Abstract
Lung cancer has attracted a lot of attention because of its high morbidity and mortality. The emergence of RFA provides a new treatment for unresectable NSCLC patients. In addition to killing in situ lung tumors, RFA also provides new immuno-activated antigens, for the treatment of lung cancer. It changes the tumor microenvironment and activates the entire immune system of patients. The peripheral blood cell count is easy to achieve and the blood cells are important in tumor immunity, which changes after RFA. On the one hand, the changes in blood cells identify the immune changes of NSCLC; on the other hand, it provides support and suspicion for the treatment of RFA.
1. Introduction
According to the cancer statistics compiled by the National Cancer Center of China and the American Cancer Society [Citation1–3], lung cancer continues to rank first in the incidence and death of malignant tumors in the world for many years. Except for small cell lung cancer, adenocarcinoma, squamous cell carcinoma and large cell lung cancer are collectively referred to as non-small cell lung cancer (NSCLC) [Citation4] about 85–90% of the incidence of lung cancer [Citation5]. Currently, only about 20% of patients can be treated surgically [Citation6]. Minimally invasive therapy, including percutaneous tumor ablation and transcatheter bronchial artery infusion chemoembolization, can be used as an effective alternative treatment for inoperable patients.
Besides cancer cells, solid tumors also contain interstitial tissues composed of nonmalignant cells, such as fibroblasts, homing epithelial cells, pericytes, myofibroblasts, vascular endothelial cells, lymphatic endothelial cells, and infiltrating immune cells. These cells together constitute the tumor microenvironment. Radiofrequency ablation is one of the earliest and most mature techniques of percutaneous tumor ablation, which has a history of clinical application and has been used for over 40 years. It is also a hyperthermia method provided over lethal temperatures. After ablation treatment, tumor tissue becomes coagulative necrosis and remains in the body Meanwhile, the tumor microenvironment and the immune state of the body change, which can stimulate the body to produce anti-tumor immune effect [Citation7].
Human blood cells mainly include red blood cells (erythrocytes), white blood cells (leukocytes), and platelets. Current studies show that these three types of cells play an important role in tumor immunity. In this paper, the changes in the immune function of various blood cells after radiofrequency ablation of non-small cell lung cancer were also reviewed.
2. The principle of radiofrequency ablation
Radiofrequency ablation (RFA) is to insert the radio frequency electrode needle (or electrode catheter) directly into the tumor center and introduce the high-frequency radio frequency wave produced by the radio frequency generator into the tumor tissue. Radio frequency wave is essentially an electromagnetic wave with a specific range of frequencies. At present, radio-frequencies usually range from 200 to 750 kHz in medicine. The alternating high-frequency current will cause rapid changes in the electromagnetic field. The positive and negative ions and polarized molecules in the nearby tissue cells move rapidly, oscillating at high speed and rubbing with each other, converting the radio frequency energy into heat energy, causing the local temperature to rise to 60–100 °C. The thermal effect can cause the intracellular and extracellular water to evaporate, dry, and pyknosis, resulting in aseptic coagulation necrosis [Citation7].
3. The beginning of radiofrequency ablation research
Radiofrequency ablation was used to therapy tumors for the first time in 1976 [Citation8]. As to its therapeutic effect and less side effects on unresectable patients, clinicians and scientific researchers have paid their attention to this new method. For lung malignant tumors, the initial study [Citation9] verified the expected degeneration of tumor cells and significant changes in tumor stroma after radiofrequency ablation. The stroma capillary wall infiltrates a large number of round cells, accompanied by degenerative changes, resulting in the supply of vessels for tumor necrosis and occlusion. The round cells in the matrix were widely infiltrated. After local necrosis and interstitial rupture, the lymphatic cistern established direct contact with the heat-injured malignant cells, resulting in continuous further destruction of the tumor. Radiofrequency ablation of the lung occurred for the first time in 1995 [Citation10], with studied for its effectiveness and complications. In 2000, Dupuy and colleagues [Citation11] reported three cases of percutaneous RFA in the treatment of lung tumors in America. In the same year, Cheng and colleagues [Citation12] reported the first experiment with CT-guided RFA to 105 patients with lung tumors in China. That opened up the prelude to the clinical RFA of lung cancer. After that, the clinical and basic research on radiofrequency ablation of the lung began to flourish.
4. The mechanism of immune response after thermal ablation
First, thermal ablation causes tumor necrosis through minimal injury, reduces the tumor load, and decreases the tumor-derived immunosuppressive factor (TDSF) produced by the malignant tumor [Citation13], so that the suppressed immune function can be restored. Second, based on previous studies [Citation14], we already know that the thermal ablation region consists of three parts. The main results are as follows: (1) The central region, coagulation necrosis can occur when the temperature is above 50 °C, where the cell membrane rupture, protein denaturation, enzyme activity and DNA polymerase function were lost, and abnormal mitochondrial function was found. Necrotic tumor fragments release intracellular antigens and inhibitors [Citation15], such as heat shock protein (HSP) and high mobility group protein B1 (HMGB1), as well as RNA and DNA, as in situ antigens, which can be ingested by dendritic cells. Furthermore, it can stimulate systemic autologous anti-tumor immune responses [Citation16,Citation17] and control distant metastasis [Citation18]. (2) Transition zone: at 41 °C to 45 °C, the tissue still has thermal damage, which is reversible and sublethal, the metabolic function may be disturbed, and the cells in this area are prone to further damage. Blood flow in the area increases. The damaged local tissue exposes hyaluronic acid and endothelial injury markers, stimulates the expression of vascular adhesion molecules and chemokines, attracts immune cells to this region and enhances immune cell function. The region contains neutrophils, macrophages, natural killer cells, dendritic cells, and CD4+ and CD8+T lymphocytes. Cell necrosis fragments can stimulate phagocytosis, and tumor cells are swallowed by antigen-presenting cells(APC). . (3) Normal tissue around the tumor: blood vessels will produce the heat sink effect, dissipating heat and reducing the ablation effect. Tumor antigens released after cell death are transferred to the surrounding lymph nodes, stimulating immature dendritic cells and immature T cells. Indirect or delayed cell injury can also occur after thermal ablation, induced continuous cell coagulative necrosis, which has been shown in preclinical and clinical studies [Citation19,Citation20]. Possible mechanisms include apoptosis, ischemic vascular injury, ischemia-reperfusion injury, lysosome release during tumor necrosis or invading granulocytes, cytokines release, and further stimulate the immune response [Citation14,Citation21].
5. The mechanism of blood cells in NSCLC immunization and the changes in blood cells after RFA
5.1. Erythrocytes
Erythrocytes not only transport O2 and CO2, and regulate acid-base balance, but are also involved in the body’s immune regulation. In 1981, Siegel and colleagues [Citation22] defined the erythrocyte immune system in the body. The immune function of erythrocytes includes the innate immune function and acquired immune function, which are in dynamic balance. Erythrocytes can adhere to tumor cells and carry them to the reticuloendothelial system (RES), where tumor cells can lyse by NK cells and swallow by macrophages. Prevent tumor cells from spreading and metastasizing through the bloodstream. Some studies [Citation23] have shown that there are a large number of peroxidases on the surface of erythrocytes. After erythrocyte immune adhesion to tumor cells, peroxidase can destroy the cell membrane of tumor cells at the adhesion spot between erythrocyte and tumor cells, and then play a role in destroying tumor cells. The receptor C3b receptor on the surface of the erythrocyte membrane can play an immune adhesion role to cancer cells. The adherent cancer cells are quickly destroyed by phagocytes. The immune function and immune activity of erythrocytes are closely related to tumorigenesis and progression. [Citation24–26] The erythrocyte adhesion function is suppressed during the process of tumor development. Some studies [Citation27] confirmed that Red Blood Cell-C3b Receptor Rate(RBC-C3bRR) in patients with lung cancer were significantly lower than those in normal controls, while the Red Blood Cell-Immune Complex Rate (RBC-ICR) was significantly higher than that in normal controls. After RFA, RBC-C3bRR was significantly increased and RBC-ICR was significantly decreased [Citation28]. The results showed that the immune function of erythrocytes recovered and strengthened after RFA.
5.2. Leukocytes
The main function of leukocytes is to participate in the body’s defense response. These include neutrophils, lymphocytes, monocytes, eosinophils, and basophils. In the early stage of tumorigenesis, it plays an anti-tumor effect. With the growth of the tumor and the change in tumor microenvironment, the immune function of leukocytes is suppressed.
5.2.1. Neutrophils
Neutrophils are an important part of innate immunity; lung tissue is an important repository of neutrophils [Citation29]. In recent years, tumor-associated neutrophils (TAN) have been found [Citation30]to regulate tumor development, and specific tumor microenvironment may show cancer-promoting or anti-tumor activity [Citation31]. In the early stages of lung cancer, tumor-infiltrating neutrophils can stimulate T cell proliferation [Citation32] and act as APC to activate T cells [Citation33], which are important inflammatory cells in the tumor microenvironment, combined with neutrophils releasing membrane-perforating agents, and water-soluble cytotoxic media, such as IL-6, IL-8 and TNF-α can help to kill the tumor cell. Various inflammatory factors released by neutrophils play important roles in tumor development and progression [Citation34–36], a small number of reactive oxygen species(ROS) play a regulatory role in cell protection and apoptosis, but a large number of ROS acting as carcinogens can induce structural changes of DNA and induce the expression of stress genes proteases, the same as reactive nitrogen species (RNS); neutrophil elastase(NE) act on tumor proliferation; IL-1β, matrix metalloprotein-9(MMP-9) and vascular endothelial growth factor(VEGF) induce tumor angiogenesis. Arginase 1 can inhibit CD8+ T cells and NK to protect tumor cells alive and metastasis cooperates with leukotrienes(LT). Engblom and his colleagues [Citation37] revealed the heterogeneity of granulocytes in the microenvironment of lung cancer, and that only neutrophils with high expression of SiglecF can promote tumor growth. Kowanetz [Citation38] suggested that neutrophils could gather in the lung earlier than tumor cells to form early metastasis. This could promote tumor angiogenesis [Citation39]. After RFA, there will be local and systemic chronic inflammation, which can activate and recruit neutrophils and release a series of pathogenic complexes, such as myeloperoxidase, NE, ROS, nuclear factor-kappa B(NF-κB), and activator protein(AP) [Citation40]. There is no clear study on the changes of neutrophils in the circulatory system of lung cancer after RFA.
5.2.2. Lymphocytes
Lymphocytes are considered to be the main effector cells in tumor immunity, especially tumor-infiltrating lymphocytes (TILs), which refer to lymphocytes collected from the blood circulation to the local part of the tumor. Participate in the formation of the tumor microenvironment and regulate local tumor immunity [Citation41]. Lymphocytes mainly include T cells, B cells, and NK cells.
T cells are divided into CD3+, CD4+, and CD8+ subsets. CD3+ represents total T lymphocytes, which directly reflects the activity and number of immunocompetent cells involved in the immune response. CD4+ helper T lymphocytes(Th) are important components of effector T cells. It can promote the secretion of antibodies by B lymphocytes and play an important role in regulating the proliferation and activation of CD8+T cells. CD8+ cytotoxic T lymphocytes (CTLs) inhibit the activation stage of the immune response, while the target cells are B lymphocytes and Th cells. CD8+ cell subsets also include human tumor-specific cytotoxic T lymphocytes [Citation42]. CD8+T cells can play an anti-tumor role in the tumor microenvironment, and the number of tumors infiltrating CTL is positively correlated with neoplasm staging. The more CD8+T cells infiltrate, the better a prognosis patients have [Citation43–45]. CD4+T cells mainly contain four subsets: type 1 helper T cells (Th1 cells), type 2 helper T cells (Th2 cells), IL-17 producing CD4+T cells (Th17 cells), regulatory T cells (Treg cells). Each subset plays a role mainly by secreting different cytokines [Citation46–48]. Th1 cells mainly secrete interferon-gamma(IFN-γ), IL-2 and tumor necrosis factor β(TNF-β) [Citation46,Citation47]. Activated Th1 cells and cytokines have strong antitumor activity and immunomodulatory effects. IFN-γ can inhibit tumor angiogenesis and induce apoptosis of tumor cells; IL-2 can stimulate the antitumor activity of NK cells; TNF-β can directly cause apoptosis of tumor cells [Citation47,Citation49]. Th2 cells mainly secrete IL-4, IL-5, IL-6, IL-10, and IL-13 [Citation47]. IL-4 and lL-10 can inhibit the response of Th1 cytokines, promoting tumor growth and antagonizing each other with Th1 cells [Citation50,Citation51]. There is an imbalance of Th1/Th2 immunity in patients with lung cancer. When Th2 cells are dominant, it can inhibit the production of Th1 cytokines and weaken the anti-tumor immune function of the body, which makes it easy for lung cancer cells to escape the surveillance of cellular immunity. This then accelerate the progress of the tumor [Citation50,Citation52,Citation53]. Th17 cells can specifically secrete IL-17, which activates signal transducer and activator of transcription 3 (STAT3) and up-regulates the expression of vascular endothelial growth factor (VEGF). It can also promote the angiogenesis of lung cancer [Citation54]. Moreover, it can induce the expression of binding zinc finger E-box binding homeobox 1 (ZEB 1), which promotes invasion and metastasis of lung cancer [Citation55]. Treg cells can inhibit normal anti-tumor function, which is related to the stage and progression of lung cancer patients [Citation56]. Tumor immune tolerance was mediated by inhibiting the activation and proliferation of CD4+ and CD8+T cells and secreting granzyme and perforin to kill effector cells. [Citation47,Citation57] Only when the ratio of CD4/CD8 cells is in a certain range can the immune function of the body be in a normal state. Tumor cells can secrete immunosuppressive factors, which can inhibit the immune function of patients by inhibiting the differentiation and proliferation of lymphocytes, reducing CD4+ antigen on the surface of lymphocytes, increasing the responsiveness of CD8+ T cells, and reducing the ratio of CD4/CD8 [Citation58]. RFA can affect the proliferation of T cells. After RFA, the levels of tumor-specific T cells, Th1 cells and Th1/Th2 ratio increased, whereas Th2, Th17, and Treg cells decreased [Citation59,Citation60]. RFA can also induce CTLs to migrate to metastatic nodules [Citation61]. These results suggest that RFA improves anti-tumor immunity mediated by T cells.
B cells originate from bone marrow and can differentiate into plasma cells via antigen stimulation. Plasma cells can synthesize and secrete the antibody–Immunoglobulin (Ig), which mainly acts on humoral immunity. Various factors released by CD4+Th cells, such as IL-4, IL-5, IL-10, and IL-13, regulate the proliferation of B cells and participate in tumor immunity in a variety of ways. The TIL in solid tumors contains a different proportion of infiltrating B cells. The data reported by Schmidt and colleagues [Citation62,Citation63] confirmed that B cell markers are the strongest prognostic factors in lung cancer and other human tumors. Biragyn and colleagues [Citation64] found that B cells play an important role in regulating the response of Treg cells. Human B cells can express CD39 and CD73 to regulate the function of T cells [Citation65]. Some studies have confirmed that the immune function of B cells is suppressed and that various types of Ig (IgA, IgG, IgM) are decreased in lung cancer [Citation17]. While some studies have shown that all types of Ig increased after RFA on the liver cancer and remained at a high level after 3 months [Citation66], but no study on lung cancer.
Natural killer cells (NK cells) account for 5 to 15% of peripheral blood lymphocytes, marked by CD3-/CD56+/CD16+ and KIR+, the c-type lectin receptor. NK cells play an important role in anti-tumor immunity and have natural cytotoxicity in tumor cells [Citation67–69]. In the early stages of tumorigenesis, tumor cells activate NK cells by up-regulating activating receptors or down-regulating inhibitory receptors. Activated NK cells can directly kill tumor cells, or indirectly eliminate tumor cells by activating other cells to inhibit tumor growth and metastasis [Citation70]. Although NK cells have a strong anti-tumor function, tumor cells can change themselves and the environment continuously, allowing some tumor cells to escape the surveillance of the immune system, including NK cells, and gradually evolve into uncontrollable malignant tumors. Due to the influence of tumor cells and tumor microenvironment, the function of a small number of NK cells infiltrating into the tumor was significantly abnormal. The main findings showed in the following aspects: the decrease of killing ability, the decline of cytokine secretion ability, the reduction of the expression of the activating receptor, the increase of inhibitory receptor expression, the impairment of proliferation ability, and even showed the phenotype of promoting tumor growth [Citation71–74]. It has been reported that in patients with lung adenocarcinoma, the number of NK cells in tumor tissues is significantly less than that in paracancerous tissues, and the decreased number of NK cells is mainly a subset of CD16+. A small number of infiltrating NK cells express lower levels of Granzyme B, CD57, and IFN- γ, suggesting that their anti-tumor ability has been damaged [Citation73,Citation74]. It has been reported that the number of NK cells in the blood of hepatocellular carcinoma and lung cancer increased significantly after RFA [Citation75], and that Hsp70 with IL-2 can stimulate NK cells against mHsp70 positive tumor cells [Citation76]. The mechanism for hyperthermia enhancing the cytotoxicity of human NK cells to tumor cells requires the NKG2D receptor to be clarified [Citation77]. It also makes NK cells become a hot spot in the biotherapy of tumors.
5.2.3. Monocyte
Monocytes are differentiated into macrophages and dendritic cells.
Macrophages are recruited and supported for growth by inflammatory chemokines, cytokines and growth factors in the tumor microenvironment [Citation78]. Tumor-associated macrophage (TAM) exists in the tumor stroma, and it is the largest number of inflammatory cells in the microenvironment [Citation79]. TAMs come from the monocytes and are transferred to primary and secondary tumors through blood at the early stage [Citation80]. There are two types: classically activated macrophages (M1) and alternatively activated macrophages (M2). M1 is usually characterized by monitoring microbial activity and proinflammatory phenotype and inducing factors to promote inflammation such as lipopolysaccharide (LPS) and IFN-γ. M2 inducing factors such as transforming growth factor β(TGF-β) and IL-4 can inhibit immunity, regulate inflammatory response, and adaptive Th2 immunity [Citation81]. TAM has phenotypic plasticity, and its phenotypic differentiation and function are affected by inducing factors in the microenvironment. M1 and M2 are the two extreme manifestations of macrophages, and their functions are in a continuous and balanced state. But with the domestication of tumor cells, they are in a state of functional imbalance, showing a functional state dominated by M2. It is one of the main factors tha promote tumor progression [Citation82]. Studies on liver cancer have shown that M2 macrophages increase significantly after RFA [Citation83], but there is no related study on the changes of macrophages after RFA in lung cancer.
Dendritic cells (DC), as the target of innate immune system immunotherapy, have always been the focus of research. DC distributed in all tissues is a professional antigen-presenting cell which can ingest, process and present tumor antigens, and can ingest all kinds of tumor-specific and tumor-associated antigen substances. After processing, it was presented to the surface of major histocompatibility complex class I (MHC-I) and MHC-II molecules to form antigen MHC molecular complex [Citation84], which combined with T cell receptor (TCR) to transmit tumor antigen information to T lymphocytes. Cytotoxic T cell reactions mediated by T cells and activated by tumor cell antigen information can specifically recognize and kill tumor cells without damaging any normal tissues [Citation85]. Therefore, DC also plays a key role in the initiation and mediation of tumor immune response and is regarded as the trigger of the immune response [Citation86]. There are three states of DCs in the body: mature, semi-mature and immature. According to their functions and properties, they can be divided into classical DCs (cDCs, also known as traditional DCs ormyeloid DCs) and plasmacytoid DCs (pDCs). pDCs morphologically resemble plasma cells but produce enormous amounts of IFN-α. They differentiate upon stimulation into immunogenic DCs that can prime T cells against viral antigen. cDCs refer to all DCs other than pDC. cDCs have an enhanced ability to sense tissue injuries, capture environmental- and cell-associated antigens, and process and present phagocytosed antigens to T lymphocytes.[87] After picking up the antigen in peripheral tissue, immature DC cells (iDC) migrated to secondary lymphoid organs through blood flow or lymphatic vessels and gradually matured, highly expressing MHC-I/II molecules, costimulatory molecules including B7 and CD40, and so on. The tumor antigen MHC molecular complex and costimulatory molecules activate the initial T cells [Citation87] by transmitting the first and second signals, which promote the amplification and specifically killing and inhibiting tumor cells. Immature or semi-mature tumor-infiltrating DCs in the tumor microenvironment of lung cancer can secrete inhibitory cytokines and participate in tumor immune escape, resulting in local immunosuppression and T cell anergy and even mediating T cell clone clearance. Tumor attracts and rearranges the biological characteristics of DCs to induce them to play the role of tumor immunosuppression or angiogenesis. Driven by tumor-related cytokines such as VEGF, IL-10 and PGE2, DCs can differentiate into regulatory DCs, which inhibit the proliferation of CD4+T cells in vitro and in vivo [Citation88]. Tumor-associated DCs can induce angiogenesis and Treg increment by producing MMP, VEGF, angiogenin, heparanase and basic fibroblast growth factor [Citation89]. The tumor itself also interferes with the maturation of DCs by secreting IL-10, and some tumor-derived factors can alter the maturation of mDCs and indirectly promote the growth of tumor cells [Citation90]. pDCs [Citation91] can induce initial CD4+T cells to differentiate into T cells with immunosuppressive function, and also play a role in tumor progression. RFA can induce DC [Citation92] and promote the maturation of DC to promote long-term immunity against NSCLC. Tissue specimens showed that DC matured after RFA [Citation17], that DCs up-regulated the expression of MHC II and CD80, and could effectively induce CTL in mouse tumor models [Citation60].
5.2.4. Eosinophils
Eosinophil(Eos) is a very important cell in the process of immune reaction and allergic reaction, which has the function of killing bacteria and parasites. A large number of Eos infiltrated around the tumor act as anti-tumor immune effector cells, inhibiting or dissolving tumor cells through a variety of mechanisms, and inducing apoptosis of tumor cells. First, Eos degranulated and secreted when it is close to the tumor, directly or indirectly inhibiting the growth of tumor cells by releasing some active mediators [Citation93]. As an antigen-presenting cell, Eos in tumor tissue promotes the activation of lymphocytes and kills tumor cells [Citation94]. Second, Eos mediates local inflammation through oxidative metabolism and the release of a number of cytotoxic proteins, which kill or inhibit the growth of tumor cells [Citation95]. Third, Eos can produce a series of cytokines, such as IL-3, IL-4, IL-5, IL-10, IL-12, GM-CSF, and tumor necrosis factor [Citation96,Citation97]. IL-3 and IL-5 can promote the proliferation and activation of Eos. IL-12 can inhibit metastasis and infiltration of tumor cells. IL-4 and GM-CSF can regulate the function of specific lymphocytes and nonspecific macrophages and kill tumor cells by strengthening their function [Citation98]. Studies have confirmed that IL-25 (or IL-17E) treatment can produce a large number of eosinophils, which is associated with tumor suppression [Citation99]. Last, Eos can integrate danger signals, respond quickly and selectively mediate anti-tumor effects [Citation100].The study also showed that the function of Eos in different tumors is different, and can also promote tumor growth. The decrease of Eos was related to the progression of lung cancer [Citation101]. After chemotherapy, the increase in Eos suggested that the prognosis was good [Citation102]. At present, there is no study on the changes in eosinophils after ablation.
5.2.5. Basophils
Basophils release histamine and other substances when they encounter specific antigens, causing allergic reactions. These produced substances induce neutrophils and eosinophils to the abnormal positions. The increase in basophils is more common in some allergic diseases, hematological diseases, malignant tumors, and some infectious diseases. Since it accounts for less than 1% of peripheral leukocytes, Anthony [Citation103] found that the basophilic cell count increased in peripheral blood of most patients with lung cancer, especially in patients with squamous cell carcinoma, which is stable and independent of leukocyte changes. Recent studies have shown that basophils decreased significantly in peripheral blood of patients with liver cancer [Citation104] and increased significantly in patients with breast cancer [Citation105]. There are no studies on the mechanism or the changes of basophils after RFA.
5.3. Platelet
The main functions of platelets are clotting and hemostasis. A series of studies have shown that the interaction between platelets and tumor cells can help tumor cells survive in the blood circulation and adhere to distant metastasis. Platelet receptors, such as adenosine diphosphate, thromboxane A2, thrombin and tumor-associated proteins, are important targets of platelet aggregation-the most basic mechanism of immune escape in tumor cells [Citation106]. Platelet aggregation is able to promote the adhesion and encapsulation of circulating tumor cells, which enhances the ability of tumor cells to escape immune attack [Citation107]. Platelets can negatively affect NK cell function by derived ectosomes [Citation108]. Platelets can induce epithelial-mesenchymal transformation and platelet-derived nucleotides and promote transepithelial migration and metastasis of tumor cells [Citation109,Citation110]. Furthermore, activated platelets can release a variety of vascular endothelial growth factors and cytokines, such as VEGF, MMP and others, thereby increasing angiogenesis in tumor tissues and ultimately promoting tumor growth [Citation111,Citation112]. In addition, these growth factors and active substances can also prevent the blood vessels in the tumor from rupturing and bleeding [Citation113]. So platelet-related parameters(platelet count, mean platelet volume and thrombocytocrit) were increased in NSCLC [Citation114]. Recent studies have shown that there is a slight decrease in platelets after RFA in the liver [Citation115]. In the same period, studies have confirmed that platelet-related parameters(platelet count, mean platelet volume and thrombocytocrit) in lung cancer after chemotherapy are also significantly reduced [Citation114]. However, there is no study expounding on the mechanism of how the platelets decrease after RFA.
6. Discussion
Lung cancer is currently the tumor with the highest incidence and mortality. RFA provides a new treatment for unresectable NSCLC patients. After treatment, in addition to killing in situ lung tumors, intracellular antigens and inhibitors are also released. That provides a variety of cytokines that changed the tumor microenvironment of patients and activated the patient’s immune system. The mainstream view is that thermal ablation assists inthe treatment of tumors. A few studies suggest that it promotes tumor progression and metastasis. In this paper, after reviewing the literature, we found that various cellular components in the blood and their related factors are involved in the immune process of lung cancer, and that they are modified and affected by tumor cells in the tumor microenvironment. Blood cells’ function is suppressed, even become accomplices in tumor progression and metastasis (as shown in ). Clinicians can monitor the blood count to direct the therapy, using antiplatelet drugs and anti-inflammatory drugs to inhibit excessive platelets and neutrophils to promote tumor growth and metastasis. Tumor microenvironment changed after RFA, while the blood cells in peripheral circulation and tumor microenvironment also changed (as shown in ): erythrocytes, platelets, B cells, T cells, and NK cells improved the function of anti-tumor immunity after RFA. Th2, Th17, Treg, TAM, and iDC, play a vital role in tumor immune escape. After RFA, iDC matured and the Th2, Th17, and Treg decreased which can enhance the effect of anti-tumor immunity. However, M2 macrophages increased to assist in tumor growth. There were no related studies on the changes in other blood cells after thermal ablation: neutrophils, basophils, and eosinophils. After RFA, clinicians can measure the blood routine to aid monitoring recurrence.
Figure 1. All types of blood cells influence NSCLC progression through multiple mechanisms. At the primary tumor site (left part), activated Th1 cells secreting IFN-γ, IL-2 and TNF-β have strong antitumor activity and immunomodulatory effects, while Th2 cells secreting IL-4 and IL-10 can inhibit the response of Th1 cytokines, promoting tumor growth (Initiation). Activated neutrophils act as carcinogens through ROS, RNS and NE promoting tumorigenesis. It secretes IL-6, IL-8 and TNF-α that help CD8+ T cell to damage pre-tumoral cells and kill the tumor cells. Mature DCs can activate the initial T cells, killing and inhibiting tumor cells. Immature or semi-mature tumor-infiltrating DCs can participate in tumor immune escape, resulting in T cell anergy. Tumor cells can secrete IL-10 to interfere with the maturation of DCs. Erythrocytes can adhere to tumor cells and transfer to macrophages and NK cells. Moreover, peroxidase on the surface of erythrocytes can destroy the cell membrane of tumor cells at the adhesion spot. As the tumor progresses, neutrophils secrete NE activating tumor proliferation, arginase-1 suppressing CD8+ T cell and NK cell responses. Treg cells can inhibit the activation and proliferation of CD4+ and CD8 + T cells and kill effector cells. Alternatively activated macrophages (M2) inducing factors such as TGF-β and IL4 can inhibit tumor immunity. B cells can regulate the response of Treg cells and regulate T cells by expressing CD39 and CD73. Eosinophils secrete IL-4 and GM-CSF, strengthening the function of T cells and macrophages. Classically activated macrophages (M1) induce factors such as LPS and IFN-γ promoting tumor immunity. As the tumor grows, neutrophils release IL-1β, MMP-9 and VEGF to induce tumor angiogenesis and metastasis cooperating with leukotrienes (LT). Th17 cells, specifically secreting IL-17, promote tumor angiogenesis and metastasis. Eos secreted IL-12 inhibits the metastasis and infiltration of tumor cells. Activated platelets can increase angiogenesis in tumor tissues. Erythrocytes can adhere to circulating tumor cells(CTC) and carry them to macrophages and NK cells to prevent tumor cells from spreading and metastasizing through the blood. Platelet aggregation can promote the adhesion and encapsulation of CTC, assisting tumor cells to escape immune attack. Neutrophils can gather in the lung earlier than tumor cells to form early metastasis (right box). Neutrophils can suppress T cells and NK cells. Platelets can protect CTC from physical clearance and immune surveillance by mononuclear phagocyte system at the metastasis site.
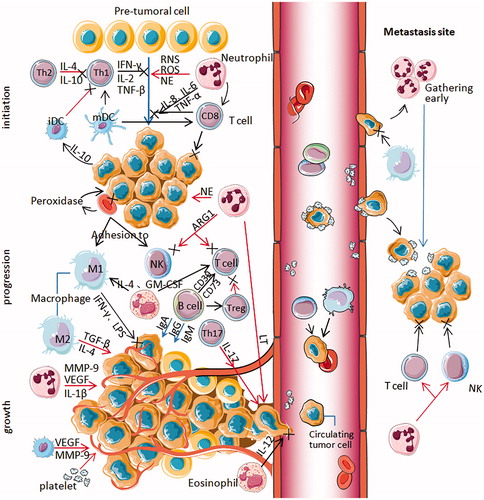
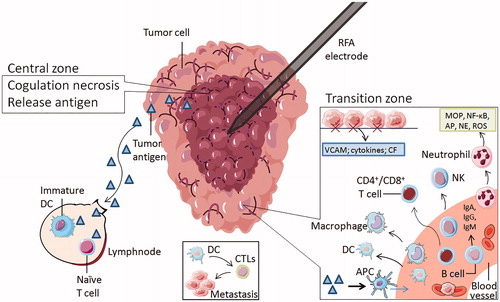
Figure 2. The zones of radiofrequency ablation to NSCLC. The RFA electrode is surrounded by two main zones. The central zone was coagulative necrosis at temperatures ≥50 °C, releasing intracellular antigens and inhibitors. Part of tumor antigen is drained to nearby lymph nodes, where it can stimulate immature DCs and naive T cells. Others are captured by antigen-presenting cells (APC), which can attract immune cells from the blood system. The transition zone at temperatures between 41 °C and 45 °C is still a heat-induced injury, but it is sublethal and reversible. The damaged local tissue exposes hyaluronic acid and endothelial injury markers, stimulate the expression of vascular adhesion molecules (VCAM), cytokines and chemotactic factor(CF), attract immune cells to this region and enhance some immune cells' antitumor function. These immune cells consist of neutrophils, B cells, macrophages, natural killer cells, dendritic cells, and CD4 + T and CD8 + T lymphocytes. Tumor cells are captured by erythrocytes and swallowed by APC. DC can induce CTLs to kill metastatic cells.
At present, tumor immunity is still in the process of research and development. Although some of the RFA results conflict, many results are still changing with the deepening of the study, and there are still many unknown areas that need further investigation. For the blood cells, many tumor-related immune mechanisms that can be further studied and explored.
Disclosure statement
The authors have no conflicts of interest to declare. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA A Cancer J Clin. 2020;70(1):7–30.
- Feng RM, Zong YN, Cao SM, et al. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond)). 2019;39(1):22.
- Schnabel PA, Junker K. Pulmonary neuroendocrine tumors in the new WHO 2015 classification: start of breaking new grounds? Pathologe. 2015;36(3):283–292.
- Poggi A, Zocchi MR. Mechanisms of tumor escape: role of tumor microenvironment in inducing apoptosis of cytolytic effector cells. Arch Immunol Ther Exp (Warsz). 2006;54(5):323–333.
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300.
- Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy[J]. Nat Rev Cancer. 2014;14(3):199–208.
- LeVeen HH, Wapnick S, Piccone V, et al. Tumor eradication by radiofrequency therapy. Responses in 21 patients. JAMA. 1976;235(20):2198–2200.
- Sugaar S, LeVeen HH. A histopathologic study on the effects of radiofrequency thermotherapy on malignant tumors of the lung. Cancer. 1979;43(2):767–783.
- Goldberg SN, Gazelle GS, Compton CC, et al. Radiofrequency tissue ablation in the rabbit lung: efficacy and complications. Acad Radiol. 1995;2(9):776–784.
- Dupuy DE, Zagoria RJ, Akerley W, et al. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol. 2000;174(1):57–59.
- Cheng QS, Zhao ZY, Liu K, et al. Percutaneous radiofrequency hyperthermia for lung cancer by the using of computer tomography-guided anchor-shaped electrode report of 105 cases[J]. J Fourth Mil Med Univ. 2000; 21(11):1399–1401.
- Mrowiet ZU. Advances in systemic therapy for psoriasis. Clin Exp Dermatol. 2001;26(4):362–367.
- Nikfarjam M, Muralidharan V, Christophi C. Mechanisms of focal heat destruction of liver tumors. J. Surg. Res. 2005;127(2):208–223.
- Slovak R, Ludwig JM, Gettinger SN, et al. Immuno-thermal ablations – boosting the anticancer immune response. J Immunother Cancer. 2017;5(1):78.
- Fagnoni FF, Zerbini A, Pelosi G, et al. Combination of radiofrequency ablation and immunotherapy. Front Biosci. 2008;13:369–381.
- Schneider T, Hoffmann H, Dienemann H, et al. Immune response after radiofrequency ablation and surgical resection in nonsmall cell lung cancer[J]. Semin Thorac Cardiovasc Surg. 2016;28(2):585–592.
- Deng Z, Zhang W, Han Y, et al. Radiofrequency ablation inhibits lung metastasis of breast cancer in mice. Zhonghua Zhong Liu Za Zhi. 2015;37(7):497–500.
- Clasen S, Krober SM, Kosan B, et al. Pathomorphologic evaluation of pulmonary radiofrequency ablation: proof of cell death is characterized by DNA fragmentation and apoptotic bodies. Cancer. 2008;113(11):3121–3129.
- Pereira PL. Actual role of radiofrequency ablation of liver metastases. Eur Radiol. 2007;17(8):2062–2070.
- Fajardo LF, Egbert B, Marmor J, et al. Effects of hyperthermia in a malignant tumor. Cancer. 1980;45(3):613–623.
- Siegel I, Lin Liu T, Gleicher N. The red-cell immune system. Lancet. 1981;318(8246):556–559.
- Anderson HL, Brodsky IE, Mangalmurti NS. The evolving erythrocyte: red blood cells as modulators of innate immunity. J Immunol. 2018;201(5):1343–1351.
- Ding Y, Guo H, Gu WJ. Progress of erythrocyte immunity in tumor research. J Liaoning Uni TCM. 2007;9(1):68–69.
- Ng T, Ryder BA, Chern H, et al. Leukocyte-depleted blood transfusion is associated with decreased survival in resected early-stage lung cancer. J Thorac Cardiovasc Surg. 2012;143(4):815–819.
- Genova C, Rijavec E, Barletta G, et al. Ipilimumab (MDX-010) in the treatment of non-small cell lung cancer. Expert Opin Biol Ther. 2012;12(7):939–948.
- Wan H, Zeng X, Yu J, et al. Red-cell immune functions, T subsets and B-cell detection and their clinical significances in patients with primary lung cancer. Zhongguo Fei Ai Za Zhi. 2001;4(5):351–353.
- Zhang WQ, Suo XH, Lin H. the effects on immune function of patients with non-small cell lung cancer after hyperthermia radiofrenquency ablation. Chin Gen Pract. 2002;5(3):197–198.
- Aulakh GK. Neutrophils in the lung: “the first responders”. Cell Tissue Res. 2018;371(3):577–588.
- Nicolás-Ávila JÁ, Adrover JM, Hidalgo A. Neutrophils in homeostasis, immunity, and cancer. Immunity. 2017;46(1):15–28.
- Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183–194.
- Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, et al. Tumor associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest. 2014;124(12):5466–5480.
- Singhal S, Bhojnagarwala PS, O'Brien S, et al. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell. 2016;30(1):120–135.
- Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17(11):1359–1370.
- Di Carlo E, Forni G, Lollini P, et al. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood. 2001;97(2):339–345.
- Jakóbisiak M, Lasek W, Gołąb J. Natural mechanisms protecting against cancer. Immunol Lett. 2003;90(2-3):103–122.
- Engblom C, Pfirschke C, Zilionis R, et al. Osteoblasts remotely supply lung tumors with cancer-promoting SiglecFhigh neutrophils. Science. 2017;358(6367):eaal5081.
- Kowanetz M, Wu XM, Lee J, et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G + Ly6C + granulocytes. Proc Natl Acad Sci USA. 2010;107(50):21248–21255.
- Dumitru CA, Fechner MK, Hoffmann TK, et al. A novel p38-MAPK signaling axis modulates neutrophil biology in head and neck cancer. J Leukoc Biol. 2012;91(4):591–598.
- Vaguliene N, Zemaitis M, Lavinskiene S, et al. Local and systemic neutrophilic inflammation in patients with lung cancer and chronic obstructive pulmonary disease. BMC Immunol. 2013; 14(1):1–11.
- Radvanyi LG. Tumor-infiltrating lymphocyte therapy: addressing prevailing questions. Cancer J. 2015;21(6):450–464.
- Song QK, Ren J, Zhou XN, et al. The prognostic value of peripheral CD4 + CD25+ T lymphocytes among early stage and triple negative breast cancer patients receiving dentritic cellscytokine induced killer cells infusion. Oncotarget. 2015;6(38):41350–41359.
- Donnem T, Hald SM, Paulsen EE, et al. Stromal CD8+ T-cell density – a promising supplement to TNM staging in non-small cell lung cancer. Clin Cancer Res. 2015;21(11):2635–2643.
- Al-Shibli KI, Donnem T, Al-Saad S, et al. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14(16):5220–5227.
- Fridman WH, Pagès F, Sautès-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306.
- Zhu JF, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112(5):1557–1569.
- Ivanova EA, Orekhov AN. T helper lymphocyte subsets and plasticity in autoimmunity and cancer: an overview. Biomed Res Int. 2015; 2015:327470.
- Gasper DJ, Tejera MM, Suresh M. CD4 T-cell memory generation and maintenance. Crit Rev Immunol. 2014;34(2):121–146.
- Boost KA, Sadik CD, Bachmann M, et al. IFN-gamma impairs release of IL-8 by IL-1beta-stimulated A549 lung carcinoma cells. BMC Cancer. 2008;(8):265.
- Nguyen AH, Berim IG, Agrawal DK. Cellular and molecular immunology of lung cancer: therapeutic implications. Expert Rev Clin Immunol. 2014;10(12):1711–1730.
- Vahl JM, Friedrich J, Mittler S, et al. Interleukin-10-regulated tumour tolerance in non-small cell lung cancer. Br J Cancer. 2017;117(11):1644–1655.
- Domagala-Kulawik J, Osinska I, Hoser G. Mechanisms of immune response regulation in lung cancer. Transl Lung Cancer Res. 2014;3(1):15–22.
- Li J, Wang Z, Mao K, et al. Clinical significance of serum T helper 1/T helper 2 cytokine shift in patients with non-small cell lung cancer. Oncol Lett. 2014;8(4):1682–1686.
- Pan B, Shen J, Cao J, et al. Interleukin-17 promotes angiogenesis by stimulating VEGF production of cancer cells via the STAT3/GIV signaling pathway in non-small-cell lung cancer. Sci Rep. 2015;(5):16053.
- Gu K, Li MM, Shen J, et al. Interhukin-17-induced EMT promotes lung cancer cell migration and invasion via NF-κB/ZEBl signal pathway. Am J Cancer Res. 2015;5(3):1169–1179.
- Hanagiri T, Fukumoto M, Koyanagi Y, et al. Regulatory T-cells and micrometastasis in lymph nodes of stage I NSCLC. Anticancer Res. 2014;34(12):7185–7190.
- Duan MC, Zhong XN, Liu GN, et al. The Treg/Th17 paradigm in lung cancer. J Immunol Res. 2014;2014:730380.
- Bold TD, Ernst JD. CD4+ T cell-dependent IFN-γ production by CD8+ effector T cells in Mycobacterium tuberculosis infection. J Immunol. 2012;189(5):2530–2536.
- Shaobin W, Yu X, Jiatian L, et al. Changes of CD4(+) T-cell subsets after radiofrequency ablation in lung cancer and its significance. J Can Res Ther. 2016;12(7):166–170.
- Higgins JP, Bernstein MB, Hodge JW. Enhancing immune responses to tumor-associated antigens[J]. Cancer Biol Ther. 2009;8(15):1440–1449.
- Kudo M. Immuno-oncology in hepatocellular carcinoma: 2017 Update. Oncology. 2017;93(1):147–159.
- Schmidt M, Hellwig B, Hammad S, et al. A comprehensive analysis of human gene expression profiles identifies stromal immunoglobulin κ C as a compatible prognostic marker in human solid tumors. Clin Cancer Res. 2012;18(9):2695–2703.
- Schmidt M, Böhm D, von TC, et al. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res. 2008; 68(13):5405–5413.
- Biragyn A, Lee-Chang C. A new paradigm for an old story: the role of regulatory B cells in cancer. Front Immunol. 2012;3:206.
- Saze Z, Schuler PJ, Hong CS, et al. Adenosine production by human B cells and B cell-mediated suppression of activated T cells. Blood. 2013;122(1):9–18.
- Ding ZH, Jiang L, Zhang K, et al. Study on the effect of LRFA under ultrasound guidance in immune function of patients with advanced hepatocellular carcinoma. J Clin Exp Med. 2017;16(15):44–47.
- Wu J, Lanier LL. Natural killer cells and cancer. Adv Cancer Res. 2003;90:127–156.
- Smyth MJ, Hayakawa Y, Takeda K, et al. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2(11):850–861.
- Hayakawa Y, Huntington ND, Nutt SL, et al. Functional subsets of mouse natural killer cells. Immunol Rev. 2006;214:47–55.
- Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9(5):495–502.
- Carrega P, Morandi B, Costa R, et al. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(-) cells and display an impaired capability to kill tumor cells. Cancer. 2008;112(4):863–875.
- Gotthardt D, Putz EM, Grundschober E, et al. STAT5 is a key regulator in NK cells and acts as a molecular switch from tumor surveillance to tumor promotion. Cancer Discov. 2016;6(4):414–429.
- Lavin Y, Kobayashi S, Leader A, et al. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell. 2017;169(4):750–765.
- Pasero C, Gravis G, Guerin M, et al. Inherent and tumor-driven immune tolerance in the prostate microenvironment impairs natural killer cell antitumor activity. Cancer Res. 2016;76(8):2153–2165.
- Mo Z, Lu H, Mo S, et al. Ultrasound-guided radiofrequency ablation enhances natural killer-mediated antitumor immunity against liver cancer. Oncol Lett. 2018;15(5):7014–7020.
- Gunther S, Ostheimer C, Stangl S, et al. Correlation of Hsp70 serum levels with gross tumor volume and composition of lymphocyte subpopulations in patients with squamous cell and adeno non-small cell lung cancer. Front Immunol. 2015;6:556.
- Dayanc BE, Beachy SH, Ostberg JR, et al. Dissecting the role of hyperthermia in natural killer cell mediated anti-tumor responses. Int J Hyperthermia. 2008;24(1):41–56.
- Bonecchi R, Locati M, Mantovani A. Chemokines and cancer: a fatal attraction. Cancer Cell. 2011;19(4):434–435.
- Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66(2):605–612.
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35.
- Coffelt SB, Hughes R, Lewis CE. Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochim Biophys Acta. 2009;1796(1):11–18.
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51.
- Guo DH, Mao ZP, Wang DY, et al. Regulation of radiofrequency ablation on polarization of type M2 macrophages in hepatic tissue of rats. J Xinxiang Med Uni. 2018;35(10):849–853.
- Sorrentino R, Morello S, Luciano A, et al. Plasmacytoid dendritic cells alter the antitumor activity of CpG-oligodeoxynucleotides in a mouse model of lung carcinoma. JI. 2010;185(8):4641–4650.
- Zhou Y, McEarchern JA, Howard E, et al. Dendritic cells efficiently acquire and present antigen derived from lung cancer cells and induce antigen-specific T-cell responses. Cancer Immunol Immunother. 2003;52(7):413–422.
- Gunzer M, Varga G, Grabbe S, et al. Dendritic cells and tumor immunity. Semin Immunol. 2001;13(5):291–302.
- Merad M, Sathe P, Helft J, et al. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604.
- Whiteside TL, Odoux C. Dendritic cell biology and cancer therapy. Cancer Immunol Immunother. 2004;53(3):240–248.
- Adam C, King S, Allgeier T, et al. DC-NK cell cross talk as a novel CD4+ T-cell-independent pathway for antitumor CTL induction. Blood. 2005;106(1):338–344.
- Herman S, Krenbek D, Klimas M, et al. Regulatory T cells form stable and long-lasting cell cluster with myeloid dendritic cells (DC). Int Immunol. 2012;24(7):417–426.
- Kassner N, Krueger M, Yagita H, et al. Cutting edge: plasmacytoid dendritic cells induce IL-10 production in T cells via the Delta-like-4/Notch axis. J Immunol. 2010;184(2):550–554.
- Yang GX, Lian ZX, Kikuchi K, et al. CD4- plasmacytoid dendritic cells (pDCs) migrate in lymph nodes by CpG inoculation and represent a potent functional subset of pDCs. J Immunol. 2005;174(6):3197–3203.
- Xu A, Zhang L, Yuan J, et al. TLR9 agonist enhances radiofrequency ablation-induced CTL responses, leading to the potent inhibition of primary tumor growth and lung metastasis. Cell Mol Immunol. 2019;16(10):820–832.
- Hogan SP, Rosenberg HF, Moqbel R, et al. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38(5):709–750.
- Caruso RA, Parisi A, Quattrocchi E, et al. Ultrastructural descriptions of heterotypic aggregation between eosinophils and tumor cells in human gastric carcinomas. Ultrastruct Pathol. 2011;35(4):145–149.
- Lee JJ, Jacobsen EA, McGarry MP, et al. Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy. 2010;40(4):563–575.
- Furbert-Harris PM, Hunter KA, VauIghn TR, et al. Eosinophils in a tri-cell multi-cellular tumor spheroid(MTS)/endothelium complex. Cell Mol Biol. 2003;49(7):1081–1088.
- Furbert-Harris PM, Parish-Gause D, Hunter KA, et al. Activated eosinophils upregulate the metastasis suppressor molecule E-cadherin on prostate tumor cells. Cell. Mol. Biol. (Noisy-le-Grand). 2003;49(7):1009–1016.
- Sakkal S, Miller S, Apostolopoulos V, et al. Eosinophils in cancer: favourable or unfavourable?. Curr Med Chem. 2016;23(7):650–666.
- Benatar T, Cao MY, Lee Y, et al. IL-17E, a proinflammatory cytokine, has antitumor efficacy against several tumor types in vivo. Cancer Immunol Immunother. 2010;59(6):805–817.
- Gatault S, Legrand F, Delbeke M, et al. Involvement of eosinophils in the anti-tumor response. Cancer Immunol Immunother. 2012;61(9):1527–1534.
- Takada K, Shimokawa M, Tanaka K, et al. Association between peripheral blood markers and immune-related factors on tumor cells in patients with resected primary lung adenocarcinoma. PLoS One. 2019;14(6):e0217991. Published 2019 Jun 4.
- Samoszuk M. Eosinophils and human cancer. Histol Histopathol. 1997;12(3):807–812.
- Anthony HM. Blood basophils in lung cancer. Br J Cancer. 1982;45(2):209–216.
- Zenan H, Zixiong L, Zhicheng Y, et al. Clinical prognostic evaluation of immunocytes in different molecular subtypes of breast cancer. J Cell Physiol. 2019;234(11):20584–20602.
- Stegner D, Dütting S, Nieswandt B. Mechanistic explanation for platelet contribution to cancer metastasis. Thromb Res. 2014;133(Suppl 2):S149–S157.
- Jurasz P, Alonso-Escolano D, Radomski MW. Platelet-cancer interactions: mechanisms and pharmacology of tumour cell-induced platelet aggregation. Br J Pharmacol. 2004;143(7):819–826.
- Sadallah S, Schmied L, Eken C, et al. Platelet-derived ectosomes reduce NK cell function. J Immunol. 2016;197(5):1663–1671.
- Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6)dju124.
- Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–590.
- Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9(2):237–249.
- Menter DG, Tucker SC, Kopetz S, et al. Platelets and cancer: a casual or causal relationship: revisited. Cancer Metastasis Rev. 2014;33(1):231–269.
- Ho-Tin-Noé B, Goerge T, Wagner DD. Platelets: guardians of tumor vasculature. Cancer Res. 2009;69(14):5623–5626.
- Tomita M, Shimizu T, Ayabe T, et al. Prognostic significance of the combined use of preoperative platelet count and serum carcinoembryonic antigen level in non-small-cell lung cancer. Gen Thorac Cardiovasc Surg. 2010;58(11):573–576.
- Mazmishvili K, Jayant K, Janikashvili N, et al. Study to evaluate the immunomodulatory effects of radiofrequency ablation compared to surgical resection for liver cancer. J Cancer. 2018;9(17):3187–3195.

