Abstract
Objective
To evaluate the safety and efficacy of ultrasound-guided percutaneous radiofrequency ablation (RFA) in patients with hepatocellular carcinoma (HCC) and liver metastases adjacent to the gallbladder (GB).
Materials and methods
A total of 113 patients with 118 liver lesions (63 HCC lesions and 55 liver metastases) adjacent to the gallbladder underwent RFA between March 2011 and June 2019. Gallbladder-related complications and technique effectiveness rates were evaluated based on the classification of liver tumors and the distance between the lesion and the gallbladder.
Results
Gallbladder-related complications were observed in 13 patients. Among the patients with HCC, there was no significant difference between the ≤0.5 cm and >0.5 cm groups (p = .282). However, among the patients with liver metastases, the incidence of gallbladder-related complications in the ≤0.5 cm group was significantly higher than that in the >0.5 cm group (p = .025). The overall incidence of complications was significantly higher in the ≤0.5 cm group than in the >0.5 cm group (p = .020). Among the patients with lesions ≤3 cm, the technical effectiveness rate in the HCC group was significantly higher than in the liver metastasis group (p = .036).
Conclusion
RFA is a safe and effective treatment option for liver tumors adjacent to the gallbladder. Patients with lesions ≤0.5 cm from the gallbladder had higher gallbladder-related complications, especially patients with liver metastases. Among patients with lesions ≤3 cm, RFA showed greater technical effectiveness for treating HCC than for treating liver metastases.
Introduction
Radiofrequency ablation (RFA) is a minimally invasive, safe, and effective treatment for malignant liver tumors [Citation1,Citation2]. However, the high recurrence rate remains a significant impediment to using RFA [Citation3,Citation4]. Difficult tumor locations that do not provide safe ablation margins, including those adjacent to the diaphragm, vessels, or gallbladder, remain an important factor in tumor recurrence [Citation5,Citation6]. Considering the advances in ablation technology, several researchers [Citation7–10] confirm that ablation of liver lesions adjacent to the gallbladder is safe and effective treatment option owing to hydrodissection and real-time temperature monitoring.
RFA has been regarded as a curative treatment modality for early-stage hepatocellular carcinoma [Citation11,Citation12] and is a comprehensive treatment method for metastatic liver cancer [Citation13–15]. However, the biological characteristics and behaviors of hepatocellular carcinoma (HCC) and liver metastases are significantly different. It has been reported [Citation16–18] that 80–90% of patients with HCC develop liver cirrhosis, but liver metastases are usually found in non-cirrhotic livers. Different liver conditions affect the ablation effect [Citation19].
To our knowledge, few reports have compared the effects of RFA for liver tumors adjacent to the gallbladder in the setting of different liver disease conditions. Hence, this study aimed to evaluate the safety and therapeutic efficacy of RFA for HCC and liver metastases adjacent to the gallbladder.
Materials and methods
Patients
This study was performed in accordance with the principles outlined in the Declaration of Helsinki. The institutional review board of the hospital approved this study (2015KT27), and the requirement for informed consent was waived owing to its retrospective nature. Between March 2011 and June 2019, the medical records of 1543 patients (2156 lesions) with HCC and liver metastases treated with ultrasound guided RFA at our hospital were retrospectively analyzed. The diagnosis of HCC was made based on the needle biopsy results or the noninvasive diagnostic criteria defined in the Asia–Pacific clinical practice guidelines on managing hepatocellular carcinoma (2017 update) [Citation20]. Liver metastases were diagnosed based on clinical findings, enhanced computed tomography (CT), and magnetic resonance imaging (MRI).
The eligibility criteria included patients with liver tumors who were not suitable for surgical treatment or who did not intend to undergo surgical treatment; tumor size ≤5 cm in diameter; distance from the tumor to the gallbladder ≤1.0 cm; conventional ultrasound or contrast-enhanced ultrasonography (CEUS) showing a liver tumor; Child-Pugh A or B liver disease; and normal coagulation function.
In total, 123 patients with 128 lesions adjacent to the gallbladder who underwent ultrasound guided RFA were identified, and ten patients with ten lesions >5 cm were excluded. A total of 113 patients (7.3%) with 118 lesions (5.5%) were included in this study ().
RFA procedure
In this study, the Valleylab system (Tyco Healthcare, North Haven, CT), the Celon Lab Power ablation system (Olympus, Germany), and the RITA Model 1500X ablation system (AngioDynamics, Latham, NY) were used. Aloka ultrasound systems (Alokaa-10, Tokyo, Japan) or GE systems (E9, GE, United States) were used to scan the lesion and guide therapy with 3.5–5.0 MHz convex probes for RFA procedures.
Enhanced CT or MRI and CEUS of the abdomen, conducted prior to RFA, were used as a reference for planned treatments. All RFA procedures were conducted under real-time US or CEUS guidance by four radiologists (CMH, YK, WW, and YW) with >5 years of experience in US-guided interventions. All patients were anesthetized with an intravenous injection of 2.5–5.0 mg of midazolam (Roche; Basel, Switzerland) and 50–100 mg of fentanyl (Fentaini; Renfu, Yichang, China). Local infiltration of anesthesia at the puncture points was achieved with 5–15 ml of 1% lidocaine (Liduokayin; Yimin, Beijing, China). The patient’s vital signs, including blood pressure, respiration, pulse, and electrocardiogram, were continuously monitored during treatment. A CEUS was performed after the RFA, and the ablation size was determined.
Ablation margins of at least 5 mm are generally necessary to minimize local tumor progression [Citation20–23]. However, obtaining a sufficient safety margin surrounding the tumor close to the gallbladder was difficult. Therefore, individualized protocols have been developed [Citation24,Citation25]. When the distance between the ablation needle and the gallbladder was within the radius of the expected ablation, the adjacent structures were protected by separating them from the gallbladder wall using hydrodissection. Overlapping and modification of the ablation zone during RFA ensure its adequacy.
Assessment of safety and therapeutic efficacy
The 118 lesions were divided into an HCC group (n = 63) and a liver metastasis group (n = 55) according to the classification of liver tumors. Each group was further subdivided into a ≤ 0.5 cm group and a > 0.5 cm group according to the distance between the lesion and the gallbladder. Complications were observed between the groups. The main outcomes were gallbladder-related complications after RFA, including the presence of gallbladder wall thickening (≥3 mm) on imaging [Citation26], clinical evaluation for cholecystitis excluding other diseases such as liver abscess and intestinal perforation, and improvement after restraint or conservative treatment.
One month after the RFA, an enhanced CT/MRI was performed to evaluate the effectiveness of RFA and minimal ablation margins. Technical effectiveness [Citation27] refers to the tumor ablation completeness on the first enhanced CT or MRI scan performed one month after RFA. The technical effectiveness of each group was compared according to tumor source (HCC and liver metastases) and tumor size (maximum diameter ≤3 cm and >3 cm). The ablation margins were measured as previously described [Citation21]. The pre-ablation and 1-month post-ablation CT/MRI images were reviewed side-by-side to measure the distance of the ablation margin from the tumor edge according to the anatomic landmarks, selecting the minimum of the multiple measures of margin values as the minimal margin. The minimal margins of the lesions were assessed and categorized as ≤5 or >5 mm. Two experienced radiologists (ZZY and WS) with more than five years of experience in abdominal imaging evaluated the technical effectiveness.
Statistical analysis
Categorical variables are described as numbers and percentages and were assessed using Fisher’s exact test or the χ2 test. Continuous variables are summarized as mean ± standard deviation and were assessed using an independent sample t-test or the nonparametric Wilcoxon Mann-Whitney test. Logistic regression analysis was used for the complication analysis. p-values less than .05 were considered statistically significant. Data analyses were performed using the SPSS software version 21.0 for Windows (SPSS, Chicago, IL).
Results
In total, 113 patients (80 males and 33 females) with a mean age of 59.8 ± 11.0 years. The mean diameter of the tumor was 2.6 ± 0.9 cm. Among them, 58 patients (63 lesions) had HCC, and 55 patients (55 lesions) had liver metastases. In patients with HCC, 38 lesions were pathologically diagnosed, and 25 were diagnosed clinically. Among the patients with liver metastases, 36 (65.5%) had primary colorectal cancer, 8 (14.5%) had lung cancer, 4 (7.3%) had breast cancer, 3 (5.5%) had gastric cancer, 1 (1.8%) had duodenal cancer, 1 (1.8%) had nasopharyngeal cancer, 1 (1.8%) had thymic cancer, and 1 (1.8%) had esophageal cancer. Among the 118 lesions analyzed, 51 (43.2%) required hydrodissection, including 28 (44.4%, 28/63) HCC and 23 (41.8%, 23/55) liver metastasis lesions.
A comparison of the basic characteristics of HCC and liver metastasis is shown in . The proportion of men in the HCC group (82.8%) was significantly higher than that in the liver metastasis group (58.2%, p = .004). Patients in the HCC group had liver cirrhosis, and those in the liver metastases group had no liver cirrhosis (p < .001). There were no significant differences in other characteristics between the two groups.
Table 1. Characteristics of the patients with HCC and liver metastases.
Assessment of safety
None of the patients experienced death due to RFA treatment. Complications occurred in 17 patients (14.4%) within 24 h of treatment, and 13 patients experienced gallbladder-related complications. All the patients showed improvement after symptomatic and conservative treatment. None of the patients in this group developed a gallbladder perforation or died ().
Table 2. Gallbladder-related complications after RFA of HCC and liver metastases.
There was no significant difference in gallbladder-related complications between patients with HCC and those with liver metastasis (7.9% vs. 14.5%, p = .253). In the ≤0.5 cm group, the rate of gallbladder-related complications in the HCC group was 11.1%, which was lower than the 25% in the liver metastases group, but the difference was not statistically significant (p = .144). In the >0.5 cm group, no significant difference was observed between the HCC and the liver metastasis groups (3.7% vs. 3.7%, p = 1.000). Among patients with HCC, there was no significant difference in the incidence of gallbladder-related complications between the ≤0.5-and >0.5 cm groups (11.1% vs. 3.7%, p = .282). Among the patients with liver metastases, the incidence of gallbladder-related complications in the ≤0.5 cm group was significantly higher than that in the >0.5 cm group (25.0% vs. 3.7%, p = .025) (). The overall incidence of complications in all patients with a distance of ≤0.5 cm between the lesion and the gallbladder was significantly higher than that in the patients with a distance of >0.5 cm between the lesion and the gallbladder (17.2% vs. 3.7%, p = .020).
Table 3. Comparison of gallbladder-related complications after RFA between HCC and liver metastases adjacent to the gallbladder.
Among the patients in the ≤0.5 cm group, those who did not undergo hydrodissection (8/28) had a higher incidence of gallbladder-related complications than those who did (3/36) (28.6% vs. 8.3%, p = .033). Among the patients in the >0.5 cm group, there was no significant difference in the incidence of gallbladder-related complications between those who did not undergo hydrodissection (2/39, 5.1%) and those who did (0/15, 0%) (p = .371).
Logistic regression showed that the Child-Pugh class (OR: 6.882, p = .041; 95% CI: 1.082–43.772), the distance between the lesion and the gallbladder (OR: 7.449, p = .016; 95% CI: 1.464–37.910), and hydrodissection (OR: 4.741, p = .035; 95% CI: 1.114–20.178) were associated with gallbladder-related complications in all patients ().
Table 4. Logistic analysis of factors influencing gallbladder-related complications after RFA between HCC and liver metastases adjacent to the gallbladder.
Assessment of technical effectiveness
One month after RFA, the technical effectiveness was evaluated using enhanced CT and MRI. Among the 113 patients (118 lesions), three patients (three lesions) were lost to follow-up. Among the 115 lesions evaluated, three were tumor residues, and 112 were successfully ablated. The technical effectiveness was 97.4% (112/115) (). The three lesions with tumor residues were all colorectal cancer liver metastases.
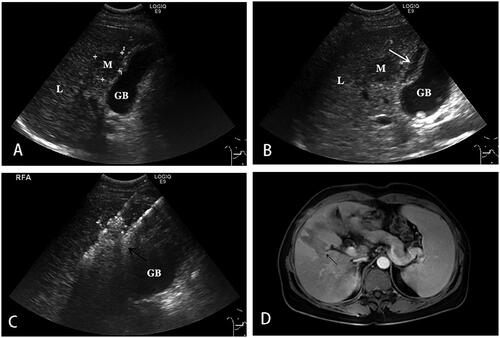
Figure 2. A 44-year-old female with HCC lesions in the S5 of the liver adjacent to the gallbladder underwent ultrasound-guided RFA.
A. The lesion in the S5 of the liver adjacent to the gallbladder, with a size of 2.6 × 2.2 cm. B. The gallbladder fossa was filled with saline (white arrow) using the hydrodissection technique. C. RFA (black arrow) with Celon double-needle for the HCC lesions near the gallbladder in the S5. D. 1 month after RFA S5 of the liver (black arrow), MRI showed no enhancement of the ablation lesion. The lesion achieved technical effectiveness.
There was no significant difference in technical effectiveness between patients with HCC and those with metastatic cancer (100% vs. 94.2%, p = .053). However, among the patients with lesions ≤3 cm, the technical effectiveness in the HCC group (100%, 48/48) was significantly higher than that in the liver metastasis group (91.2%, 31/34) (p = .036). The technical effectiveness in patients with HCC (n = 15) and those with liver metastases (n = 18) in the >3 cm group was 100% (). In patients with HCC, the technical effectiveness in both the ≤3 cm group (n = 48) and the >3 cm group (n = 15) was 100%. In patients with liver metastases, there was no significant difference in technical effectiveness between the ≤3 cm group (n = 34) and the >3 cm group (n = 18, p = .194).
Table 5. Technical effectiveness of radiofrequency ablation for liver tumors adjacent to the gallbladder.
Among all lesions that were analyzed, 73 (63.5%) had an ablation margin ≤5 mm, of which 95.9% achieved technical effectiveness, and 42 (36.5%) had an ablation margin >5 mm, of which all (100%) achieved technical effectiveness; there was no significant difference between the groups (p = .298). Among the HCC lesions, those with an ablation margin ≤5 mm and those with >5 mm ablation margins achieved technical effectiveness (100% vs. 100%, p = 1.000). Among patients with liver metastases, all patients with an ablation margin ≤5 mm had a lower technical effectiveness rate than those with an ablation margin >5 mm (88.5% vs. 100%), but the difference was not significant (p = .235).
Discussion
In this study, we found that percutaneous radiofrequency ablation of HCC lesions and liver metastases adjacent to the gallbladder was a safe and effective treatment option. A more stable and safer ablation zone can be created in patients with HCC than in those with liver metastases.
The distance between the lesion and the gallbladder is an important factor affecting the risk of complications [Citation28]. In this study, we reached different conclusions regarding HCC and metastatic lesions. Among the patients with liver metastases, the incidence of complications in the ≤0.5 cm group was significantly higher than in the >0.5 cm group. However, among patients with HCC, there was no significant difference between the ≤0.5-and >0.5 cm groups. We first considered that these differences might be related to liver disease conditions and function. In this study, multivariate analysis showed that liver function classification was essential for gallbladder-related complications (p = .041). All patients with HCC had a history of cirrhosis, and seven (12.1%) patients had grade B liver function, whereas none of the patients with metastases had liver cirrhosis or grade A liver function. A previous study [Citation29] found that the cirrhotic tissue had the ‘oven effect’ in RFA treatment, which refers to the hypothesis that the liver with fibrous cirrhosis plays a role in heat insulation, and can concentrate the heat in the tumor tissue to obtain a higher ablation temperature and a more uniform ablation area. Liu et al. [Citation30] showed that the lower thermal conductivity of background tissues significantly increases the temperatures within a defined ablation target. These findings provide insights into the oven effect. Patients with HCC generally have a history of liver cirrhosis, which reduces the thermal conductivity of the liver. The internal temperature of the tumor increases, and heat is prevented from spreading out, thereby reducing damage to the gallbladder wall. This may also be related to tumor morphology or characteristics, as several studies [Citation22,Citation31] have confirmed by biopsy that microsatellite lesions were found within 5 mm of the lesion margin in patients with metastatic cancer, which could not be detected by imaging. Therefore, it is possible that metastases spread to the gallbladder fossa more frequently than HCC tumors. We will further explore the reasons for this finding in a subsequent study.
Hydrodissection was associated with gallbladder-related complications in all patients. When the distance between the tumor and gallbladder was ≤0.5 cm, the patients without hydrodissection had a higher incidence of gallbladder-related complications than those with hydrodissection. This shows that hydrodissection reduces gallbladder-related complications and protects the gallbladder wall, consistent with previous studies [Citation8,Citation10,Citation32]. The main reason is that water injection into the gallbladder bed separates the gallbladder wall from the lesion and reduces the risk of thermal damage to the gallbladder wall, particularly in patients with lesions less than 0.5 cm from the gallbladder.
The technical effectiveness reported in this study was 97.4%, which is similar to that reported in previous studies [Citation33]. All three residual lesions were observed in the patients with liver metastases. In addition, the technical effectiveness of RFA for treating HCC was better than that of RFA for treating liver metastases (p = .053). Although there was no statistically significant difference between the two groups, the p-value was close to .05. We hypothesize that the expansion of the sample size may lead to significant differences between the two groups for the following reasons: first, liver metastases occur in non-cirrhotic tissue, which does not accumulate more energy to form a ‘hot-furnace’ effect; second, colorectal liver metastases may have worse biological behavior than HCC [Citation34]. Therefore, in clinical practice, using more energy or a longer ablation time for liver metastases is necessary.
Creating sufficient ablation margins is essential for thermal ablation and remains the most important technical factor for the ablation effect [Citation22,Citation23]. In this study, the ablation margin was not a significant factor affecting technical effectiveness, which may be related to the fact that high technical effectiveness was achieved both with ablation margin >5 mm and ≤5 mm groups (p = .192). The relationship between ablation margin, recurrence, and survival will be further investigated during long-term follow-up.
Previous studies [Citation35–37] have shown that lesion size is an important factor affecting prognosis; however, the two groups with lesions >3 cm and ≤3 cm achieved similar short-term ablation outcomes, consistent with the outcomes reported in previous reports [Citation38,Citation39]. This may be due to the application of multipoint, multiple, and overlapping ablation strategies for larger tumors [Citation40]. Subgroup analysis showed that among patients with lesions ≤3 cm, the technical effectiveness among patients with HCC was higher than that among those with liver metastases, possibly because a history of liver cirrhosis affected the ablation effect.
In this study, 65.5% of patients with liver metastases had primary colorectal cancer, and 35.5% of patients had other cancers, including one with thymic cancer and one with esophageal cancer. At present, there are fewer studies on radiofrequency ablation for the treatment of tumors with liver metastases than for colorectal cancer, despite RFA having a shorter recovery period and preserving more of the organ parenchyma; therefore, it is important to expand treatment options for patients with cancers that may not be suitable for resection. It has also been used to treat metastatic cancer [Citation1]. In the current study, researchers also confirmed that RFA could improve the survival rate of patients with metastatic cancer [Citation41–44].
This study has some notable limitations. First, it was based on short-term follow-up results and did not include an analysis of factors that might affect long-term outcomes, such as chemotherapy regimens. Second, the sample size was relatively small; therefore, further studies with larger sample sizes are needed to confirm the results. Third, histological specimens could not be obtained to confirm gallbladder wall damage because patients showed improvement or healed after observation or conservative treatment.
In conclusion, percutaneous radiofrequency ablation for HCC lesions and liver metastases adjacent to the gallbladder is a safe and effective treatment option. Patients with a distance of ≤0.5 cm between the lesion and gallbladder had higher gallbladder-related complications than patients with a distance of >0.5 cm, especially those with liver metastases. Among patients with lesions ≤3 cm, RFA showed greater technical effectiveness in treating HCC than in treating liver metastases.
Author contributions
Yuanfeng Meng and Binbin Jiang contributed equally to the study. Kun Yan contributed to the conception and design of the study. All authors contributed to the manuscript revision and read and approved the submitted version.
Acknowledgments
We are grateful to Dr. Zhao Yang for statistical assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The approval of data and material was obtained from participants.Consent for publication Not applicable.
Additional information
Funding
References
- Mansur A, Garg T, Shrigiriwar A, et al. Image-guided percutaneous ablation for primary and metastatic tumors. Diagnostics. 2022;12(6):1300.
- Deng Q, He M, Fu C, et al. Radiofrequency ablation in the treatment of hepatocellular carcinoma. Int J Hyperth. 2022;39(1):1052–1063.
- Yang G, Wang G, Sun J, et al. The prognosis of radiofrequency ablation versus hepatic resection for patients with colorectal liver metastases: a systematic review and meta-analysis based on 22 studies. Int J Surg. 2021;87:105896.
- Wang LJ, Zhang ZY, Yan XL, et al. Radiofrequency ablation versus resection for technically resectable colorectal liver metastasis: a propensity score analysis. World J Surg Onc. 2018;16(1):207.
- Vietti Violi N, Duran R, Demartines N, et al. Local recurrence rate in patients with colorectal cancer liver metastasis after wedge resection or percutaneous radiofrequency ablation. Int J Hyperthermia. 2018;34(7):1020–1028.
- Toshimori J, Nouso K, Nakamura S, et al. Local recurrence and complications after percutaneous radiofrequency ablation of hepatocellular carcinoma: a retrospective cohort study focused on tumor location. Acta Med Okayama. 2015;69(4):219–226.
- Levit E, Bruners P, Günther RW, et al. Bile aspiration and hydrodissection to prevent complications in hepatic RFA close to the gallbladder. Acta Radiol. 2012;53(9):1045–1048.
- Azevedo A, Falsarella P, Rocha R, et al. Percutaneous cholecystostomy and hydrodissection in radiofrequency ablation of liver subcapsular leiomyosarcoma metastasis adjacent to the gallbladder: protective effect. J Radiol Case Rep. 2016;10(10):24–32.
- Fan J, He S, Zheng Y. Analyses of clinical efficacy of ultrasound-guided radiofrequency ablation in liver cancer adjacent to the gallbladder and its prognosis. J Buon. 2019;24(6):2411–2417.
- Garnon J, Koch G, Caudrelier J, et al. Hydrodissection of the gallbladder bed: a technique for ablations located close to the gallbladder. Cardiovasc Intervent Radiol. 2019;42(7):1029–1035.
- Benson AB, D’Angelica MI, Abbott DE, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Network. 2021;19(5):541–565.
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380.
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422.
- Yoshino T, Arnold D, Taniguchi H, et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol. 2018;29(1):44–70.
- Benson AB, Venook AP, Al-Hawary MM, et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Comp Cancer Network. 2021;19(3):329–359.
- Kew MC. Epidemiology of chronic hepatitis B virus infection, hepatocellular carcinoma, and hepatitis B virus-induced hepatocellular carcinoma. Pathol Biol. 2010;58(4):273–277.
- Kanda T, Goto T, Hirotsu Y, et al. Molecular mechanisms driving progression of liver cirrhosis towards hepatocellular carcinoma in chronic hepatitis B and C infections: a review. Int J Mol Sci. 2019;20(6):1358.
- Konyn P, Ahmed A, Kim D. Current epidemiology in hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2021;15(11):1295–1307.
- Wang H, Lee JC, Cao K, et al. What is the difference in ablation zone of multi-bipolar radiofrequency ablation between liver cirrhosis and normal liver background? – A prospective clinical study. Int J Hyperther. 2020;37(1):1248–1259.
- Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370.
- Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of Colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36(1):166–175.
- Sotirchos VS, Petrovic LM, Gönen M, et al. Colorectal cancer liver metastases: biopsy of the ablation zone and margins can be used to predict oncologic outcome. Radiology. 2016;280(3):949–959.
- Shady W, Petre EN, Do KG, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol. 2018;29(2):268–275.e261.
- Yan K, Yang W, Chen MH, et al. Clinical application of hepatic wedge ablation for treating liver malignancies of the inferior margin: a new ablation technique. Int J Hyperth. 2017;33(2):203–211.
- Chen MH, Wei Y, Yan K, et al. Treatment strategy to optimize radiofrequency ablation for liver malignancies. J Vasc Interv Radiol. 2006;17(4):671–683.
- Chopra S, Dodd GD, 3rd, Chanin MP, et al. Radiofrequency ablation of hepatic tumors adjacent to the gallbladder: feasibility and safety. AJR Am J Roentgenol. 2003;180(3):697–701.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology. 2014;273(1):241–260.
- Lee J, Rhim H, Jeon YH, et al. Radiofrequency ablation of liver adjacent to body of gallbladder: histopathologic changes of gallbladder wall in a pig model. AJR Am J Roentgenol. 2008;190(2):418–425.
- Livraghi T, Goldberg SN, Lazzaroni S, et al. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210(3):655–661.
- Liu Z, Ahmed M, Weinstein Y, et al. Characterization of the RF ablation-induced ‘oven effect’: the importance of background tissue thermal conductivity on tissue heating. Int J Hyperther. 2006;22(4):327–342.
- Hayashi H, Nabeshima K, Hamasaki M, et al. Presence of microsatellite lesions with colorectal liver metastases correlate with intrahepatic recurrence after surgical resection. Oncol Rep. 2009;21(3):601–607.
- Tewari SO, Petre EN, Osborne J, et al. Cholecystokinin-assisted hydrodissection of the gallbladder fossa during FDG PET/CT-guided liver ablation. Cardiovasc Intervent Radiol. 2013;36(6):1704–1706.
- Petit A, Hocquelet A, N'kontchou G, et al. No-Touch multi-bipolar radiofrequency ablation for the treatment of subcapsular hepatocellular carcinoma ≤5 cm not puncturable via the non-tumorous liver parenchyma. Cardiovasc Intervent Radiol. 2020;43(2):273–283.
- Liu C, He J, Li T, et al. Evaluation of the efficacy and postoperative outcomes of hydrodissection-assisted microwave ablation for subcapsular hepatocellular carcinoma and colorectal liver metastases. Abdom Radiol. 2021;46(5):2161–2172.
- Lee DH, Lee JM, Lee JY, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014;270(3):900–909.
- Yang W, Yan K, Goldberg SN, et al. Ten-year survival of hepatocellular carcinoma patients undergoing radiofrequency ablation as a first-line treatment. World J Gastroenterol. 2016;22(10):2993–3005.
- Kim GA, Shim JH, Kim MJ, et al. Radiofrequency ablation as an alternative to hepatic resection for single small hepatocellular carcinomas. Br J Surg. 2015;103(1):126–135.
- Orlacchio A, Chegai F, Del Giudice C, et al. Radiofrequency thermoablation of HCC larger than 3 cm and less than 5 cm proximal to the gallbladder without gallbladder isolation: a single center experience. Biomed Res Int. 2014;2014:896527.
- Bai XM, Cui M, Yang W, et al. The 10-year survival analysis of radiofrequency ablation for solitary hepatocellular carcinoma 5 cm or smaller: primary versus recurrent HCC. Radiology. 2021;300(2):458–469.
- Chen MH, Yang W, Yan K, et al. Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients–mathematic model, overlapping mode, and electrode placement process. Radiology. 2004;232(1):260–271.
- Pan C, Wu P, Yu J, et al. CT-guided radiofrequency ablation prolonged metastatic survival in patients with liver metastases from nasopharyngeal carcinoma. Int J Hypertherm. 2011;27(6):549–554.
- Chen YP, Chan ATC, Le QT, et al. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80.
- Jin Y, Cai YC, Cao Y, et al. Radiofrequency ablation combined with systemic chemotherapy in nasopharyngeal carcinoma liver metastases improves response to treatment and survival outcomes. J. Surg. Oncol. 2012;106(3):322–326.
- Hoshino S, Furukawa M, Aragane K, et al. Successful multimodal treatment in a patient with thymoma accompanied by hepatic metastasis. J Thorac Oncol. 2008;3(1):98–100.