 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective: In magnetic particle hyperthermia, a promising least-invasive cancer treatment, malignant regions in proximity with magnetic nanoparticles undergo heat stress, while unavoidably surrounding healthy tissues may also suffer from heat either directly or indirectly by the induced eddy currents, due to the developed electric fields as well. Here, we propose a facile upgrade of a typical magnetic particle hyperthermia protocol, to selectively mitigate eddy currents' heating without compromising the beneficial role of heating in malignant regions.
Method: The key idea is to apply the external magnetic field intermittently (in an ON/OFF pulse mode), instead of the continuous field mode typically applied. The parameters of the intermittent field mode, such as time intervals (ON time: 25-100 s, OFF time: 50-200 s, Duty Cycle:16-100%) and field amplitude (30-70 mT) are optimized based on evaluation on healthy tissue and cancer tissue phantoms. The goal is to sustain in cancer tissue phantom the maximum temperature increase (preferably within 4-8°C above body temperature of 37°C), while in the healthy tissue phantom temperature variation is suppressed far below the 4°C dictating the eddy current mitigation.
Results: Optimum conditions of intermittent field (ON/OFF: 50/100 in s, Duty Cycle: 33%, magnetic field: 45mT) are then examined in ex-vivo samples verifying the successful suppression of eddy currents. Simultaneously, a well-elaborated theoretical approach provides a rapid calculation of temperature increase and, furthermore, the ability to quickly simulate a variety of duty cycle times and field controls may save experimental time.
Conclusion: Eventually, the application of an intermittent field mode in a magnetic particle hyperthermia protocol, succeeds in eddy current mitigation in surrounding tissues and allows for the application of larger field amplitudes that may augment hyperthermia efficiency without objecting typical biomedical applicability field constraints such as Brezovich criterion.
Introduction
Cancer is still the second largest cause of death worldwide with 9.6 million deaths in 2018 [Citation1]. Magnetic particle hyperthermia (MPH) is an adjunct cancer treatment based on biomedical nanotechnology that complements the established therapies (chemotherapy, radiation therapy) increasing the therapeutic efficacy of the latter and at the same time reducing their side effects [Citation2–4].
The combination of magnetic fields (MF) and magnetic nanoparticles (MNPs) is currently implemented in diagnosis (Magnetic Resonance Imaging, MRI) [Citation5], in therapy (drug deposition, magnetic hyperthermia) and for handling cancerous tissues (biolabeling, bioseparation) [Citation6]. MNPs can penetrate biological tissues, since they easily cross many biological barriers and are considered relatively safe for humans [Citation7]. Accordingly, MPH has been proposed in a clinical trial together with radiation therapy in patients with recurrent glioblastoma, reducing radiation session duration by 50% and increasing life expectancy by 100% [Citation8,Citation9].
MPH is based on the heat production by MNPs (expressed by the Specific Loss Power, SLP, in W/g) under alternating magnetic field (AMF) exposure in the radio frequency range (100–1000 kHz) [Citation10], due to magnetic hysteresis losses and/or relaxation mechanisms [Citation11]. The result may be gradual apoptosis or even necrosis of cancer cells that are subjected to heat shocks within the hyperthermia window of 41–45 °C, while surrounding healthy tissues typically sustain such temperature ranges [Citation12–14].
The major challenges of MPH for its widespread clinical use against cancer are: production of the appropriate AMF, accurate tissue thermometry, long-term biocompatible behavior of the MNPs, spatially homogeneous distribution of the AMF and MNPs in the tumor, the effect of biological matter on the MNPs heating capacity and finally tolerance and/or safety of patient exposure to AMF, directly related to eddy current side effects [Citation15–17]. MPH clinical studies are already under way in Europe in conjunction with radiotherapy [Citation8,Citation18,Citation19], while recently, a new magnetic hyperthermia center specializing in brain tumors opened at the Independent Public Clinical Hospital No. 4 in Lublin, Poland [Citation20].
Eddy currents are induced by the AMF and it has been shown that they limit MPH treatment efficacy in clinical trials [Citation15–17]. The product Ho×f, typically known as the Brezovich criterion (where Ho is the amplitude and f is the frequency of the magnetic field) provides a safety threshold for magnetic field driven therapy. It was initially proposed in 1988 [Citation21] as the upper limitation for major discomfort in patients undergoing AC magnetic field driven therapies. For micron-sized magnetic implants, it was reported as Ho×f = 4.85 × 108 A m−1 s−1, but since, magnetic carriers are shrinking in size, down to nanoscale elements, nowadays it seems rather stringent, and requires reconsideration [Citation22]. As Ho×f increases, the desired heating rate in cancer tumor regions augments accordingly, yet such increase is directly reflected to the heating of surrounding healthy tissues, due to eddy currents, a direct side-effect. Thus, application of MPH should follow certain field constraints, unless the effect of eddy currents is safely mitigated. Hergt et al. [Citation23] revisited the Brezovich criterion and proposed one order of magnitude greater value for the limit (Ho×f ≤ 5 × 109 A m−1 s−1), while Bellizzi et al. [Citation24,Citation25] recently used a 3 D realistic human head model and proposed, numerically calculating, an optimization criterion by examining its reliability and feasibility. It is worthwhile mentioning that in these cases [Citation24,Citation25] the Ho×f product was two orders of magnitude larger than the Brezovich criterion.
It is widely accepted that to prevent unwanted heating in normal tissue the field strength frequency product H0×f should be limited. In MPH treatment where AMF coils are employed for MNPs excitation, another crucial parameter that affects the strength of eddy currents is the radial position r within the coil in which the phantom (or the tissue) exists and defines the radius of the eddy current path within the tissue. The phantom can be placed at any point inside the coil along the radial direction from 0 to R, where R is the radius of the coil, while the absorbed power density at each point within the tissue, due to eddy currents, changes as a function of r2 [Citation26]. Thus, for large tissues or small coils, where r ≈ R (i.e., the location of the tissue is close to coil surface), the undesirable eddy currents’ heating is exacerbated. To sum up, the magnitude of eddy currents depends not only on the AMF source (H0 and f), but also on the size of the tissue exposed to the field. However, according to Herrera et al. [Citation27] the magnitude of eddy currents also depends on the size of the nanoparticles. More specifically, they showed that small-sized MNPs (d < 20 nm) as in our case have negligible appearance of eddy currents in contrast to larger-sized MNPs, wherein the occurrence of eddy currents is significant.
Alternative approaches to circumvent eddy current constraints during magnetically driven theranostics schemes are on the way. For example, Ivkov et al. [Citation28] proposed the application of ON/OFF cycles, i.e., an intermittent exposure mode with an AMF of 153 kHz frequency and 40–130 mT amplitude aiming to reduce the power of eddy currents in mice through heat dispersion and dissipation during the OFF-time interval. They have shown that reducing unwanted heating of healthy tissues is not only a function of the amplitude of the applied field and the timing of the ON/OFF intervals, but also significantly depends on the relative duration of the ON and OFF time, which is called ‘duty cycle’ [Citation29]. Another attempt to introduce clinically applicable techniques in order to address the heating issue of healthy tissues is reported by Stigliano et al. [Citation26]. Firstly, the hyperthermia coil, i.e., a large electric field region, is selectively displaced away from healthy tissues. Secondly, the coil is in constant motion with respect to the tissue under treatment, so that successive heat losses due to eddy currents are dispersed in a larger tissue volume. Studies on tissue models have shown that displacement and motion reduce the maximum temperature by 74% and 19% in simulation and 77% and 33% experimentally, respectively [Citation26]. Moreover, Nieskoski and Trembly [Citation30] proposed an optimized AMF single-coil instead of a Helmholtz coil, to optimize the heating of the MNPs which is limited by the current-induced heating. They determined a power ratio which is the ratio of MNPs’ induced heat to surface eddy current heating in order to compare the geometries of the coil and showed that it depends on the target depth of MNPs. Also, they demonstrated that by increasing the power ratio, the concentration of MNPs necessary for therapeutic efficacy in tissue, decreases [Citation30]. Other reports [Citation31,Citation32] have also suggested improved design of AMF coils, while Kumar et al. [Citation33] examined the performance of skin surface cooling application to reduce maximum temperature, due to eddy currents. They used a water jacket during small animals’ exposure to high AMF amplitude which almost had no interaction with magnetic field and did not affect its desired amplitude [Citation33].
In this manuscript, we evaluate the role of an intermittently applied AMF on the thermal response of iron oxide (Fe3O4) MNPs with respect to field parameters and in direct comparison with the corresponding continuously applied AMF. This work aims to reduce the heating of healthy tissues, due to eddy currents, without compromising the beneficial role of MPH in cancerous cells. Thus, applied field amplitude Ho may augment, even surpassing the clinically acceptable constraint of Brezovich criterion, without worrying for side-effect of regional potential heating of nonmalignant sites. This may be achieved by intermittent field application, multiple ON/OFFs, within an optimized time window, in phantom and ex vivo samples, where heating (i.e., temperature increase) is restricted to selected specific sites in excellent agreement with the corresponding simulated MPH sequences.
Methods
Sample preparation
MNPs with a mean diameter of 10 nm were prepared by aqueous co-precipitation of ferric and ferrous salts in alkaline conditions [Citation34]. Details on sample synthesis and their physical characterization (Supplementary Figure S1) are presented in supporting information.
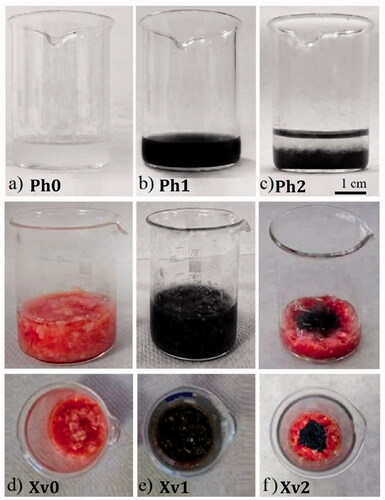
To proceed with phantom sample preparation, we followed the simple idea of gels, specifically those made of agarose, which are routinely used as phantom models, while they comprise the only transparent porous materials which successfully simulate animal tissues [Citation35]. More specifically, 3–4% agarose gel content mimics the microstructure of hard tissues [Citation36], while lower content gels have porosities similar to soft tissues such as the human brain [Citation35]. Accordingly, three different phantoms based on agarose solutions were synthesized: (1) healthy tissue phantom: Ph0, (2) cancer tissue phantom Ph1, and (3) two layer tumor–healthy tissue interface Ph2, composed of a cancer tissue phantom layer (bottom) and a healthy phantom tissue layer (top) as shown in , respectively. Accordingly, three ex vivo samples based on veal tissue were prepared: (1) veal tissue sample, named Xv0 (), which is the reference one, (2) veal tissue sample with MNPs, named Xv1 (), in two different concentrations 4 mg/mL (Xv1a) and 8 mg/mL (Xv1b), and (3) two-region tumor–healthy tissue interface named Xv2 (), where the MNPs (8 mg/mL) were placed in the center of the beaker and dark area within the sample’s center represents tumor region, while the surrounding red colored area healthy tissue. More details on phantom sample fabrication are given in supporting information section synthesis and properties.
Figure 1. Samples under study (a) Ph0: Reference: agarose solution corresponding to healthy tissue phantom, (b) Ph1: agarose with MNPs, corresponding to cancer tissue phantom (c) Ph2: Tumor–healthy tissue interface, (d) Xv0: Reference: Veal tissue sample corresponding to healthy tissue, (e) Xv1: Veal tissue sample with MNPs (Xv1a: 4 mg/mL, Xv1b: 8 mg/mL) corresponding to cancer tissue, (f) Xv2: Tumor (central region)–healthy (peripheral region) tissue interface.
Magnetic particle hyperthermia experiment
Magnetic particle hyperthermia was carried out in a commercial AMF generator (1.2 kW Ambrell Easyheat 0112) under the frequency of 375 kHz and variable magnetic field amplitude of 30–70 mT. Sample’s temperature was recorded in 0.4 s intervals for ample time (i.e., 900 s) with an Optic Fiber Temperature sensor immersed in the central region of sample under study. To start with, we record the MPH reference, which is a typical experimental sequence performed in the MNPs aqueous solution with the same concentration and under the same field conditions followed hereafter. Schematics of experimental protocol (Supplementary Figure S2) now appear in supporting information magnetic particle hyperthermia section together with corresponding details on measuring protocol. A representative graph of aqueous solution MNPs heating efficiency is depicted in Supplementary Figure S3 at concentration 4 mg/mL and 60 mT/375 kHz, showing the facile entrance within the hyperthermia window at the first 50 s, thus yielding a technologically exploitable SLP value of 546 W/g. Error bars in values of sample’s temperature increase (ΔΤ), resulting from MPH experiments, are estimated by uncertainty assessment that arises from the temperature recording method, such as the resolution and sensitivity of optic fiber, and the not ideally uniform temperature distribution within the sample [Citation37].
Duty cycle
The crucial factor in the intermittent mode of applying the AMF is the so-called duty cycle, which correlates the field ON/OFF time durations, and is expressed by the following equation:
(1)
(1)
where ‘Field ON time (s)+Field OFF time (s)’ is defined as the total duration time of one operation cycle (period of an operation cycle). gives the duty cycle (%) values and the corresponding ON/OFF field duration in s.
Table 1. Duty cycles and the corresponding field ON/OFF values (in s).
In all samples, the intermittent modes with the duty cycles of were examined, while continuous operation was serving as the reference of a typical hyperthermia sequence. Finally, the total duration of the phantom study measurement was 30 min (15 min for the heating phase and 15 min for the cooling phase, when the sample returns to its initial temperature in the absence of a field). In the case of the ex vivo samples these intervals were doubled to 60 min (30 min heating phase, 30 min cooling phase) in order to provide a measurable signal.
Theoretical approach
The objective of the theoretical approach was the evaluation and validation of the experimental optimum conditions by obtaining the numerical temperature increase curves and comparing them with the corresponding experimental ones. So, a simple algorithm, based on the work by Neufeld et al. [Citation38], was chosen for the fast estimation of the temperature development in the case of intermittent external field application. Thus, we follow a safety assessment model to predict temperature increase during MRI. The model is based on theoretical considerations like relating peak temperatures in the presence or absence of local thermoregulation, as well as on data extracted from simulations involving anatomical models to determine a characteristic time constant τ which is a characteristic parameter of the tissue and ranges between 100 and 600 s, depending on the tissue type and the temperature increase during MPH treatment [Citation31]. The main assumption of Neufeld et al. is that the temperature increase exhibits exponential behavior and eventually tends to equilibrium. This increase is given by applying the following analytical relationship:
(2)
(2)
where
is the temperature increase at a specific time ti,
is the temperature increase at the next time interval,
is the time interval (step),
is the maximum temperature achieved during AMF application and τ is the time constant. In order to extract the temperature sequence in the case of intermittent AMF application, we modified EquationEquation (2)
(2)
(2) by means of the following expression:
(3)
(3)
where κ is a constant that equals to 1, when the AMF is ON and to 0, when the AMF is OFF, resulting to the local heating and cooling of the phantom, respectively.
To start with, we performed a fitting function based on EquationEquation (2)(2)
(2) to the experimental temperature increase curve for the case of continuously applied AMF to estimate the maximum temperature
for a specific duration of time, and the corresponding time constant τ. The time step
was set to 0.1 s. This method of limited time interval selecting ensures that the fitting parameters are accompanied with a reasonably small standard deviation [Citation39].
After substituting the estimated and τ values in EquationEquation (3)
(3)
(3) we got the temporal evolution of temperature during the intermittent application of the AMF. Initially, temperature increase
is zero. At each time step,
is updated by EquationEquation (3)
(3)
(3) offering a rapid calculation of
and, furthermore, the ability to quickly simulate a large number of different duty cycles. The above technique was applied in both Ph1 and Ph2 phantoms and also in Xv1 and Xv2 samples.
In order to outline the validity of our approach, we employed a combined electromagnetic-thermal model based on COMSOL Multiphysics simulations of the MPH set up and phantom samples. This model is analyzed and presented in the supporting information of the manuscript, where the numerical results of electromagnetic and heat transfer simulations are shown in Supplementary Figures S6–S10. The geometry of the model, illustrated in Supplementary Figure S6, is following the experimental MPH apparatus. Simulations were conducted for two samples. The first is the healthy tissue phantom (Ph0) and the second is the cancer tissue phantom (Ph1). Throughout the COMSOL simulations, we utilized a concise model that offers a combination of the heat transfer equations with Maxwell’s time-harmonic equations, the definition of dynamic heating and electromagnetic boundary conditions, and the employment of a robust, fully coupled solver ensuring: a) an accurate estimation of the magnetic field shown in Supplementary Figures S7 and S8, b) a detailed description of the heating process shown in Supplementary Figure S9 revealing the not ideally homogeneous temperature spatial distribution within the sample and c) a precise estimation of the temperature increase curves for both Ph0 and Ph1 when continuous or intermittent AMFs are applied as depicted in Supplementary Figure S10.
In Supplementary Figure S10(a), the validation of EquationEquation (2)(2)
(2) (continuously applied AMF) is achieved after the comparison of the temperature increase curves obtained by this approximation with the ones obtained numerically after the full simulation with COMSOL. This comparison is employed for both Ph0 and Ph1 phantoms and the maximum temperature increase deviation between the two methods (approximation and simulation) is found equal to ΔΤmax ∼0.5 °C. This small deviation clearly illustrates the validity of EquationEquation (2)
(2)
(2) . Similarly, Supplementary Figure S10(b) depicts the validation of EquationEquation (3)
(3)
(3) (intermittently applied AMF), for Ph0 and Ph1 phantoms. The maximum temperature increase deviation between this equation and the numerical solution is even lower than in the case of continuously applied AMF and equals to ΔΤmax ∼0.3 °C, a value that safely validates EquationEquation (3)
(3)
(3) .
The whole procedure of the theoretical approach followed is depicted in the flow chart shown in Supplementary Figure S5 of supporting information.
Results and discussion
The proof of principle was verified in the phantom study, where the parameters applied were optimized, and subsequently evaluated in ex vivo samples. As far as we know, the in vivo and ex vivo studies, conditions and effect may differ dramatically because of biological interactions with the nanoparticles and also perfusion cannot be neglected in in vivo models, as in ex vivo [Citation40]. One of the major challenges is to reduce heating, due to eddy currents during MPH. Thus, we demonstrate agarose models namely phantoms (Ph0, Ph1, Ph2) and ex vivo models in veal tissues (Xv0, Xv1 and Xv2) in order to study the impact of operation cycle, duty cycle and magnetic field amplitude on field-induced eddy currents. Finally, a theoretical approach was undertaken to check the thermal response, in order to compare our experimental results in both models. Consequently, the results of this approach may be implemented in any stage of biomedical application as means of fine tuning (augmenting) MPH heating efficiency.
Phantom study
The aim of this study was to find the optimum conditions, in which the heating of healthy tissues, due to eddy currents is reduced, when an intermittent AMF is applied. At the same time, the appropriate MNPs’ heat release must be maintained at such levels to guarantee hyperthermia temperatures. For these reasons three different agarose phantoms (Ph0, Ph1, Ph2) were used. Initially, we wanted to determine the right operation cycle, then the appropriate duty cycle and, finally, the proper magnetic field amplitude for Ph0 and Ph1 phantoms. From these three studies, the optimal conditions were verified via a theoretical approach and tested in the Ph2 phantom. Τhe latter phantom experiment took place to study how healthy tissues may be affected by surrounding heating imposed by MNPs on cancer tissues.
Operation cycle
Firstly, it was crucial to study the operation cycle in phantoms by varying the duration of time and applying an intermittent mode ON/OFF (25/50, 50/100, 75/150, 100/200 in s), while the duty cycle remained stable at 33%. shows the ΔΤ curves as a function of time for Ph0 and Ph1 phantoms. The black line corresponds to continuous operation of AMF (ON/OFF: 900/0). For the case of intermittent mode, ΔΤ coincides with the maximum temperature increase observed, i.e., the highest ΔΤ peak. Field discontinuity is readily visualized with the zigzag form of the heating curves and is illustrated by the different colored lines. In all cases, the magnetic field and the frequency were kept constant at 60 mT and 375 kHz, respectively. Also, in order to have a better understanding of the maximum ΔΤ for each experiment in the two phantoms is summarized in .
Figure 2. Experimental ΔΤ curves for all operation cycles used in (a) Ph0: reference: healthy tissue and (b) Ph1: cancer tissue, phantoms. The black line represents the continuous mode of AMF ON/OFF: 900/0 (in s) and purple, red, blue and dark cyan curves depict the intermittent mode ON/OFF: 25/50, 50/100, 75/150, 100/200 (in s), respectively. The applied experimental conditions were: AMF amplitude: 60 mT/375 kHz and duty cycle: 33%; the shaded bands illustrate the hyperthermia window (ΔΤ = 4–8 °C).
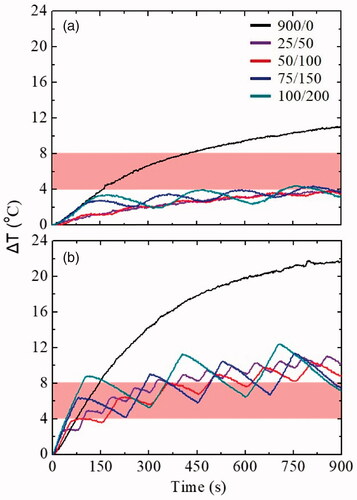
Table 2. Operation cycle experiments in phantoms (Ph0: reference: healthy tissue, Ph1: cancer tissue).
As it is obvious, considering a body temperature equal to 37 °C, a temperature increase of 4–8 °C (shaded area with red color) appears sufficient to enter the MPH window (41–45 °C) a prerequisite for subsequent clinical application. In the curves shown in , the measured ΔΤ in the Ph0 phantom is a result of the eddy currents, while in the Ph1 phantom the increase is also due to the presence of the MNPs. Observing , for both phantoms the period of each operation cycle was not a critical factor as, differences between ΔΤ values were not significant. On the contrary, the intermittent application of the magnetic field as compared to continuous application led to a smaller ΔΤ (reduction of approximately 60%) not only in the Ph0, but also in the Ph1 phantom. In Ph1 the goal was to achieve the maximum possible reduction of ΔΤ due to eddy currents, while at the same time, maintaining a satisfactory heat release from the MNPs, so that the treatment of hyperthermia could be effectively applied. Based on this principle, the time duration of one operation cycle ON/OFF:50/100 (in s) was chosen as the optimum in order to move on with the duty cycle study.
Duty cycle
Another important parameter which can significantly influence the heating rate is the duty cycle. Thus, the ‘ON’ time was kept constant at 50 s, according to the preceding results, while the time ‘OFF’ was varied from 50 to 250 s. shows the ΔΤ curves of intermittently applied AMF with different duty cycles, resulting from varying the ‘OFF’ time. In all cases, the magnetic field and the frequency were kept constant at 60 mT and 375 kHz, respectively. For a better understanding of the curves depicted in , the maximum ΔΤ for each duty cycle experiment in both phantoms is presented in .
Figure 3. ΔΤ curves of each duty cycle experiment in (a) Ph0: reference: healthy tissue and (b) Ph1: cancer tissue phantoms. The black, red, blue, dark cyan and pink curves depict the intermittent mode ON/OFF: 50/50, 50/100, 50/150, 50/200 and 50/250 (in s), respectively. The applied experimental conditions were: AMF: 60 mT/375 kHz and the hyperthermia window is illustrated with a shaded band.
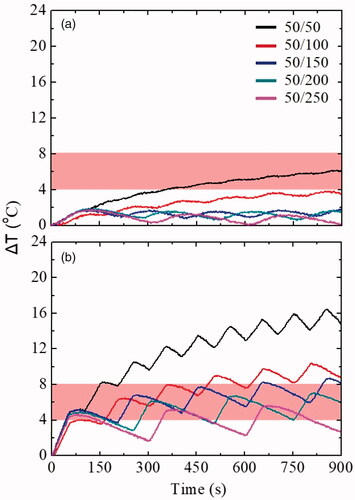
Table 3. Duty cycle experiments in a) Ph0: reference: healthy tissue and b) Ph1: cancer tissue phantoms.
Comparing , there are differences between the Ph0 and Ph1 phantoms. In general, the ΔΤ of Ph0 did not enter the hyperthermia window except of 50% duty cycle, i.e., ON/OFF: 50/50 (in s). On the contrary, the ΔΤ in all duty cycles of Ph1 reached it, and this was due to the presence of MNPs. The 33% duty cycle (red line) was considered as the optimum one, since it maintained the ΔΤ at a satisfactory level (10.4 °C, corresponding to a clinical body temperature of 47.4 °C [Citation41]). Despite the relative high ΔΤ, surpassing the MPH window, such a duty cycle may be exploited clinically, since ΔΤ naturally undergo different decrease levels due to perfusion [Citation42,Citation43].
Magnetic field amplitude
Τhe next step of this study was the selection of the optimal magnetic field by examining four different AMF amplitudes: 30, 45, 60 and 70 mT. The following experiments were carried out using the optimal operation cycle and duty cycle conditions (ON/OFF: 50/100 in s and 33%, respectively). displays the ΔΤ curves of the two optimum magnetic field amplitude values (45 mT and 60 mT at constant frequency of 375 kHz) in Ph0 and Ph1. Moreover, ΔΤ curves for 30 mT and 70 mT are presented in Supplementary Figure S4 (supporting information). provides the maximum ΔΤ for each magnetic field amplitude experiment in both phantoms.
Figure 4. ΔΤ curves of the two optimum magnetic field amplitude values [(a) 45 mT/375 kHz, (b) 60 mT/375 kHz] in Ph0 (reference: healthy tissue) and Ph1 (cancer tissue) phantoms. Dark cyan and red curves represent the intermittent mode ON/OFF: 50/100 (in s) in Ph1 and Ph0, respectively, while blue and black curves illustrate the continuous mode ON/OFF: 900/0 (in s) in Ph1 and Ph0, respectively. Shaded bands denote the hyperthermia window.
![Figure 4. ΔΤ curves of the two optimum magnetic field amplitude values [(a) 45 mT/375 kHz, (b) 60 mT/375 kHz] in Ph0 (reference: healthy tissue) and Ph1 (cancer tissue) phantoms. Dark cyan and red curves represent the intermittent mode ON/OFF: 50/100 (in s) in Ph1 and Ph0, respectively, while blue and black curves illustrate the continuous mode ON/OFF: 900/0 (in s) in Ph1 and Ph0, respectively. Shaded bands denote the hyperthermia window.](/cms/asset/9c61ef08-7b4d-46b4-9cdb-0f710cdac906/ihyt_a_1899310_f0004_c.jpg)
Table 4. Magnetic field experiments in a) Ph0: reference: healthy tissue and b) Ph1: cancer tissue, phantoms.
As expected, an increase in magnetic field amplitude results in the increase of the maximum temperature reached. From at 30 mT, the ΔΤ of Ph0 phantom decreased considerably, the heating due to eddy currents also reduced almost to zero. Nevertheless, the corresponding value in Ph1 phantom did not managed to reach to the required ΔΤ value to enter the hyperthermia window. At 70 mT except that the field is rather high, during continuous mode AMF application resulting to excessive ΔΤ values (>70 °C), the Ph0 and Ph1 agarose phantoms began to decompose. So, these two magnetic fields amplitudes were rejected for subsequent evaluation. On the contrary, the applied magnetic field amplitude of 45 mT suppresses ΔΤ from 9.1 to 2.9 °C in Ph0 (healthy tissue phantom), when the field mode was changed from continuous to intermittent. Accordingly, in Ph1 (cancer tissue phantom), ΔΤ decreased from 17.9 to 7.2 °C yet sustained within the MPH window. In general, ΔΤ ranging from 4 to 8 °C outlines the MPH window by considering an initial body temperature equal to 37 °C. Thus, the ΔΤ of 7.2 °C would result to temperature to 44.2 °C, rendering this MPH, effective. Similar features were observed also for the 60 mT, where ΔΤ in Ph0 and Ph1 were 3.8 and 10.4 °C, respectively.
From , we see that ΔΤ of Ph0 (healthy tissue phantom) was 2.9 and 3.8 °C for 45 and 60 mT, respectively. Thus, 45 mT leads to a larger reduction of eddy currents’ heating. These values compare relatively well with the Ph0 reference ΔΤ for continuous mode of 30 mT which is 3.4 °C. On the contrary, the therapeutic capacity of the MNPs was enhanced, since the Ph1 (cancer tissue phantom) ΔΤ of 5.3 °C by the 30 mT continuous mode was much lower than the corresponding ΔΤs of 7.2 °C and 10.4 °C observed when 45 mT and 60 mT were applied, respectively. Thus, we may surmise that doubling the field from 30 to 60 mT the ΔΤ in Ph1 (cancer tissue phantom) also doubled (from 5.3 to 10.4 °C), without increasing the side effects on healthy tissues, ΔΤ in Ph0 increased from 3.4 to 3.8 °C. From the above analysis it is clear that with the utilization of intermittent exposure AMF, it is possible to exceed the limit set by Brezovich (Ho×f was equal to 1.35 × 1010 A m−1 s−1 when Ho=45 mT and 1.79 × 1010 A m−1 s−1 when Ho=60 mT) and yet to attain milder side effects and efficient treatment. More specifically, by using an intermittent mode AMF we managed to increase the applied AMF amplitude and at the same time to achieve a reduction in eddy currents’ heating and an increase in the therapeutic capacity of the MNPs.
Theoretical approach
After the experimental results in phantoms Ph0 and Ph1, a theoretical approach was attempted in order to verify the choice of the optimal conditions (magnetic field amplitude, duty cycle) by estimating numerically the temperature rise curves. Following the framework, described in methods, the curves for both cases of continuous and intermittent magnetic field mode were occurred. depicts the theoretical and experimental curves for Ph0 and Ph1 phantoms in the case of continuous (900/0 in s) and intermittent mode (50/100 in s) for the two best magnetic field amplitudes (45 and 60 mT). Experimental (blue, dark cyan) and theoretical (black, red) curves were found to be in good agreement for both phantoms and magnetic field conditions.
Figure 5. Theoretical approach of Ph0: reference: healthy tissue phantom (a) 45 mT and (c) 60 mT under continuous and intermittent mode, and Ph1: cancer tissue phantom (b) 45 mT and (d) 60 mT under continuous and intermittent mode. In continuously applied AMF mode theoretical and experimental curves were in good agreement. In the case of intermittent mode, the peaks of theoretical and experimental curves were very close for both Ph0 and Ph1 phantoms. The hyperthermia window is depicted with the shaded bands.
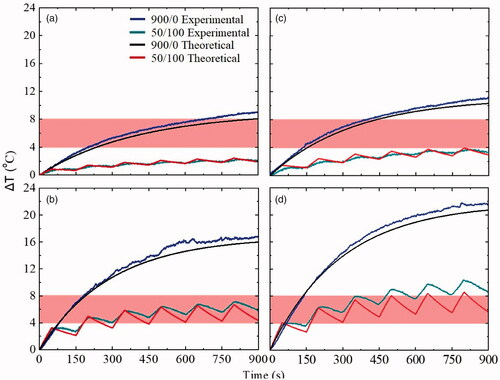
For the continuous mode and field amplitudes of 45 mT and 60 mT and after 900 s of treatment, a comparison was made between theoretical, obtained from EquationEquation (2)(2)
(2) , and experimental temporal evolution of the ΔΤ in both phantoms (Ph0 and Ph1). At 45 mT the estimated time constant τ and maximum temperature
were estimated at 580 s and 9 °C, respectively for Ph0, and 495 s and 16 °C, respectively for Ph1. At 60 mT the ΔΤ was evaluated to be 10.5 °C and 20.6 °C for Ph0 and Ph1, respectively, with the corresponding time constants being equal to 533 and 456 s, respectively. In the case of intermittent mode magnetic field, the ΔΤ curves in Ph0 and Ph1 were obtained theoretically by substituting in EquationEquation (3)
(3)
(3) the values of time constant and
obtained for the continuous mode. The theoretical curves calculated by EquationEquation (3)
(3)
(3) were in good agreement with the corresponding experimental curves. This outcome verifies that there is a reliable way of numerically predicting the temporal evolution of temperature in the intermittent mode, when it is possible to assess the respective ΔΤ curves for the continuous mode. In this way, such an approach may not only evaluate an experimental sequence but gain significant experimental time to quickly optimize treatment protocol parameters of the intermittent mode.
Tumor–healthy tissue system interface (Ph2)
The above experimental and theoretical results were obtained inside homogeneous phantoms mimicking either healthy or MNPs loaded tumor tissue. It is important to examine how healthy tissues are affected by local heating of MNPs after magnetic field application. Thus, a phantom of the tumor–healthy tissue interface (Ph2) was examined in two different operating modes, namely continuous mode (900/0 in s) and intermittent mode with 33% duty cycle (50/100 in s). The magnetic field and the frequency were kept constant at 45 mT and 375 kHz, for both operating modes. Measurements were taken at three different points (P.1, P.2, P.3) as shown in . More specifically, P.1 was in the surface of the healthy tissue layer, P.2 was at the interface of the two layers and P.3 was in the cancer tissue layer. illustrates the ΔΤ curves taken at the three aforementioned points. For the continuous mode (900/0 in s) of P.1, P.2, P.3 the curves are depicted with dark cyan, blue and pink, respectively. Also, for the intermittent mode (50/100 in s) of P.1, P.2, P.3 the curves are portrayed with red, black and olive, respectively. Aditionally, summarizes the results obtained from the Ph2 phantom at the three aforementioned positions (P.1, P.2, P.3).
Figure 6. (a) The three measuring points (P.1, P.2, P.3) inside the Ph2 phantom, (b) ΔΤ curves measured at these points (P.1, P.2, P.3) for continuous and intermittent operating modes. Hyperthermia window is denoted with a shaded band.
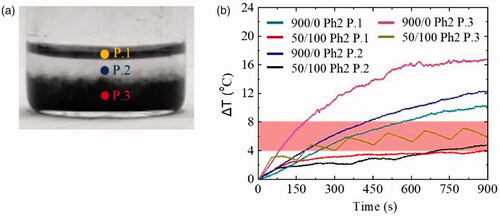
Table 5. Continuous and intermittent mode magnetic fields experiments in Ph2 phantom.
shows that there was a ΔT increase in all positions (P.1, P.2, P.3) for the continuous application of AMF. For the intermittent exposure with a duty cycle of 33% (50/100 in s), the measurements at positions P.1 and P.2 were very different from all the experiments performed above. Their behavior was neither that of the continuous one, nor that of the intermittent mode magnetic field. In fact, in position P.2 (black curve) the peaks that appeared were not at defined positions and distances from each other as they were in our previous experiments. In position P.1 (red curve) the peaks were almost non-existent. Finally, comparing P.3 with P.1 and P.2, ΔΤ increased faster in both operating modes, a phenomenon that is clearly due to the presence of MNPs.
Results collected in demonstrate clearly the proof of principle of this study since at P.1 (healthy tissue phantom) the ΔΤ due to eddy currents decreased substantially, from 10.5 °C to 4.0 °C by exchanging the field mode from continuous to intermittent one. On the other hand, at P.3 (cancer tissue phantom), the ΔΤ of 7.2 °C resided within the hyperthermia temperature window contrary to the corresponding much greater decrease of 17.0 °C in the continuous field mode. The most important finding from the was that the ΔΤ in intermittent mode at P.2 was minimal (from 4.0 to 4.9 °C), so we may surmise that the healthy–cancer tissue interface is not practically affected by the released heat.
Ex vivo study
Until now, only a few studies [Citation44,Citation45] have assessed MNPs thermal behavior inside biological tissues to achieve the translation from laboratory setups to clinical application. In order to proceed further, phantom study was followed by an ex vivo study. In this stage, experiments on veal tissues were conducted to determine whether the previous optimum operating parameters (ON/OFF: 50/100 in s, duty cycle: 33%, magnetic field: 45 mT) were applicable in ex vivo conditions. Thus, we examined: (a) a reference veal tissue in order to simulate healthy tissue, Xv0, (b) veal tissue with MNPs to simulate cancer tissue, Xv1, (c) tumor–healthy tissue interface, Xv2. Finally, a theoretical approach took place only in the Xv2 system in order to be compared with our experimental results.
Application of optimum conditions
After finding the ideal operating parameters from the agarose phantoms, the application of them in ex vivo tissues came next. is intended to represent the ΔΤ curves in healthy veal tissue, Xv0, and cancer tissue, Xv1. Two different concentration values of MNPs were utilized for the Xv1 tissue leading to Xv1a, which contained 4 mg/mL, and Xv1b, containing 8 mg/mL to acquire measurable signals in the ex vivo studies in accordance with in vivo studies [Citation46]. It should be noted that the magnetic field and the frequency were kept constant at 45 mT and 375 kHz, respectively. summarizes the experimental results obtained from the ΔΤ curves.
Figure 7. ΔΤ curves of continuous and intermittent operating modes in ex vivo veal tissues without MNPs (Xv0) and with MNPs (a) Xv1: 4 mg/mL MNPs, and (b) Xv1: 8 mg/mL MNPs. The hyperthermia window is illustrated by the shaded bands.
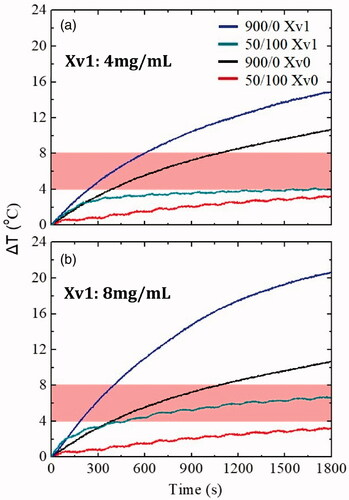
Table 6. Continuous and intermittent mode magnetic fields in ex vivo veal tissues (without MNPs: Xv0, with MNPs: Xv1).
In general, a similar behavior with the Ph0 healthy tissue phantom was observed. However, considering the first results, the duration time for the ex vivo experiments was doubled in order to raise the ΔΤ and enter the hyperthermia window, since tissue-like samples were involved. shows that in the intermittent operating mode and the tissue with 4 mg/mL, ΔΤ approached the hyperthermia window in the last seconds (1200–1800s). On the contrary, in the case of 8 mg/mL, this happened after the first 350 s (350–1800s).
From , following the same selection criteria as for the other operating parameters, the concentration of 8 mg/mL was chosen as the most appropriate one. Not only a significant ΔΤ decrease in temperature due to eddy currents was observed, but also a sufficiently high ΔΤ for hyperthermia treatment was maintained via the intermittent field mode application.
Ex vivo tumor–healthy tissue interface system
The last step of this work involved an ex vivo system that was designed in such a way that the core of the sample represents a putative tumor (Xv2 P.1), i.e., tissue loaded with MNPs, surrounded by pure veal tissue that would imitate the healthy tissues (Xv2 P.2). The purpose of this system was to investigate not only the response of this particular system to the different modes of AMF application, but also to examine if healthy tissues (Xv2 P.2) are affected by the neighboring heating of the MNPs (Xv2 P.1) located in the tumor area. Ιt should be mentioned that in this experiment, the magnetic field and the frequency were kept constant at 45 mT and 375 kHz, respectively. shows the ΔΤ curves in the ex vivo Xv2 and summarizes the experimental results.
Figure 8. (a) Points (P.1, P.2) where magnetic hyperthermia measurements were performed, (b) ΔΤ curves measured at these points (P.1, P.2) in continuous and intermittent mode of magnetic field application in the ex vivo tumor–healthy tissue interface system Xv2. Hyperthermia window appears as a shaded band.
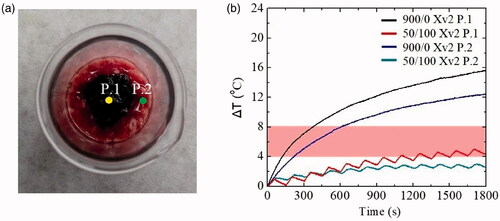
Table 7. Continuous and intermittent mode magnetic field in Xv2 system.
Observing image 8 (b), it seems that the hyperthermia curves in the continuous mode, of P.1 rose faster than P.2. As for the multiple one, P.1 entered the hyperthermia limit, while P.2 did not. With the help of the , it is clear what happened when a continuous and intermittent mode was applied. Once again, ΔΤ due to eddy currents in P.2 decreased quite a bit from 12.5 to 3.0 °C when intermittent mode, was applied. Concurrently, MNPs’ therapeutic efficacy was maintained, as ΔΤ in P.1 reached the hyperthermia range. Τhe reduction noted here was from 15.6 °C in continuous mode to 5.1 °C by applying intermittent mode. This latest experiment reaffirms the advantages of intermittent operation mode over the continuous one as not only the side effects in healthy tissues are reduced but at the same time the therapeutic ability of the MNPs is retained.
Theoretical approach
The purpose here is to bestow a direct quantitative evaluation and validation of the experimental optimum conditions by the direct comparison of the numerically calculated values with the experimental ones. Under this framework, a comparison between theoretical (red and black curves) and experimental (black and dark cyan curves) data, took place for the optimum duty cycle (33%). The good agreement between the theoretical approach and the experimental findings is outlined in . This outcome constitutes an additive proof of the suitability and validity of the optimum conditions emerged from the experimental procedure.
Figure 9. Theoretical approach of the ex vivo model mimicking a tumor–healthy tissue interface (a) point Xv2 P.2 without MNPs, (b) point Xv2 P.1, with MNPs, for both application modes of the AMF. Hyperthermia window is shown with a shaded band.
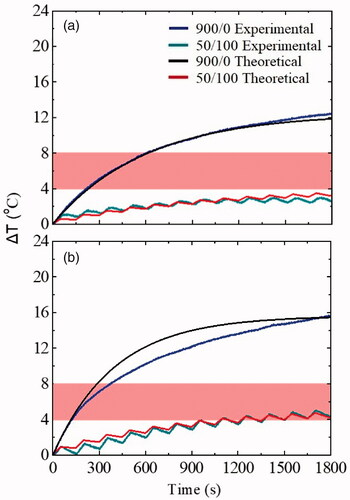
For the continuous magnetic field mode of 45 mT field amplitude and after 1800s of treatment, a comparison between theoretical and experimental temperature temporal evolution in both tissue samples (Xv2 P.2 and Xv2 P.1) was performed. The estimated time constant τ and maximum temperature increase were found equal to 556 s and 12οC, respectively, for Xv2 P.2 and 525 s and 15.5οC, respectively, for Xv2 P.1. ΔΤ curves obtained theoretically using EquationEquation (3)
(3)
(3) in the case of intermittent AMF application were in good agreement with the corresponding experimental ones, indicating again the suitability of the numerical methodology introduced for predicting the temporal evolution of temperature under discontinuous application of AMFs.
The application of intermittent operation mode resulted in induced eddy currents with very short duration and so in mitigating the undesired healthy tissue heating, even when reaching Ho×f values much higher than Brezovich limit (1.35 × 1010 A m−1 s−1 for Ho=45 mT). This outcome permits the use of higher AMF amplitudes and thus allowing for a significant enhancement of the MNPs’ treatment efficacy.
Finally, in this work, based on the size of the 8-turn coil (inner diameter of 2.54 cm), the phantom diameter is restricted to 3 cm, while future goal concerns the use of larger diameter coils and thus the use of larger diameter phantoms (i.e., 10 cm), for subsequent in vivo studies.
Conclusions
In a MPH protocol, the intermittent magnetic field mode is superior to the continuous magnetic field mode with respect to side-effect overheating naturally field application. The reason is that smaller temperature variation, due to eddy currents is obtained and at the same time MPH efficiency is practically maintained, resulting in a much more successful, effective treatment with milder side-effects. This outcome was confirmed by phantoms and ex vivo MPH experiments. In addition, the numerically validated theoretical approach, proposed in this study, could be easily and rapidly applied for the establishment of an optimum protocol. The optimal conditions for achieving this goal were ON/OFF: 50/100 (in s), duty cycle 33%, magnetic field amplitude 45 mT in both healthy/cancer tissue phantoms and ex vivo experiments. For the latter, the optimal MNPs concentration was 8 mg/mL. Furthermore, it has been shown that by doubling the magnetic field amplitude, the ΔΤ in the cancer tissue phantom was also doubled, thus having better therapeutic potential, sparing side effects in healthy tissues. Therefore, we could argue that by applying an intermittent mode magnetic field it is possible to overcome the Brezovich criterion.
ihyt_a_1899310_sm8933.pdf
Download PDF (1.3 MB)Disclosure statement
The author has no conflict of interests to declare.
Reference
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
- Makridis A, Topouridou K, Tziomaki M, et al. In vitro application of Mn-ferrite nanoparticles as novel magnetic hyperthermia agents. J Mater Chem B. 2014;2:8390–8398.
- Dutz S, Hergt R. Magnetic particle hyperthermia-a promising tumour therapy? Nanotechnology. 2014;25:452001.
- Singh A, Jain S, Sahoo SK. Magnetic nanoparticles for amalgamation of magnetic hyperthermia and chemotherapy: an approach towards enhanced attenuation of tumor. Mater Sci Eng C. 2020;110:110695.
- Wankhede M, Bouras A, Kaluzova M, et al. Magnetic nanoparticles: an emerging technology for malignant brain tumor imaging and therapy. Expert Rev Clin Pharmacol. 2012;5:173–186.
- Chang D, Lim M, Goos JA, et al. Biologically targeted magnetic hyperthermia: potential and limitations. Front. Pharmac. 2018;9:831.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
- Maier-Hauff K, Ulrich F, Nestler D, et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol. 2011;103:317–324.
- Gupta R, Sharma D. Evolution of magnetic hyperthermia for glioblastoma multiforme therapy. ACS Chem Neurosci. 2019;10:1157–1172.
- Bauer LM, Situ SF, Griswold MA, et al. High-performance iron oxide nanoparticles for magnetic particle imaging – guided hyperthermia (hMPI). ). Nanoscale. 2016;8:12162–12169.
- Angelakeris M. Magnetic Particle hyperthermia. In: Sattler KD, editor. 21st century nanoscience handbook. Boca Raton (FL): CRC Press; 2020.
- Perigo EA, Hemery G, Sandre O, et al. Fundamentals and advances in magnetic hyperthermia. Appl. Phys. Rev. 2015;2:041302.
- Shetake NG, Balla MM, Kumar A, et al. Magnetic hyperthermia therapy: an emerging modality of cancer treatment in combination with radiotherapy. J Radiat Cancer Res. 2016;7:13–17.
- Rosensweig RE. Heating magnetic fluid with alternating magnetic field. J Magn. Magn. Mater. 2002; 252:370–374.
- Johannsen M, Thiesen B, Wust P, et al. Magnetic nanoparticle hyperthermia for prostate cancer. Int J Hyperthermia. 2010;26:790–795.
- Johannsen M, Gneveckow U, Taymoorian K, et al. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: results of a prospective phase I trial. Int J Hyperthermia. 2007;23:315–323.
- Wust P, Gneveckow U, Johannsen M, et al. Magnetic nanoparticles for interstitial thermotherapy-feasibility, tolerance and achieved temperatures. Int J Hyperthermia. 2006;22:673–685.
- Mahmoudi K, Bouras A, Bozec D, et al. Magnetic hyperthermia therapy for the treatment of glioblastoma: a review of the therapy’s history, efficacy and application in humans. Int J Hyperthermia. 2018;34:1316–1328.
- Southern P, Pankhurst QA. Commentary on the clinical and preclinical dosage limits of interstitially administered magnetic fluids for therapeutic hyperthermia based on current practice and efficacy models. Int J Hyperthermia. 2018;34:671–686.
- Schier P, Barton C, Spassov S, et al. European research on magnetic nanoparticles for biomedical applications: standardisation aspects. In: Korbicz J, Maniewski R, Patan K, et al., editors. Polish Conference on Biocybernetics and Biomedical Engineering. Vol. 1033. Cham (Switzerland): Springer. 2019. p. 316–326.
- Brezovich IA. Low frequency hyperthermia: capacitive and ferromagnetic thermoseed methods. Med Phys Monograph. 1988; 16:82–111.
- Angelakeris M. Magnetic nanoparticles: a multifunctional vehicle for modern theranostics. Biochim Biophys Acta Gen Subj. 2017;1861:1642–1651.
- Hergt R, Dutz S, Röder M. Effects of size distribution on hysteresis losses of magnetic nanoparticles for hyperthermia. J Phys Condens Matter. 2008;20:385214.
- Bellizzi G, Bucci OM. On the optimal choice of the exposure conditions and the nanoparticle features in magnetic nanoparticle hyperthermia. Int J Hyperthermia. 2010;26:389–403.
- Bellizzi G, Bucci OM, Chirico G. Numerical assessment of a criterion for the optimal choice of the operative conditions in magnetic nanoparticle hyperthermia on a realistic model of the human head. Int J Hyperthermia. 2016;32:688–703.
- Stigliano RV, Shubitidze F, Petryk JD, et al. Mitigation of eddy current heating during magnetic nanoparticle hyperthermia therapy. Int J Hyperthermia. 2016;32:735–748.
- Herrera LJM, Bruvera IJ, Scaffardi LB, et al. Sizing and Eddy currents in magnetic core nanoparticles: an optical extinction approach. Phys Chem Chem Phys. 2017;19:3076–3083.
- Ivkov R, DeNardo SJ, Daum W, et al. Application of high amplitude alternating magnetic fields for heat induction of nanoparticles localized in cancer. Clin Cancer Res. 2005;11:7093s–7103s.
- Attaluri A, Kandala SK, Zhou H, et al. Magnetic nanoparticle hyperthermia for treating locally advanced unresectable and borderline resectable pancreatic cancers: the role of tumor size and eddy-current heating. Int J Hyperthermia. 2020;37:108–119.
- Nieskoski MD, Trembly BS. Comparison of a single optimized coil and a Helmholtz pair for magnetic nanoparticle hyperthermia. IEEE Trans Biomed Eng. 2014;61:1642–1650.
- Lerch IA, Kohn S. Radiofrequency hyperthermia: the design of coil transducers for local heating. Int J Radiat Oncol Biol Phys. 1983;9:939–948.
- Gneveckow U, Jordan A, Scholz R, et al. Description and characterization of the novel hyperthermia and thermoablation- system for clinical magnetic fluid hyperthermia. Med Phys. 2004;31:1444–1451.
- Kumar A, Attaluri A, Mallipudi R, et al. Method to reduce non-specific tissue heating of small animals in solenoid coils. Int J Hyperthermia. 2013;29:106–120.
- Simeonidis K, Liébana-Viñas S, Wiedwald U, et al. A versatile large-scale and green process for synthesizing magnetic nanoparticles with tunable magnetic hyperthermia features. RSC Adv. 2016;6:53107–53117.
- Salloum M, Ma RH, Weeks D, et al. Controlling nanoparticle delivery in magnetic nanoparticle hyperthermia for cancer treatment: experimental study in agarose gel. Int J Hyperthermia. 2008;24:337–345.
- Chen ZJ, Broaddus WC, Viswanathan RR, et al. Intraparenchymal drug delivery via positive-pressure infusion: experimental and modeling studies of poroelasticity in brain phantom gels. IEEE Trans Biomed Eng. 2002;49:85–96.
- Makridis A, Curto S, Van Rhoon GC, et al. A standardisation protocol for accurate evaluation of specific loss power in magnetic hyperthermia. J Phys D Appl Phys. 2019;52:255001.
- Neufeld E, Fuetterer M, Murbach M, et al. Rapid method for thermal dose-based safety supervision during MR scans. Bioelectromagnetics. 2015;36:398–407.
- Bordelon DE, Cornejo C, Grüttner C, et al. Magnetic nanoparticle heating efficiency reveals magneto-structural differences when characterized with wide ranging and high amplitude alternating magnetic fields. J Appl. Phys. 2011;109:124904.
- Rodrigues HF, Mello FM, Branquinho LC, et al. Real-time infrared thermography detection of magnetic nanoparticle hyperthermia in a murine model under a non-uniform field configuration. Int J Hyperthermia. 2013;29:752–767.
- Dabbagh A, Hedayatnasab Z, Karimian H, et al. Polyethylene glycol-coated porous magnetic nanoparticles for targeted delivery of chemotherapeutics under magnetic hyperthermia condition. Int J Hyperthermia. 2019;36:104–114.
- Sumser K, Neufeld E, Verhaart RF, et al. Feasibility and relevance of discrete vasculature modeling in routine hyperthermia treatment planning. Int J Hyperthermia. 2019;36:800–810.
- Sebeke L, Deenen DA, Maljaars E, et al. Model predictive control for MR-HIFU-mediated, uniform hyperthermia. Int J Hyperthermia. 2019;36:1039–1049.
- Maniotis N, Myrovali E, Kalpaxidou Z, et al. Ex-vivo evaluation of magnetite magnetic nanoparticles as magnetic hyperthermia carriers. Proceedings of the 1st EMF-Med World Conference on Biomedical Applications of Electromagnetic Fields and COST EMF-MED, Final Event with 6th MCM. 2018. p. 1–2.
- Harabech M, Kiselovs NR, Maenhoudt W, et al. Experimental ex-vivo validation of PMMA-based bone cements loaded with magnetic nanoparticles enabling hyperthermia of metastatic bone tumors. AIP Adv. 2017;7:056704.
- Efremova MV, Nalench YA, Myrovali E, et al. Size-selected Fe3O4-Au hybrid nanoparticles for improved magnetism-based theranostics. Beilstein J Nanotechnol. 2018;9:2684–2699.

