev. 2015;41:742–753.
- Babjuk M, Böhle A, Burger M, et al. EAU guidelines on non–muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol. 2017;71:447–461.
- Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM classification of malignant tumours. 7th ed. Hoboken: Wiley-Blackwell; 2009.
- Ba M, Cui S, Wang B, et al. Bladder intracavitary hyperthermic perfusion chemotherapy for the prevention of recurrence of non-muscle invasive bladder cancer after transurethral resection. Oncol Rep. 2017;37:2761–2770.
- Ito A, Shintaku I, Satoh M, et al. Prospective randomized phase II trial of a single early intravesical instillation of pirarubicin (THP) in the prevention of bladder recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma: the THP monotherapy study group trial. J Clin Oncol. 2013;31:1422–1427.
- Tan WS, Kelly JD. Intravesical device-assisted therapies for non-muscle-invasive bladder cancer. Nat Rev Urol. 2018;15:667–685.
- Kunimoto S, Miura K, Takahashi Y, et al. Rapid uptake by cultured tumor cells and intracellular behavior of 4'-O-tetrahydropyranyladriamycin. J Antibiot. 1983;36:312–317.
- Kang M, Jeong CW, Kwak C, et al. Single, immediate postoperative instillation of chemotherapy in non-muscle invasive bladder cancer: a systematic review and network meta-analysis of randomized clinical trials using different drugs. Oncotarget. 2016;7:45479–45488.
- Matsumura Y. Cooperative study of therapy of superficial bladder cancer by intravesical instillation of pirarubicin. Am J Clin Oncol. 1990;13:S5–S10.
- Yamamoto Y, Nasu Y, Saika T, et al. The absorption of pirarubicin instilled intravesically immediately after transurethral resection of superficial bladder cancer. BJU Int. 2001;86:802–804.
- Maruyama T, Higuchi Y, Suzuki T, et al. Double short-time exposure to pirarubicin produces higher cytotoxicity against T24 bladder cancer cells. J Infect Chemother. 2011;17:11–16.
- Arends TJH, Nativ O, Maffezzini M, et al. Results of a randomised controlled trial comparing intravesical chemohyperthermia with mitomycin C versus bacillus Calmette-Guérin for adjuvant treatment of patients with intermediate- and high-risk non–muscle-invasive bladder cancer. Eur Urol. 2016;69:1046–1052.
- Colombo R, Da Pozzo LF, Salonia A, et al. Multicentric study comparing intravesical chemotherapy alone and with local microwave hyperthermia for prophylaxis of recurrence of superficial transitional cell carcinoma. J Clin Oncol. 2003;21:4270–4276.
- Colombo R, Salonia A, Leib Z, et al. Long-term outcomes of a randomized controlled trial comparing thermochemotherapy with mitomycin-C alone as adjuvant treatment for non-muscle-invasive bladder cancer (NMIBC). BJU Int. 2011;107:912–918.
- Tan WS, Panchal A, Buckley L, et al. Radiofrequency-induced thermo-chemotherapy effect versus a second course of bacillus Calmette-Guérin or institutional standard in patients with recurrence of non–muscle-invasive bladder cancer following induction or maintenance Bacillus Calmette-Guérin Therapy (HYMN): a phase III, open-label, randomised controlled trial. Eur Urol. 2019;75:63–71.
- Sousa A, Piñeiro I, Rodríguez S, et al. Recirculant hyperthermic Intravesical chemotherapy (HIVEC) in intermediate–high-risk non-muscle-invasive bladder cancer. Int J Hyperthermia. 2016;32:374–380.
- Sousa A, Inman BA, Piñeiro I, et al. A clinical trial of neoadjuvant hyperthermic intravesical chemotherapy (HIVEC) for treating intermediate and high-risk non-muscle invasive bladder cancer. Int J Hyperthermia. 2014;30:166–170.
- Ekin RG, Akarken I, Cakmak O, et al. Results of intravesical chemo-hyperthermia in high-risk non-muscle invasive bladder cancer. Asian Pacif J Cancer Prev. 2015;16:3241–3245.
- Soria F, Milla P, Fiorito C, et al. Efficacy and safety of a new device for intravesical thermochemotherapy in non-grade 3 BCG recurrent NMIBC: a phase I–II study. World J Urol. 2016;34:189–195.
- Ekin RG, Akarken I, Zorlu F, et al. Intravesical bacillus Calmette-Guérin versus chemohyperthermia for high-risk non-muscle-invasive bladder cancer. Can Urol Assoc J. 2015;9:278.
- Tan WS, Palou J, Kelly J, et al. Safety and tolerability analysis of hyperthermic intravesical mitomycin to mitomycin alone in HIVEC I AND HIVEC II: an analysis of 307 patients [abstract MP15-18]. J Urol. 2017;197:e177.