?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objectives
To compare the therapeutic effects of locoregional deep hyperthermia combined with intravesical chemotherapy with those of intravesical chemotherapy alone in patients with intermediate-/high-risk non-muscle invasive bladder cancer (NMIBC). To evaluate the impact of thermal dose in hyperthermia treatment.
Methods
We analyzed data retrieved from the medical records of patients with intermediate-/high-risk NMIBC treated with intravesical mitomycin (IM group) or locoregional deep hyperthermia combined with intravesical mitomycin (CHT group) at a single tertiary care hospital between May 2016 and June 2019. The primary and secondary endpoints were the recurrence-free survival rate and progression-free survival rate, respectively. Thermal dose was evaluated and adverse events were also recorded.
Results
In total, 43 patients (CHT: 18 patients, IM: 25 patients) were enrolled. The median follow-up durations were 14 and 23 months, respectively. The recurrence rate at 12 months was significantly lower in the CHT group than in the IM group (11.1% vs. 44%, p = .048); this trend persisted at 24 months (CHT: 11.1%, IM: 48%; p = .027). The recurrence-free survival was also significantly higher in the CHT group than in the IM group (p = .028). No tumor recurrence was noted in patients who received a thermal dose of ≥4 CEM43. All adverse events were well tolerated, and there was no treatment-related mortality.
Conclusions
Intravesical chemotherapy combined with locoregional deep hyperthermia for intermediate-/high-risk papillary NMIBC can significantly decrease the recurrence rate relative to that observed after intravesical chemotherapy alone.
Introduction
Bladder cancer was reported to be the ninth most common cancer worldwide and among Taiwanese men in 2012 [Citation1,Citation2]. Diagnosed patients mostly exhibit non-muscle invasive (NMIBC) bladder cancer [Citation3]. However, the patients still require adjuvant treatment, cystoscopy, and follow-up imaging studies because NMIBC is characterized by a high recurrence rate [Citation4]. Consequently, a long-term follow-up protocol is inevitable, and the cost per patient for bladder cancer is the highest among the costs for all cancers [Citation5]. Even though patients receive adjuvant therapy such as intravesical chemotherapy, approximately 10%–15% will progress to muscle-invasive disease within 5 years [Citation6]. In the event of progression to muscle-invasive or metastatic bladder cancer, the survival rate becomes significantly lower than that for NMIBC [Citation7]. Thus, the high recurrence and progression rates for NMIBC remain important unresolved issues.
For patients with intermediate- and high-risk bladder cancer, adjuvant treatment with intravesical bacillus Calmette–Guérin (BCG) after transurethral resection of the tumor is the standard treatment because it is more effective than intravesical chemotherapy [Citation8]. However, because of a shortage of BCG in Taiwan, intravesical mitomycin has replaced BCG for the adjuvant treatment of bladder cancer [Citation9]. According to per-protocol analysis in a randomized controlled trial with a 2-year follow-up period, the recurrence rate for patients with intermediate- and high-risk papillary bladder cancer was significantly lower after microwave-induced hyperthermia combined with mitomycin instillation than after BCG instillation alone [Citation10]. In addition to microwave-induced heating, conductive heating fluid and locoregional hyperthermia can be used for bladder hyperthermia therapy [Citation11]. The BSD-2000 deep regional hyperthermia system (Sigma Ellipse, Pyrexer medical, USA), is a unique system for locoregional hyperthermia therapy; it uses electromagnetic waves to increase the bladder wall temperature [Citation11]. The safety of intravesical chemotherapy combined with hyperthermia therapy [chemohyperthermia (CHT)] using the BSD-2000 has been reported in previous studies [Citation12,Citation13]. However, to our knowledge, comparative studies for evaluating the efficacy of CHT using the BSD-2000 are lacking. Accordingly, we designed this retrospective study to compare the therapeutic effects of CHT using the BSD-2000 with those of intravesical mitomycin instillation alone in patients with intermediate- or high-risk papillary NMIBC.
Methods
Patients
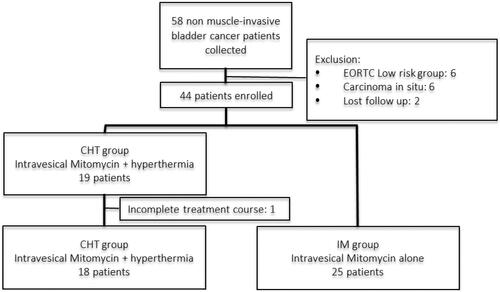
This study was approved by the Institutional Review Board of Taipei Medical University (Protocol Number: N201906031). Requirement for informed consent could be waived. We retrospectively reviewed the medical records of patients diagnosed with and treated for bladder cancer at a single tertiary care hospital between May 2016 and June 2019. The patients were stratified into low-, intermediate-, and high-risk groups according to risk stratification defined by the European Association of Urology guidelines [Citation14]. Patients diagnosed with intermediate or high risk NMIBC and aged between 20 and 99 years old were included in this study, while those diagnosed with low-risk NMIBC, those with carcinoma in situ noted in their pathological report, those who were lost to regular follow-up, and those who did not completed six courses of hyperthermia therapy were excluded.
Treatment protocol
The included patients were divided into two groups according to the treatment: intravesical mitomycin instillation combined with locoregional deep hyperthermia (CHT group) and intravesical mitomycin instillation alone (IM group). In all patients, mitomycin (30 mg of mitomycin dissolved in 30 ml of normal saline) was instilled within 24 h after surgery, with or without hyperthermia. Secondary transurethral resection of bladder tumor (TURBT) was performed if the resected specimen did not show a muscle layer in histopathology. All patients received weekly intravesical mitomycin instillation or CHT within 1 month after completing TURBT. In the CHT group, patients received locoregional deep hyperthermia combined with intravesical mitomycin instillation every week for 6 weeks. In the IM group, patients received intravesical mitomycin therapy every week for 6 weeks. After 6 weeks, all patients in both groups were scheduled to receive maintenance therapy with intravesical mitomycin (30 mg of mitomycin dissolved in 30 ml of normal saline) once every month for 10 months.
Hyperthermia therapy
The BSD-2000 deep regional hyperthermia system was used in the CHT group. Treatment was designed on the basis of computed tomography findings within 1 month before treatment. On the day of treatment, a 14-French urethral catheter was inserted and clamped after mitomycin instillation (30 mg of mitomycin dissolved in 30 ml of normal saline) into the bladder. For temperature monitoring during treatment, thermometers were set at the suprapubic skin, urinary bladder, and rectum. During hyperthermia therapy, the intravesical temperature was maintained at 40 °C to 43 °C for 60 min. The urethral catheter was removed after hyperthermia therapy completed. Details of the treatment procedure using the BSD-2000 have been described by Yuan et al. [Citation15] Patients were carefully instructed to report any discomfort during treatment. Hyperthermia treatment was stopped if patients experienced intolerable discomfort or refused to continue treatment.
Thermal dose
We used the cumulative equivalent minutes of exposure at 43 °C (CEM43) as a relevant parameter for monitoring the hyperthermia treatment [Citation16]. The following equation was used for calculation: thermal dose at 43 °C
Here, Tave is the average temperature in degrees Celsius during the measurement time interval Δt in minutes, with R = 0.5 at or above 43 °C and R = 0.25 below 43 °C [Citation16]. This metric quantifies thermal exposure in terms of the number of minutes of heating at 43 °C needed to obtain equivalent effects in biological tissues. The temperature profile in each CHT session was recorded and used to derive an average maximum temperature and the temperature achieved in 90% time of all treatments.
Treatment efficacy and toxicity evaluation
After the 6-week treatment duration, all patients underwent cystoscopy every 3 months for 2 years and every 6 months thereafter until 5 years. The primary endpoint was the recurrence-free survival rate, and the secondary endpoint was the progression-free survival rate. TURBT was performed if tumors were detected in cystoscopy or imaging studies. Recurrence was defined as the development of urothelial carcinoma diagnosed by pathologists. Progression was defined as tumor invasion into the muscle layer or lymph nodes or the occurrence of distant metastasis. Treatment-related toxicity was recorded according to the Common Terminology Criteria for Adverse Events (CTCAE) v5.0 [Citation17].
Statistical analysis
All statistical analyses were performed using SPSS ver. 21.0 (SPSS Inc., IBM Corporation, Somers, NY, USA). The independent t-test for continuous variables and the chi-square test for categorical variables were used to compare the IM and CHT groups in terms of age, sex, T stage, median time to recurrence, and basic characteristics of patients with recurrence. Follow-up duration was calculated using the reverse Kaplan-Meier method. Differences in the European Association of Urology (EAU) risk classification and history of bladder cancer between the two groups were determined using the Mann–Whitney U test, while differences in the recurrence rate at 12 and 24 months were determined using the chi-square test. Cox proportional hazards regression was used to determine the recurrence risks in the two groups. Recurrence-free survival was estimated by the Kaplan–Meier method. A p-value of <.05 was considered statistically significant.
Results
Baseline data
In total, data for 58 patients with NMIBC were retrieved, following which 15 patients were excluded (six patients with low-risk NMIBC, six with concomitant carcinoma in situ, two lost to follow-up, and one who decided to discontinue the treatment). Eventually, there were 18 and 25 patients in the CHT and IM groups, respectively (). No patient received secondary TURBT, and all completed the induction and maintenance treatments. The median age of patients was 66.5 years in the CHT group and 75 years in the IM group. Male patients were predominant in both groups. Most patents exhibited high-risk bladder cancer (n = 32; 76.7%). The cancer was recurrent in 13 patients in the CHT group (72.2%), while none of the patients in the IM group had a previous history of cancer recurrence (p < .001). The median follow-up durations were 14 (9–25) and 23 (20–26) months in the CHT and IM groups (p = .482), respectively. The baseline characteristics of both groups are listed in .
Table 1. Baseline data.
Treatment efficacy
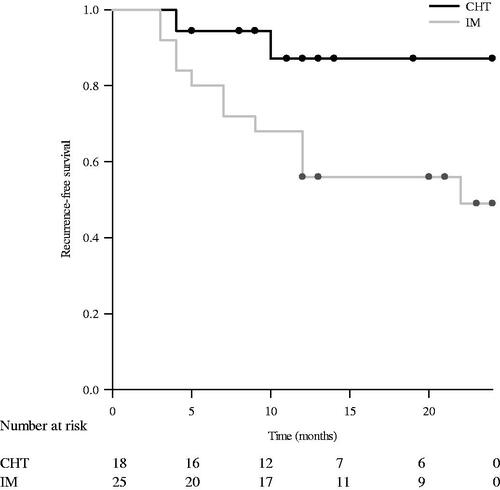
At 12 months, 2 and 11 patients exhibited local recurrence in the CHT and IM groups, respectively. At 24 months, one more patient in the IM group and no patient in the CHT group exhibited recurrence. The recurrence rates at 12 (11.1% vs. 44%; p = .048) and 24 (11.1% vs. 48%; p = .027) months were significantly lower in the CHT group than in the IM group (). The recurrence-free survival rate calculated by the Kaplan–Meier method was also significantly higher in the CHT group than in the IM group (p = .028; ). Cox proportional hazards regression analyses demonstrated that the tumor recurrence risk was significantly lower in the CHT group than in the IM group (hazard ratio: 0.22, 95% confidence interval: 0.05–0.99; p = .049;); the risk of recurrence in the CHT group was 78% lower than that in the IM group. Recurrence developed in 2 male patients and no female patient in the CHT group and 9 male patients and 3 female patients in the IM group (Supplementary Table 1). The initial pathological stage was Ta in the 2 patients with recurrence in the CHT group, and the number of patients with pathological stage T1 and tumor recurrence was higher in the IM group than in the CHT group (57.1% vs. 0%, p = .033; Supplementary Table 1). The average thermal dose ranged from 1.6 to 23.27 CEM43, with a median of 9.12 CEM43. 6 of the 18 patients received a thermal dose of <4 (ranged 1.60–3.82) CEM43, and 2 of them developed recurrence. None of the patients who received a thermal dose of ≥4 (ranged 4.18–23.27) CEM43 developed recurrence. Only one patient in both groups showed tumor progression and the difference was insignificant.
Table 2. Recurrence rate according to Treatment.
Table 3. Adverse events of both groups.
Treatment safety
12 patients in the CHT group (66.7%) and 17 in the IM group (68%) experienced increased urinary frequency, while gross hematuria occurred in 2 and one patient, respectively. Suprapubic soreness occurred after hyperthermia treatment in 2 patients. All adverse events could be treated with medication, with a good response. There were none grade 3 or worse adverse events in either group (CTCAE grading scale) ().
Discussion
The findings of this study demonstrated that the recurrence-free survival rate for intermediate- or high-risk papillary NMIBC was significantly higher with locoregional deep hyperthermia combined with intravesical mitomycin chemotherapy (CHT group) than with intravesical mitomycin chemotherapy alone (IM group). All treatment-related toxicities were mild (less than grade 3) and manageable with medications. Thus, in regions with a shortage of BCG, CHT would be a better choice than intravesical mitomycin alone for intermediate- or high-risk papillary NMIBC.
The recurrence rate for NMIBC remains high despite adjuvant intravesical chemotherapy [Citation18,Citation19]. In a previous study, 46.4% patients exhibited tumor recurrence at 26 months after adjuvant intravesical mitomycin instillation [Citation18]. The recurrence rate for patients with intermediate- or high-risk NMIBC treated with mitomycin alone in the present study (48% at 2 years) was consistent with the previously reported rate. The possible mechanisms underlying the high recurrence rate for NMIBC include an insufficient drug concentration reaching the tumor cells and low cytotoxicity of chemotherapy [Citation19]. Hyperthermia has the potential to increase the treatment efficacy by increasing the tumor cell permeability of mitomycin and promoting increased mitomycin accumulation in tumor cells [Citation20,Citation21]. We used the BSD-2000 hyperthermia system with the first six weekly instillations of intravesical mitomycin in order to enhance the treatment effects of mitomycin. At the 24-month follow-up, a decreased recurrence risk of 78% was observed in the CHT group. None of the patients discontinued CHT because of adverse events, and treatment-related adverse events of grade 3 or worse were not observed in any patient. Thus, our findings provide evidence that CHT is an effective and safe treatment for patients with intermediate- or high-risk papillary NMIBC.
Multiple and large tumors increase the risk of recurrence in patients with NMIBC [Citation22,Citation23] and CHT should be considered for patients with tumors >3 cm or multiple tumors. In the present study, 5 patients with treatment-naïve NMIBC and a high risk of tumor recurrence received CHT; among these patients, four exhibited high-grade, large tumors (>3 cm) and one exhibited multiple papillary tumors (>8 sites). None of the patients developed tumor recurrence. However, 3 of 5 patients with multiple tumors (>8 sites) and 2 of 3 patients with tumors >3 cm in the IM group developed recurrence during the follow-up period. Pathological T1 stage is also another risk factor compared to pathological Ta stage. None of the 8 patients with T1 stage in the CHT group developed tumor recurrence, whereas 4 of 7 patients with T1 stage in the IM group developed recurrence during the follow-up period (57.1% vs 0%, p = .033, Supplementary Table 1). These results suggest that CHT effectively lowers the risk of recurrence in patients with large tumors, multiple tumors or tumors with higher pathological stage and should be preferred over mitomycin instillation alone for NMIBC with a high recurrence risk.
The number of patients with recurrent NMIBC was higher in the CHT group (72.2%) than in the IM group (0%) because CHT is one of the second-line treatment options at our hospital. Considering the shortage of BCG in Taiwan, there are limited treatment choices for patients with recurrent NMIBC. Alternative intravesical chemotherapy, device-assisted intravesical therapy, and radical cystectomy are available sequential treatments for patients who do not respond to mitomycin [Citation24–26]. A prospective study with a 10-year follow-up period found recurrence-free survival rates of 53% and 15% for the CHT and IM groups, respectively [Citation27]. Meanwhile, an open-label, phase III randomized controlled trial showed that the recurrence-free survival time was comparable between radiofrequency-induced CHT and institutional standard second-line therapy for NMIBC refractory to BCG [Citation28]. In the present study, 2 of the 13 patients (15.4%) with recurrent tumors treated by CHT developed further recurrence, and the 2-year recurrence-free survival rate in this group was 88.9%. However, a longer follow-up duration is needed for evaluating the therapeutic effects of CHT using a locoregional deep hyperthermia device.
Thermal dose calculations permit the conversion of temperature and time data to reference values that quantify the thermal effect by calculating the required time of heating at a specified reference temperature for obtaining the same thermal effects. CEM43 is used as an indicator of thermal exposure to hyperthermia because the reference temperature of 43 °C is most commonly used to convert all thermal exposures to equivalent-minutes [Citation16]. In the present study, there was a positive correlation between the thermal dose of CHT and the treatment efficacy. Specifically, 50% patients with <4 CEM43 developed recurrence, while none of the patients with ≥4 CEM43 developed recurrence. This correlation has been reported for other types of cancer. In a meta-analysis of thermal data for breast tumors, the local tumor control rates were significantly better when higher intratumoral temperatures were achieved [Citation29]. The thermal dose may be used as one of the more relevant parameters for monitoring hyperthermia treatment.
The technique for hyperthermia therapy in most published studies involved intravesical microwave-induced heating using the Synergo system (Medical Enterprises, Amsterdam, Netherlands) [Citation11,Citation27,Citation28]. Locoregional deep hyperthermia is another option for patients with NMIBC; it allows a well-designed treatment area, targets deep tissues, allows real-time monitoring of the treatment temperature, and can be used to treat other types of cancer. For patients with NMIBC, there is a dearth of long-term studies comparing CHT involving locoregional hyperthermia with intravesical mitomycin alone [Citation11]. Our findings demonstrated the therapeutic efficacy of locoregional hyperthermia using the BSD-2000 for primary or recurrent, intermediate-/high-risk papillary NMIBC, with evidence that CHT using the BSD-2000 results in better oncological outcomes with acceptable adverse events than does intravesical mitomycin instillation alone.
This study has some limitations. First, the sample size was insufficient to draw definitive conclusions regarding the therapeutic efficacy of CHT. Second, this was a retrospective study, and all patients with recurrent bladder cancer received CHT. Therefore, there was a selection bias. A prospective randomized clinical trial is needed to clarify the treatment efficacy of locoregional deep hyperthermia for intermediate- or high-risk papillary NMIBC. Third, the therapeutic effect is not different between 30 mg and 40 mg of intravesical mitomycin [Citation30]. The dosage of mitomycin was 30 mg per instillation in the present study, and the therapeutic efficacy of other dosages (such as 40 mg) should be evaluated in further studies. Fourth, the median follow-up duration was not more than 2 years, and a longer follow-up duration is necessary. Fifth, only one patient in both groups showed tumor progression after recurrence. Accordingly, the impact of hyperthermia on tumor progression should be evaluated in future studies. Sixth, hyperthermia was used only in the induction course of intravesical chemotherapy. The optimal dose and duration of hyperthermia should be evaluated in future studies.
In conclusion, intravesical chemotherapy combined with locoregional hyperthermia for intermediate-/high-risk papillary NMIBC can significantly decrease the recurrence rate (78%) relative to that observed after intravesical chemotherapy alone
Supplemental Material
Download PDF (11.4 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Antoni S, Ferlay J, Soerjomataram I, et al. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71(1):96–108.
- Hung CF, Yang CK, Ou YC. Urologic cancer in Taiwan. Jpn J Clin Oncol. 2016;46(7):605–609.
- Kamat AM, Bağcıoğlu M, Huri E. What is new in non-muscle-invasive bladder cancer in 2016? Turk J Urol. 2017;43(1):9–13.
- Woldu SL, Bagrodia A, Lotan Y. Guideline of guidelines: non-muscle-invasive bladder cancer. BJU Int. 2017;119(3):371–380.
- Sievert KD, Amend B, Nagele U, et al. Economic aspects of bladder cancer: what are the benefits and costs? World J Urol. 2009;27(3):295–300.
- van Rhijn BW, Burger M, Lotan Y, et al. Recurrence and progression of disease in non-muscle-invasive bladder cancer: from epidemiology to treatment strategy. Eur Urol. 2009;56(3):430–442.
- Alfred Witjes J, Lebret T, Comperat EM, et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur Urol. 2017;71(3):462–475.
- Babjuk M, Bohle A, Burger M, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol. 2017;71(3):447–461.
- Choo SH, Nishiyama H, Kitamura H, et al. Practice pattern of non-muscle invasive bladder cancer in Japan, korea and Taiwan: a web-based survey. Int J Urol. 2019;26(12):1121–1127.
- Arends TJ, Nativ O, Maffezzini M, et al. Results of a randomised controlled trial comparing intravesical chemohyperthermia with mitomycin C versus bacillus Calmette-Guerin for adjuvant treatment of patients with intermediate- and high-risk Non-Muscle-invasive bladder cancer. Eur Urol. 2016;69(6):1046–1052.
- Liem EI, Crezee H, de la Rosette JJ, et al. Chemohyperthermia in non-muscle-invasive bladder cancer: an overview of the literature and recommendations. Int J Hyperthermia. 2016;32(4):363–373.
- Juang T, Stauffer PR, Craciunescu OA, et al. Thermal dosimetry characteristics of deep regional heating of non-muscle invasive bladder cancer. Int J Hyperthermia. 2014;30(3):176–183.
- Inman BA, Stauffer PR, Craciunescu OA, et al. A pilot clinical trial of intravesical mitomycin-C and external deep pelvic hyperthermia for non-muscle-invasive bladder cancer. Int J Hyperthermia. 2014;30(3):171–175.
- Babjuk M, Burger M, Comperat EM, et al. European association of urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ) – 2019 update. Eur Urol. 2019;76(5):639–657.
- Yuan Y, Cheng KS, Craciunescu OI, et al. Utility of treatment planning for thermochemotherapy treatment of nonmuscle invasive bladder carcinoma. Med Phys. 2012;39(3):1170–1181.
- van Rhoon GC. Is CEM43 still a relevant thermal dose parameter for hyperthermia treatment monitoring? Int J Hyperthermia. 2016;32(1):50–62.
- National Cancer Institute; [cited 2021 Nov 2]. Available from: https://ctep.cancer.gov/protocolDevelopment/adverse_effects.htm.
- Bohle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal Meta-analysis of comparative studies on recurrence and toxicity. J Urol. 2003;169(1):90–95.
- Volpe A, Racioppi M, D'Agostino D, et al. Mitomycin C for the treatment of bladder cancer. Minerva Urol Nefrol. 2010;62(2):133–144.
- van Valenberg H, Colombo R, Witjes F. Intravesical radiofrequency-induced hyperthermia combined with chemotherapy for non-muscle-invasive bladder cancer. Int J Hyperthermia. 2016;32(4):351–362.
- van Valenberg FJP, van der Heijden AG, Lammers RJM, et al. Intravesical radiofrequency induced hyperthermia enhances mitomycin C accumulation in tumour tissue. Int J Hyperthermia. 2018;34(7):988–993.
- Bryan RT, Collins SI, Daykin MC, et al. Mechanisms of recurrence of Ta/T1 bladder cancer. annals. 2010;92(6):519–524.
- Jancke G, Damm O, Rosell J, et al. Risk factors for local recurrence in patients with pTa/pT1 urinary bladder cancer. Scand J Urol Nephrol. 2008;42(5):417–421.
- Şanlı Ö, Lotan Y. Alternative therapies in patients with non-muscle invasive bladder cancer. Turk J Urol. 2017;43(4):414–424.
- Stasi D, Riedl SM. C.: updates in intravesical electromotive drug administration of mitomycin-C for non-muscle invasive bladder cancer. World J Urol. 2009;27(3):325–330.
- Parker WP, Smelser W, Lee EK. Utilization and outcomes of radical cystectomy for high-grade non-muscle-invasive bladder cancer in elderly patients. Clin Genitourin Cancer. 2017;2017:2.
- Colombo R, Salonia A, Leib Z, et al. Long-term outcomes of a randomized controlled trial comparing thermochemotherapy with mitomycin-C alone as adjuvant treatment for non-muscle-invasive bladder cancer (NMIBC). BJU Int. 2011;107(6):912–918.
- Tan WS, Panchal A, Buckley L, et al. Radiofrequency-induced thermo-chemotherapy effect versus a second course of Bacillus Calmette-Guérin or Institutional Standard in Patients with Recurrence of Non-muscle-invasive Bladder Cancer Following Induction or Maintenance Bacillus Calmette-Guérin Therapy (HYMN): a Phase III, open-label, randomised controlled trial. Eur Urol. 2019;75(1):63–71.
- Sherar M, Liu FF, Pintilie M, et al. Relationship between thermal dose and outcome in thermoradiotherapy treatments for superficial recurrences of breast cancer: data from a phase III trial. Int J Radiat Oncol Biol Phys. 1997;39(2):371–380.
- Jeong CW, Jeon HG, Kwak C, et al. Comparison of 30 mg and 40 mg of mitomycin C intravesical instillation in korean superficial bladder cancer patients: prospective, randomized study. Cancer Res Treat. 2005;37(1):44–47.