Abstract
Purpose
Radiotherapy (RT) is the primary treatment for prostate cancer (PCa); however, the emergence of castration-resistant prostate cancer (CRPC) often leads to treatment failure and cancer-related deaths. In this study, we aimed to explore the use of microwave hyperthermia (MW-HT) to sensitize PCa to RT and investigate the underlying molecular mechanisms.
Methods
We developed a dedicated MW-HT heating setup, created an in vitro and in vivo MW-HT + RT treatment model for CRPC. We evaluated PC3 cell proliferation using CCK-8, colony experiments, DAPI staining, comet assay and ROS detection method. We also monitored nude mouse models of PCa during treatment, measured tumor weight, and calculated the tumor inhibition rate. Western blotting was used to detect DNA damage repair protein expression in PC3 cells and transplanted tumors.
Results
Compared to control, PC3 cell survival and clone formation rates decreased in RT + MW-HT group, demonstrating significant increase in apoptosis, ROS levels, and DNA damage. Lower tumor volumes and weights were observed in treatment groups. Ki-67 expression level was reduced in all treatment groups, with significant decrease in RT + MW-HT groups. The most significant apoptosis induction was confirmed in RT + MW-HT group by TUNEL staining. Protein expression levels of DNA-PKcs, ATM, ATR, and P53/P21 signaling pathways significantly decreased in RT + MW-HT groups.
Conclusion
MW-HT + RT treatment significantly inhibited DNA damage repair by downregulating DNA-PKcs, ATM, ATR, and P53/P21 signaling pathways, leading to increased ROS levels, aggravate DNA damage, apoptosis, and necrosis in PC3 cells, a well-established model of CRPC.
Introduction
Prostate cancer (PCa) is commonly diagnosed in males (14.1%) and is the fifth leading cause of cancer death worldwide [Citation1], where castration-resistant prostate cancer (CRPC) contributes significantly. Unlike advanced PCa, which is sensitive to androgen deprivation therapy, CRPC patients eventually develop biochemical and clinical evidence of treatment resistance [Citation2–4], which results in poor clinical efficiency and prognosis. Surgery, radiation therapy, immunotherapy, targeted therapy, and chemotherapy are common clinical therapies that aim to overcome CRPC [Citation5–8]; however, more than half of these patients develop resistance after therapy, leading to pernicious consequences. Furthermore, these treatments have serious subsidiary side effects such as urinary incontinence and erectile dysfunction [Citation9, Citation10]. Therefore, there is an urgent need to develop improved treatment strategies for CRPC.
Radiotherapy (RT) has historically been the treatment of choice for PCa due to its demonstrated safety and feasibility in the treatment of primary tumors. In addition, it has the potential to improve oncological outcomes in patients with CRPC. Unfortunately, CRPC is often resistant to RT, making it an incurable disease [Citation11, Citation12]. The limitations of RT are mainly reflected in the radiation insensitivity of hypoxic tumor cells and the DNA damage response (DDR). An appealing strategy to potentially supplement systemic radiosensitizers is the use of non-ablative hyperthermia (HT), often termed as moderate HT (40–45 °C), as a localized radiosensitizer [Citation13]. Numerous clinical studies have suggested that HT can be widely used in combination chemotherapy and RT to improve the effect of comprehensive tumor treatment [Citation14–19]. Several phase I and II studies of HT + RT for PCa have been conducted, with some showing promising results for overall survival [Citation20, Citation21].
In the case of PCa, published data have shown an oncological benefit of additional HT combined with definitive RT in advanced PCa that is accompanied by excellent tolerability [Citation13, Citation22]. In addition to clinical studies, various fundamental studies have been conducted on the mechanisms of hyperthermic sensitivity to RT. HT can sensitize hypoxic tumor cells and inhibit the repair of DNA damage induced by RT, and cells in the radiation-resistant ‘S’ phase are sensitive to HT [Citation23]. However, most current methods for basic studies of HT are water baths, which do not reflect actual clinical HT. To solve this problem, we developed special microwave radiation equipment [Citation24] that can provide microwave irradiation at a certain temperature. Our basic research found that Microwave-Hyperthermia (MW-HT) combined with photothermal agents can accurately target CPRC [Citation25]. In this study, this system was used to explore the related mechanism of the effect of MW-HT combined with RT on PCa.
This study aimed to explore the molecular mechanism of MW-HT sensitized RT and provide guidance and further information for clinical treatment of PCa. In this study, a self-made MW-HT device was used to simulate clinical HT, and the radiation sensitivity of MW-HT combined with RT (MW − HT + RT) on PCa was measured, the extent of DNA damage and ROS content in PCa cells was detected, and the potential changes in the protein levels of ATM, ATR, and DNA-PKcs were investigated. We found that MW-HT in combination with RT increases ROS levels in PC3 cells and inhibits DNA damage repair after RT by down-regulating DNA-PKCs, ATM, ATR, and P53/P21 mediated signaling pathways, thereby inducing apoptosis in tumor cells.
Materials and methods
Cell lines and animals
Human PC3 PCa cells were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). PC3 cells were cultured in DMEM (Sigma-Aldrich, St. Louis, MI, USA) supplemented with fetal bovine serum (10%, Gibco, NY, USA.), penicillin (10,000 units/mL), and streptomycin (10 mg/mL).
Balb/c Nu/Nu male mice (6 weeks old, 16–18 g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd (China), and all experimental procedures involving animal studies were approved by the Zhejiang Chinese Medical University Animal Care and Use Committee. Aliquots of 1.5 × 107 PC3 cells were injected subcutaneously 1 cm into the dorsum of the mouse. After tumors became palpable at approximately 7–9 mm in diameter, the mice were randomly assigned to control, 43 °C MW-HT (MW-43 °C), 10 Gy dose of radiotherapy (RT-10Gy), and RT-10Gy + MW-43 °C (n = 5) groups. The specific treatment plan for PCa nude mice is shown in . Tumor volume was assessed every 2 days with a caliper and computed using the following formula: Volume (mm3) = (L × W2)/2, where L and W designate the length and width of the tumor, respectively. The study was terminated based on the welfare of the experimental subjects, the progression of the tumor, and the recommended cycle length of treatment. After treatment, tumor tissues of the mice in each group were fixed with paraformaldehyde for histological analysis and stained with hematoxylin and eosin.
Radiation and microwave-hyperthermia therapy treatment system
PC3 cells were exposed to homogeneous X-rays using an X-RAD225 Biological Irradiator (Precision, X-ray Inc, USA; equipment parameter: 225 kV/s; 13.3 mA; filter: 2 mm AI; source-to-skin distance (SSD): 40 cm)), as shown in . Cell culture plates or dishes were placed in the X-RAD225 Biological Irradiator. PCa nude mice were anesthetized using sodium pentobarbital solution (40 mg/kg, ip). As shown in , the mice were placed in the X-RAD225 Biological Irradiator (equipment parameter: 225 kV/s; 13.3 mA; filter: 2.5 mm AI/0.1 mm Cu; SSD: 35 cm) and were irradiated with a total dose of 10 Gy to the tumor area while shielding the rest of the body. Mouse RT schedules were modified and adapted from previously reported studies of Lin et al. [Citation26] and Rajkumar et al. [Citation27] for PCa pre-clinical models.
Figure 1. Microwave hyperthermia or radiotherapy devices were used for in vitro and in vivo experiments. (A) An X-RAD225 Biological Irradiator. (B) Cell culture plates in the irradiator. (C) Prostate cancer (P Ca) nude mice in the irradiator. (D) A microwave hyperthermia device. (E) Cell culture plates under the MW generator. (F) P Ca nude mice under the MW generator. (G) Schematic diagram of MW-HT starting work according to temperature. (H) The change trend of frontal temperature and rectal temperature with MWHT work. (J) The change trend of tumor epidermal temperature and tumor core temperature with MW-HT work.
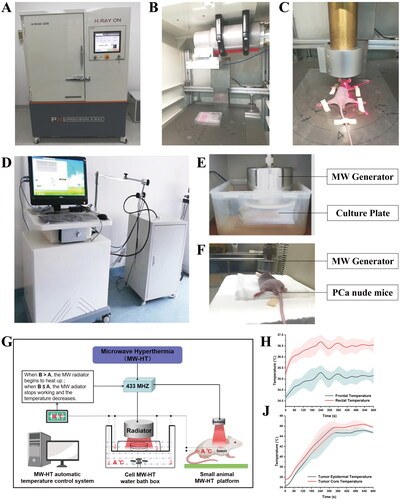
In this study, homemade MW-HT devices were used for both in vitro and in vivo experiments. The self-made MW-HT device included four main components: microwave generator, microwave radiator, automatic control system, and optical fiber temperature probes 1 and 2 (not affected by electromagnetic waves), as shown in . The working diagram of the MW-HT devices is shown in .When the temperature of the cell is lower than the set temperature, the MW radiator starts to heat up. When the cell temperature is greater than or equal to the set temperature, the MW radiator stops working and the temperature decreases.
As illustrated in , prior to initiating MW-HT, the temperature of the surrounding circulating water is adjusted to approximately 0.5 °C below the set point, and the cell culture plate, microwave radiator, and optical fiber temperature probe are accurately positioned. Subsequently, the MW-HT device is securely sealed, and the microwave generator is activated. Typically, the temperature will reach the set point within approximately 5 min. Throughout this period, it is important to ensure that the MW-HT device is operating within normal parameters. After treatment with MW-HT, the cell culture plate was removed and placed in an incubator for further culture.
As shown in , following the mice being placed under anesthesia, the microwave radiation probe was gently positioned about 5–10 mm away from the transplanted tumor. The parameters of the MW-HT device were set as follows: output power: 25 W, hyperthermia time: 10 min). This study used noninvasive frontal temperature detection instead of anal temperature detection and tumor epidermal temperature instead of tumor center temperature. showed that frontal temperature and tumor epidermal temperature were in agreement, indicating that this method of temperature measurement is feasible. The forehead and tumor skin temperatures of naked mice were measured at 0, 60, 120, 180, 240, 360, 480, and 600 s during MW-HT treatment. Forehead temperature was measured using a forehead thermometer and the temperature of the tumor epidermis was measured using a Testo-872 infrared thermometer (Testo, Germany; thermal sensitivity < 0.06 °C). Throughout the HT process, considering the skin temperature of the mice should be avoided, as it is too extreme. After confirming that all mice in each group were awake, they were kept in an experimental animal room for routine feeding.
Cell viability assay
The viability of PC3 cells was detected using the Cell Counting Kit-8 (MedChem Express, Princeton, NJ, USA) assay according to the manufacturer’s instructions. Briefly, approximately 5 × 103 PC3 cells/well were seeded into 96-well plates and divided into the different experimental groups (control, MW (41, 43, 45 °C), RT (2, 4, 6, 8, 10, 12 Gy) and MW + RT (41 °C + 4Gy, 43 °C + 4Gy, 45 °C + 4Gy). After treatment for 24, 48, 72, and 96 h, the absorbance was measured at 550 nm using a Multiskan Spectrum spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
Colony forming assay
PC3 cells were exposed to the indicated treatments and seeded into 6-well plates. The number of implants per well in cloning experiments of PC3 cells with different RT doses and HT combined with radiotherapy is shown in Supplementary Material Table S1. After culturing for 2 weeks, the cells were fixed with 4% paraformaldehyde for 20 min at room temperature. The cells were then washed with PBS and stained with crystal violet solution for 20 min. The results were analyzed using ImageJ software (version 1.5).
Measurement of intracellular ROS levels
The production of intracellular ROS was detected using the fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA, Beyotime Biotechnology, China). Briefly, 8 × 105 PC3 cells were seeded into 6-well plates and then divided into different experimental groups (control, MW-43 °C, RT-4 Gy, and 43 °C + 4Gy). Following treatment for 6 h, the cells were incubated with 10 μm DCFH-DA in FBS-free DMEM for 20 min at 37 °C and then washed three times for 10 min with PBS. Subsequently, the cells were mounted under a fluorescence microscope (Olympus BX61; Olympus, Tokyo, Japan) at an excitation wavelength of 488 nm and emission wavelength of 525 nm. Using Image-Pro Plus software (Media Cybernetics, Inc., Rockville, MD, USA), the average fluorescence intensity of the images was evaluated and normalized to obtain the relative ratio between the experimental groups.
Alkaline single-cell gel electrophoresis (comet assay)
A detailed procedure was followed to evaluate the potential DNA damage in PC3 cells. First, a total of 6 × 105 cells were plated in each well of a 6-well plate and allowed to adhere for 24 h. The cells were then distributed into different experimental groups: control, MW-43 °C, RT-4 Gy, and 43 °C + 4Gy. Subsequently, 0.65% low-melting agarose, mixed with cells, was added onto a slice of 0.65% normal-melting agarose, pre-coated on a glass slide. The slide was then placed in lysis buffer containing 1% Triton X-100 at 4 °C for 1 h and subsequently transferred to lysis buffer containing 0.5 mg/mL DNase-free proteinase K(Amresco, OH) at 37 °C for 2 h. This was followed by immersion of the slide in ice-cold alkaline electrophoresis solution for 20 min to unwind the DNA in the cells before electrophoresis. The electrophoresis was carried out at a constant current of 300 mA for 20 min. Following electrophoresis, the cells were neutralized in Tris buffer (0.4 M, pH = 7.5) for 15 min. The DNA damage was subsequently evaluated by staining the comets with Gel-red and capturing the images with a fluorescence microscope (Nikon, Tokyo, Japan). The number of comets was quantified using the CASP 1.2.2 software (Krzysztof Konca, Wroclaw, Poland) for each sample. A total of approximately 100 comets were counted for each sample.
DAPI staining
DAPI staining was used to detect chromatin condensation and nuclear fragmentation, which are known signatures of apoptosis. PC3 cells (6 × 105 cells per well) were inoculated into 6-well plates, allowed to attach for 24 h, and then divided into the different experimental groups (control, MW-43 °C, RT-4Gy, and 43 °C + 4Gy). After treatment for 24, 48, and 72 h, the medium was removed and the cells were washed with PBS. The cells were fixed with 4% paraformaldehyde at room temperature for 15 min. The cells were then gently washed three times for 15 min with 4% paraformaldehyde. DAPI (Beyotime Biotechnology) solution (5 µg/ml) was added in the dark and incubated for 20 min. Cells were observed under a fluorescence microscope and photographed under a suitable field of view. Chromatin condensation was observed under fluorescence microscopy, and apoptosis was counted three times in 100 randomly selected cells.
Western blot analysis
The differently treated cells and tumor tissues were lysed at 4 °C using RIPA buffer (R0010, Solarbio, Beijing, China) mixed with protease and phosphatase inhibitors. The protein concentration was determined using a BCA Protein Assay kit (P0012, Beyotime Biotechnology). The PVDF membranes were incubated in 5% nonfat milk for 1–2 h and probed with primary antibodies overnight at 4 °C. Western blot analysis was performed using the following antibodies: anti-DNA-PKcs (38168s, 1:1000, Cell Signaling Technology (CST), Danvers, MA, USA), anti-Ku80 (2753s, 1:1000, CST), anti-Ku70 (4588s, 1:1000, CST), anti-p53 (9282s, 1:1000, CST), anti-p21 (2947s, 1:1000, CST), anti-p21 (2947s, 1:1000, CST), anti-ATM (2873s, 1:1000, CST), anti-phosphorylated(p-)ATM (5883s, 1:1000, CST), anti-Chk2 (6334s, 1:1000, CST), anti-p-Chk2 (2197s, 1:1000, CST), anti-ATR (2790s, 1:1000, CST), anti-p-ATR (2853s, 1:1000, CST), anti-Chk1 (2360s, 1:1000, CST), anti-p-Chk1 (2348s, 1:1000, CST), and anti-GAPDH (5174s, 1:1000, CST). After washing with TBST three times, the membranes were incubated with goat anti-rabbit IgG HRP-linked secondary antibody (7074s, 1:5000, CST) for 1–2 h at room temperature. The BeyoECL plus kit (Beyotime, P0018) was used for protein bands signal testing. Protein images were captured using an Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE, USA).
TUNEL assay
The TUNEL assay was performed using the Colorimetric TUNEL Apoptosis assay kit (C1091, Beyotime Biotechnology), according to the manufacturer’s manual. Tumor sections were treated with 3% hydrogen peroxide and TUNEL assay solution. Following three washes with PBS, the samples were stained with DAB and counterstained with hematoxylin. The number of TUNEL-positive cells in three random fields was counted using an Olympus microscope.
Immunohistochemistry (IHC) analysis
The tumor sections were rehydrated and treated with H2O2 at room temperature for 15 min. After blocking with goat serum blocking solution for 60 min, the sections were incubated with anti-ki67 (9449s, 1:200, CST) at 4 °C overnight, followed by incubation with a peroxidase-conjugated secondary antibody for 30 min at room temperature. After the tombstone reaction developed, the sections were re-stained with hematoxylin. To determine the expression of Ki67, a scoring system was employed using the Image-ProPlus 4.5 software (Media Cybernetics, Silver Spring, MD, USA). The immunohistochemical study evaluation criteria was as follows: 0 (no area dyed), 1 (< 25% of the area dyed), 2 (26–50% of the area dyed), 3 (51–75% of the area dyed), and 4 (76–100% of the area dyed). A score < 3 indicated weak expression, while a score ≥ 3 indicated strong expression.
Statistical analyses
Statistical analysis was performed using GraphPad Prism 5.0 and SPSS 22.0. Data are expressed as the mean ± standard deviation (SD) of at least three independent trials. T-test was used for within-group comparisons, while the one-way ANOVA with Tukey’s post hoc analysis was employed for between-group comparisons. A P-value less than 0.05 was deemed statistically significant.
Results
Radiosensitization effect of MW-HT in vivo
To explore the potential of HT in improving radiosensitivity for PCa, we conducted in vivo experiments to evaluate the impact of MW-HT on RT outcomes. The therapeutic efficacy was assessed in mice subjected to various experimental conditions (control, MW-43 °C, RT-10Gy, and 43 °C + 10Gy groups), as depicted in the animal experiment design shown in the . During the treatment, tumor temperature rose with increasing MW-HT treatment duration in both the MW-43 °C and 10 Gy + MW-43 °C groups. The temperature of the transplanted tumor reached 41 °C at 180 s, whereas the skin temperature of the tumor area remained approximately 43 °C after 360 s (). As shown in , there was no significant disparity in the weight of the mice among the groups. However, after undergoing MW-HT treatment, mice in the MW-43 °C and 10 Gy + MW-43 °C groups exhibited a slight increase in weight compared to the control and RT-10Gy groups. After the third session of MW-HT treatment, the volume of PCa xenografts was reduced in groups treated with MW-43 °C, RT-10Gy, and 10 Gy + MW-43 °C. The 10 Gy + MW-43 °C group had the strongest inhibitory effect on the growth of prostate cancer xenografts, which was significantly different from other groups (p < 0.01, ).
Figure 2. Experimental results of hyperthermia sensitization radiotherapy in vivo. (A) Schematic illustration of the in vivo experimental design. (B–C) Frontal and graft skin temperature of P Ca-bearing nude mice during microwave hyperthermia. (D) Body weight of mice in each group during microwave hypothermia (MW-HT) combined with radiotherapy (RT). (E) Relationship between primary tumor volume and days after different treatments. (F, G, H) Tumor weight and photographs of different treatment groups at the end of the experiment. The all data are expressed as the mean ± SD, n = 3–5, #P < 0.05 or ##P < 0.01 compared to the control, *P < 0.05 or **P < 0.01 compared to 10 Gy + MW-43 °C.
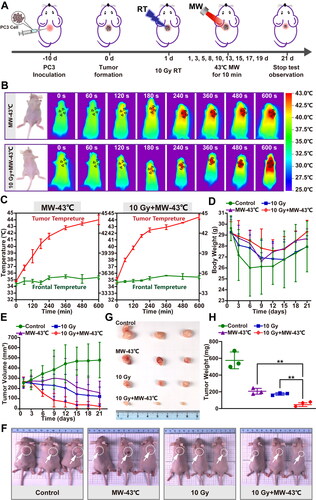
As shown in Supplementary Material Figure S1,the protocol for a mouse tumor experiment was executed with specific health parameters and treatment sequences, and the survival data of mice were systematically evaluated to plot a survival curve. Following the experiment, the transplanted neoplastic tissue was extracted, evaluated for weight, and captured in photographs. The findings demonstrated that the combination of MW-HT and RT significantly inhibited the growth of PCa xenografts compared to treatment with RT or HT alone (p < 0.01, ).
Radiation treatment inhibits PC3 cell viability
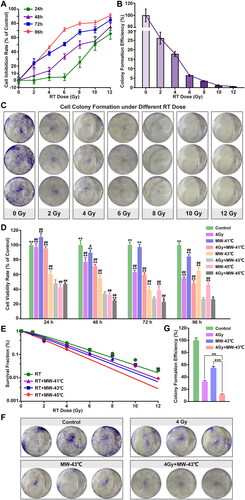
PC3 cells were treated with 2 Gy, after treatment for 24, 48, 72, and 96 h, the inhibitory rates of proliferation were −1.88 ± 5.10%, 6.72 ± 9.41%, 24.20 ± 6.37%, and 22.54 ± 4.29%, respectively (). The results showed that when the radiation dose was 4 Gy, the cell inhibition rates at 24, 48, 72, and 96 h were 4.15 ± 4.45%, 13.66 ± 4.66%, 37.95 ± 3.15%, and 46.31 ± 2.12%, respectively. The inhibition rate of PC3 cells was over 50% at 96 h for radiation doses of 6, 8, 10, and 12 Gy. The clone formation rate of PC3 cells was lower for higher radiation doses (). When the radiation dose was 2, 4, 6, 8, 10, and 12 Gy, the clone formation rates were 16.07 ± 1.93%, 10.93 ± 0.82%, 3.98 ± 0.30%, 2.18 ± 0.09%, 0.74 ± 0.17%, and 0.37 ± 0.05%, respectively. Based on the experimental data and screening principle of the radiosensitizer, we chose 4 Gy as the radiation dose for the in vitro experiments.
Figure 3. Inhibitory effect of radiotherapy on PCa PC3 cells AND in vitro radiosensitization effect of MW-HT. (A) Inhibitory effect of different radiation doses on the proliferation of P Ca P C3 cells analyzed using the CCK-8 assay. (B, C) Cloning of P C3 cells under different radiation doses. (D) The combined effect of MW-HT and 4Gy on the proliferation inhibition of P C3 cells was detected using the CCK-8 method. (E) Clonogenic survival of P Ca P C3 cells. The cells were exposed to X-ray irradiation and then treated with MW-HT. Subsequently, cells were incubated for two weeks and the number of colonies with >50 cells was scored. A multi-target click mathematical model simulated the P Ca P C3 cell SF curve. (F–G) Cloning of P Ca P C3 cells with MW-43 °C combined with RT-4Gy.The results are expressed as the mean ± SD, n = 6, **P < 0.01 or ***P < 0.001 compared to 4 Gy + MW-43 °C.
Radiosensitization effect of MW-HT in vitro
To investigate the combined effects of HT and RT on the growth of PC3 cells, cell viability was assessed using the CCK-8 assay. As shown in , compared with the control group, the proliferation of PC3 cells was significantly inhibited in the group treated with MW-HT and RT (p < 0.01). It was found that the effect of 4 Gy + MW-41 °C on PC3 cell proliferation was not stable, and short-term treatment was not excellent. Treatment with 4 Gy + MW-45 °C significantly inhibited the proliferation of PC3 cells (p < 0.01). Treatment with 4 Gy + MW-43 °C had a stable inhibitory effect on PC3 cells, and combined therapy with HT and RT significantly increased the inhibitory rate of PC3 compared with RT alone (p < 0.01). After correcting for the cell survival fraction in the HT group, SER > 1. It has been proposed that HT has a radiosensitive effect on PC3 cells. The radio sensitizing effect of MW-45 °C on PC3 cells was the strongest, SER (D0) = 1.287 and SER (Dq) =4.137 ( and Supplementary Material Table S2). As shown in , tumor tissues are difficult to reach at 45 °C in clinical MW-HT; hence, MW-43 °C combined with RT-4Gy was selected using cloning formation assay. The results showed that compared with the control group, the clone formation rate in all experimental groups decreased significantly (p < 0.01); the clone formation rates of the 4 Gy, MW-43 °C, and 4 Gy + MW groups were 12.30%, 16.64%, and 1.00%, respectively.
MW-HT combined with RT increases ROS levels
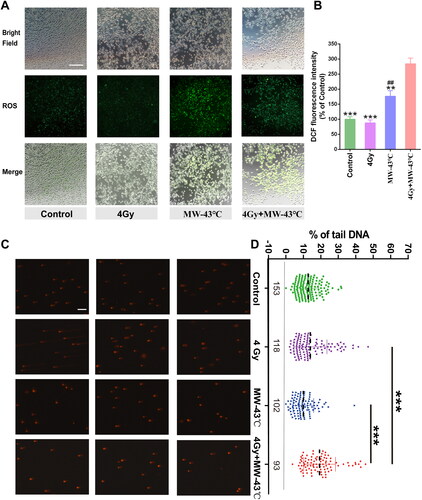
To study whether oxidative stress can promote MW-HT sensitization RT, we used DCFH-DA fluorescent probe to measure intracellular ROS production, which indicates total cell ROS. As shown in , ROS production in PC3 cells increased 2.85-fold after 6 h following combined treatment with MW-HT and RT and 1.77-fold in the MW-HT group. There were significant differences compared with the control group (, p < 0.01). The RT group did not produce excessive ROS compared to the control group.
Figure 4. Effect of MW-HT with RT on ROS levels and DNA damage in PCa PC3 cells. (A) Representative photographs of DCF fluorescence staining of P C3 cells. Scale bar = 200 μm. (B): Quantitative analyses of the mean fluorescence intensity. The data are expressed as the mean ± SD, n = 6, ##P < 0.01 compared to control, **P < 0.01 or ***P < 0.001 compared to 10 Gy + MW-43 °C. (C) The comet assay was used to investigate the effects of combining MW-HT and RT-4 Gy on DNA fragmentation in P Ca P C3 cells, Scale bar = 200 μm. (D) Mann-Whitney Rank Sum test were used to compare the difference between Control, 4Gy, MW-43 °C and MW-43 °C + 4Gy groups, ***P < 0.001 compared to 4 Gy + MW-43°C.
MW-HT enhances RT’s potential to induce notable DNA damage
In order to examine whether MW-HT could augment the effect of radiation therapy by enhancing DNA damage in PC3 cells, the comet assay was utilized. As evident from , a significant increase in DNA damage was observed in PC3 cells following 24-h treatment with MW-HT and radiation therapy, when compared with the treatment group alone (, p < 0.001). Notably, the DNA damage induced by MW-HT was not found to be significantly higher than the control group. In conclusion, our findings indicate that MW-HT can potentiate the effect of radiation therapy by inducing DNA damage in PC3 cells.
MW-HT adds DNA damage and weakens DNA repair efficiency under irradiation
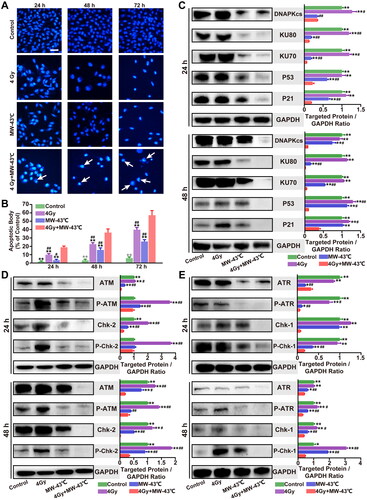
To investigate whether the MW-HT-induced effects on the viability of PC3 cells are due to induced apoptosis, chromatin condensation, and morphological changes in the nucleus, DAPI staining, which specifically interacts with DNA, was performed. To evaluate the degree of apoptosis, images of 100 cells from five random fields were captured using a fluorescence microscope (magnification: 200x). The apoptotic proportion of PC3 cells in the 4 Gy + MW-43 °C group at 24h, 48h, and 72h was 18.33%, 36.50%, and 57.17%, respectively. The 4 Gy + MW-43 °C group had a significantly higher percentage of apoptotic PC3 cells than other groups. These results indicate that MW-HT combined with RT induces a decline in PC3 cell viability, which is closely associated with apoptosis (p < 0.01, ).
Figure 5. Combined treatment with MW-HT and RT induces apoptosis by inhibiting ATM/ATR- Chk2/Chk1 and Ku70/ Ku80-DNA-PKcs-P53-P21 signaling pathways in PC3 cells. (A) Effect of combined treatment with MW-HT and RT on apoptosis of P Ca P C3 cells. Scale bar = 10 μm. (B) Graphs showing the quantification of apoptotic bodies in P C3 cell. Data are expressed as the mean ± SD, n = 6. (C–E) Western blot analysis of the signaling pathway-related proteins of DNA-P KCs, AT M, AT R, and P53/P21 in P C3 cells. GAP DH expression was included as an internal control. Data are expressed as the mean ± SD, n = 3. #P < 0.05 or ##P < 0.01 compared to control, *P < 0.05 or **P < 0.01 compared to 10 Gy + MW-43°C.
We performed histopathological analysis via hematoxylin-eosin (H&E) staining. The results demonstrated that the tumor tissue of 10 Gy + MW-43 °C-treated mice exhibited extensive nuclear condensation and fragmentation and was considerably different from the staining pattern of samples from the other groups (). Immunohistochemical analysis demonstrated that when HT was combined with RT, Ki-67 in the 10 Gy + MW-43 °C group was significantly suppressed (p < 0.01, ). In addition, 10 Gy + MW-43 °C-treated mice showed increased cancer cell apoptosis rate compared with the other groups (p < 0.05, ).
Figure 6. Combined treatment with MW-HT and RT induces apoptosis by inhibiting ATM/ATR-Chk2/Chk1 and Ku70/ Ku80-DNA-PKcs-P53-P21 signaling pathways in vivo. (A) H&E, Ki-67, and T UNEL staining of tumor tissues in different treatment groups. Scale bar = 50 μm. (B) Ki-67 positive cells were detected by immunohistochemistry and analyzed using the Image-ProPlus 6.0 software. (C) Cell apoptosis rate was calculated as the percentage of T UNEL-positive cells relative to the total cells and analyzed using the Image-ProPlus 6.0 software. (D–E) Western blot analysis of the signaling pathway-related proteins of DNA-P KCs, AT M, AT R, and P53/P21 in vivo. GAP DH expression was included as an internal control. Data are expressed as the mean ± SD, n = 3–5, #P < 0.05 or ##P < 0.01 compared to control, *P < 0.05 or **P < 0.01 compared to 10 Gy + MW-43°C.
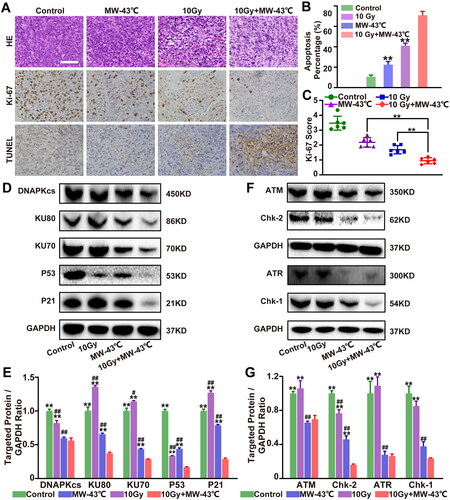
To investigate in detail the combined effect of RT and MW-HT on apoptosis in PC3 cells, ATM/ATR-Chk2/Chk1 and Ku70/Ku80-DNAPKcs-P53-P21 signaling pathway-associated proteins were examined by western blot analysis. As shown in , compared with the control and single treatment groups, the protein expression levels of DNA-PKcs, KU80, KU70, P53, and P21 in the RT + MW-HT group were significantly decreased in the PC3 cells (p < 0.05 or p < 0.01). In PCa xenografts, we found that levels of DNA-PKcs, KU80, KU70, P53, and P21 were significantly lower in the RT + MW-HT group than in the alternative group (p < 0.05 or p < 0.01(). In vitro experiments, we found significant decline in protein expression and phosphorylation levels of ATM, Chk2, ATR, and Chk1 in the RT + MW-HT group (p < 0.05 or p < 0.01, ). In vivo experiments demonstrated that ATM, Chk2, ATR, and Chk1 were significantly lower in the RT + MW-HT group than in the control group (p < 0.05 or p < 0.01, ).
Discussion
This study explored the mechanisms associated with the sensitivity of MW-HT to RT. Clinical studies have shown that HT has a positive effect on tumor treatment; however, the underlying biological mechanism of HT remains unclear [Citation28, Citation29]. In preclinical studies, water baths are the most common heating system used to study HT due to lack of specialized heat therapy instruments. Due to lack of basic research on HT instruments, it is difficult to explain the anti-tumor mechanism of HT-sensitive RT. Electromagnetic heating devices that operate at frequencies of 8–915 MHz have been proposed for cancer HT [Citation30]. We developed the current microwave generator combined with a water bath HT device [Citation24], which is used for HT experiments on tumors in vivo and in vitro, to better fit the clinical treatment plan and simulate the clinical MW-HT scenario. The application of electromagnetic microwaves results in the disturbance of the water molecules in the surrounding tissues, causing a change in the polarity of polar molecules. The resultant frictional forces result in the development of high temperatures, which can exceed the threshold for biological tissues. These elevated temperatures lead to the denaturation and inactivation of bioactive substances, including proteins and enzymes, ultimately leading to cellular death [Citation31, Citation32]. We found that the current self-developed 433 MHz MW-HT instrument combined with RT had a significant inhibitory effect on PC3 cells and a mouse transplant tumor model.
The limitations of clinical tumor RT are mainly reflected in the radiation insensitivity and DDR of tumor hypoxic cells [Citation33, Citation34]. DNA double-strand breaks (DSB) are the main cause of cancer cell death induced by radiation therapy [Citation35, Citation36]; however, DNA repair of tumor cells is one of the most influential factors that leads to radiation resistance [Citation37]. How to effectively inhibit DSB repair and enhance tumor cell radiosensitivity is a key issue to be addressed in this discipline. The DSB repair pathways in eukaryotic cells mainly include non-homologous end joining (NHEJ), homologous recombination (HR), and alternative end joining [Citation38]. It has been found that DNA-PKcs, ATM, and ATR are critical regulators of DNA damage repair-related pathways [Citation39]. DNA-PKcs and ATM mediate the repair of DNA double-strand breaks mainly through NHEJ and HR, respectively, whereas ATR responds to stalled DNA replication forks and DNA single-strand breaks [Citation40, Citation41]. DNA-PKcs play a core role in the selection of the DSB repair mode in tumor cells induced by RT and are also an essential link between RT-induced DSB damage and radiation sensitivity of tumor cells. In this study, MW-HT in combination with RT effectively inhibited Ku70/80 and DNA-PKcs expression in PC3 cells and tissues, resulting in NHEJ repair failure. As Ku70/80 is not activated after inhibition and cannot be removed from the DSB site, the DSB end is occupied by Ku70/80, and HR repair is blocked [Citation42]. Combining MW-HT and RT to kill two birds with one stone, where damaged DNA cannot be repaired by NHEJ and HR, results in cell death and thus increased radiosensitivity in PC3 cells. Thus, one way by which MW-HT can sensitize prostate cancer to RT is by inhibiting Ku70/80 and DNA-PKcs expression.
After radiation-induced DNA damage, ATR and ATM quickly detect this damage, induce complex signal cascades, activate downstream CHK1/2 to promote the repair of damaged DNA, and block the cell cycle [Citation43, Citation44]. If irreparable DNA damage occurs, the cell initiates apoptosis. We found that MW-HT in combination with RT was effective in inhibiting the expression of ATM and ATR signaling pathways and the associated protein phosphorylation in PC3 cells and tissues, thereby inhibiting DNA damage repair and promoting apoptosis in PCa. RS or DSB activate PIKK proteins ATR, DNA-PKcs, and/or ATM, causing activation of target proteins p53, Chk1, and Chk2, which regulate the G1/S, intra-S, or G2/M checkpoints, respectively [Citation45]. As shown in Supplementary Material Figures S2 and S3, MW-HT in combination with RT subsequently induced cell cycle arrest and apoptosis in PC3 cells.
As a transcription factor, P53 has the ability to activate the expression of numerous genes directly involved in regulating the cell cycle, repairing DNA damage, and influencing processes such as cell senescence, differentiation, and apoptosis [Citation46]. The present study investigated the protein expression levels of p53 and p21 in the PC3 cell line, as p21 is an established downstream target of p53 and its expression is directly influenced by the activity of p53. Our research findings unequivocally establish that the combination of MW-HT and RT effectively suppresses the activation of the p53 signaling pathway, suggesting that this combinatorial therapy could potentially retard PCa progression by interfering with this critical regulatory pathway.
Hypoxic tumors are generally more resistant to radioactivity [Citation47], and it has been found that excessive intracellular ROS is a mechanism of radiation-induced DNA damage in tumor cells. We found that MW-HT induces substantial ROS production, sensitizes them to radiation therapy, inhibits DNA damage repair, and induces apoptosis in PC3 cells.
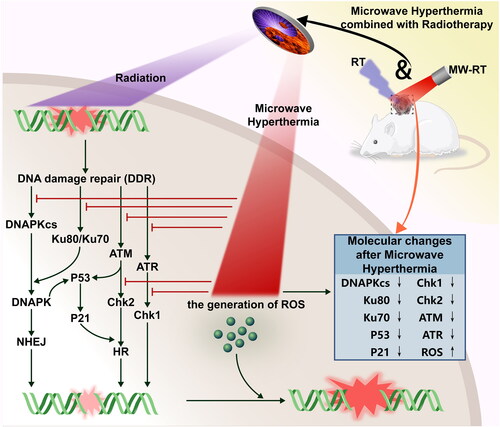
In conclusion, in the present study, we used the MW-HT device specifically designed by our research group for the first time to combine radiation therapy for PCa in vitro and in vivo. As shown in , our study found that MW-HT increased ROS production, suppressed the expression of DNA-PKcs and ATM-related proteins, rendered damaged DNA inoperable for repair by NHEJ or HR, and caused apoptosis in PC3 cells. Meanwhile, MW-HT inhibits DNA damage repair by modulating the ATR signaling pathway to block the cell cycle. In addition, MW-HT in combination with RT can suppress the expression of P53 and P21 to induce cell cycle arrest and apoptosis. Our results show that MW-HT + RT significantly inhibits DNA damage repair after RT and induces apoptosis and necrosis in PC3 cells by downregulating the DNA-PKcs, ATM, ATR, and P53/P21 signaling pathways. This pre-clinical study helps provide a laboratory basis for the application of MW-HT in combination with RT in clinical practice.
Figure 7. Schematic illustration of the treatment process of radiotherapy with microwave hyperthermia sensitivity for PCa and the associated radio-sensitive mechanism. This study found that MW-HT sensitization mainly includes the following parts: (1) MW-HT can induce the increase of ROS in P Ca cells, thereby reducing the RT resistance of hypoxic tumor cells and inducing apoptosis of P Ca cells. (2) MW-HT effectively prevents RT-induced DNA damage repair (by NHEJ and HR) by downregulating DNA-P Kcs and AT M signaling pathways, thereby sensitizing the RT effect. (3) MW-HT can inhibit the AT R signaling pathway and downregulate P53/P21, thereby blocking cell cycle to inhibit DNA damage repair and promote apoptosis of P Ca cells.
Supplemental Material
Download Zip (1.6 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):1–14. doi: 10.3322/caac.21660.
- Teo MY, Rathkopf DE, Kantoff P. Treatment of advanced prostate cancer. Annu Rev Med. 2019;70(1):479–499. doi: 10.1146/annurev-med-051517-011947.
- McNevin CS, Baird AM, McDermott R, et al. Diagnostic strategies for treatment selection in advanced prostate cancer. Diagnostics (Basel). 2021;11(2):11. doi: 10.3390/diagnostics11020345.
- Jin J. Screening for prostate cancer. JAMA. 2018;319(18):1946. doi: 10.1001/jama.2018.4972.
- Derks YHW, Löwik DWPM, Sedelaar JPM, et al. PSMA-targeting agents for radio and fluorescence-guided prostate cancer surgery. Theranostics. 2019;9(23):6824–6839. doi: 10.7150/thno.36739.
- Valle LF, Lehrer EJ, Markovic D, et al. A systematic review and meta-analysis of local salvage therapies after radiotherapy for prostate cancer. Eur Urol. 2021;80(3):280–292. doi: 10.1016/j.eururo.2020.11.010.
- Fay EK, Graff JN. Immunotherapy in prostate cancer. Cancers (Basel). 2020;12(7):12. doi: 10.3390/cancers12071752.
- Abou D, Benabdallah N, Jiang W, et al. Prostate cancer theranostics—an overview. Front Oncol. 2020;10:884. doi: 10.3389/fonc.2020.00884.
- Nguyen DD, Berlin A, Matthew AG, et al. Sexual function and rehabilitation after radiation therapy for prostate cancer: a review. Int J Impot Res. 2021;33(4):410–417. doi: 10.1038/s41443-020-00389-1.
- Campodonico F, Ennas M, Zanardi S, et al. Management of prostate cancer with systemic therapy: a prostate cancer unit perspective. Curr Cancer Drug Targets. 2021;21(2):107–116. doi: 10.2174/1568009620666201021163919.
- Catton C, Lukka H. The evolution of fractionated prostate cancer radiotherapy. Lancet. 2019;394(10196):361–362. doi: 10.1016/S0140-6736(19)31338-8.
- Sachdev S, Carroll P, Sandler H, et al. Assessment of postprostatectomy radiotherapy as adjuvant or salvage therapy in patients with prostate cancer: a systematic review. JAMA Oncol. 2020;6(11):1793–1800. doi: 10.1001/jamaoncol.2020.2832.
- Beck M, Ghadjar P, Mehrhof F, et al. Salvage-radiation therapy and regional hyperthermia for biochemically recurrent prostate cancer after radical prostatectomy (results of the planned interim analysis). Cancers (Basel). 2021;13(5):1133. doi: 10.3390/cancers13051133.
- Datta NR, Jain BM, Mathi Z, et al. Hyperthermia: a potential game-changer in the management of cancers in low-middle-income group countries. Cancers (Basel). 2022;14(2):315. doi: 10.3390/cancers14020315.
- Datta NR, Marder D, Datta S, et al. Quantification of thermal dose in moderate clinical hyperthermia with radiotherapy: a relook using temperature-time area under the curve (AUC). Int J Hyperthermia. 2021;38(1):296–307.)[doi: 10.1080/02656736.2021.1875060.
- Wang Y, Hong W, Che S, et al. Outcomes for hyperthermia combined with concurrent radiochemotherapy for patients with cervical cancer. Int J Radiat Oncol Biol Phys. 2020;107(3):499–511. doi: 10.1016/j.ijrobp.2020.03.006.
- Gani C, Lamprecht U, Ziegler A, et al. Deep regional hyperthermia with preoperative radiochemotherapy in locally advanced rectal cancer, a prospective phase II trial. Radiother Oncol. 2021;159:155–160. doi: 10.1016/j.radonc.2021.03.011.
- Hu Y, Li Z, Mi DH, et al. Chemoradiation combined with regional hyperthermia for advanced oesophageal cancer: a systematic review and meta-analysis. J Clin Pharm Ther. 2017;42(2):155–164. doi: 10.1111/jcpt.12498.
- Dharmaiah S, Zeng J, Rao VS, et al. Clinical and dosimetric evaluation of recurrent breast cancer patients treated with hyperthermia and radiation. Int J Hyperthermia. 2019;36(1):986–992. doi: 10.1080/02656736.2019.1660810.
- Hurwitz Mark D, Hansen Jorgen L, Prokopios-Davos S, et al. Hyperthermia combined with radiation for the treatment of locally advanced prostate cancer: long-term results from Dana-Farber cancer institute study 94-153. CANCER. 2011;117(3):510–516. doi: 10.1002/cncr.25619.
- Maluta S, Dall’Oglio S, Romano M, et al. Conformal radiotherapy plus local hyperthermia in patients affected by locally advanced high risk prostate cancer: preliminary results of a prospective phase II study. Int J Hyperthermia. 2007;23(5):451–456. doi: 10.1080/02656730701553260.
- Le Guevelou J, Chirila ME, Achard V, et al. Combined hyperthermia and radiotherapy for prostate cancer: a systematic review. Int J Hyperthermia. 2022;39(1):547–556. doi: 10.1080/02656736.2022.2053212.
- Datta NR, Kok HP, Crezee H, et al. Integrating loco-regional hyperthermia into the current oncology practice: SWOT and TOWS analyses. Front Oncol. 2020;10:819. doi: 10.3389/fonc.2020.00819.
- Wu ZB, Ma SL, Zhu J, et al. An experimental device for heating tumor cell. CN Patent 2015205415415. Filed July 23, 2015; issued December 2, 2015.
- Liu P, Wu Y, Xu X, et al. Microwave triggered multifunctional nanoplatform for targeted photothermal-chemotherapy in castration-resistant prostate cancer. Nano Res. 2023;16:9688–9700. doi: 10.1007/s12274-023-5541-1.
- Lin L, Kane N, Kobayashi N, et al. High-dose per fraction radiotherapy induces both antitumor immunity and immunosuppressive responses in prostate tumors. Clin Cancer Res. 2021;27(5):1505–1515. doi: 10.1158/1078-0432.CCR-20-2293.
- Rajkumar AW, Wang J, Chennupati DV, et al. Optimizing STING activity in prostate cancer pre-clinical models. Int J Radiat Oncol, Biol, Phys. 2021;3S:111. doi: 10.1016/j.ijrobp.2021.07.796.
- Peeters H, van Zwol EM, Brancato L, et al. Systematic review of the registered clinical trials for oncological hyperthermia treatment. Int J Hyperthermia. 2022;39(1):806–812. doi: 10.1080/02656736.2022.2076292.
- Liebl CM, Kutschan S, Dörfler J, et al. Systematic review about complementary medical hyperthermia in oncology. Clin Exp Med. 2022;22(4):519–565. doi: 10.1007/s10238-022-00846-9.
- Stauffer PR. Evolving technology for thermal therapy of cancer. Int J Hyperthermia. 2005;21(8):731–744. doi: 10.1080/02656730500331868.
- Walter J, Hader M, Sengedorj A, et al. Broadband microwave spiral applicator (105-125 MHz) for in vitro examinations of hyperthermia-induced tumor cell death forms—first analyses with human breast cancer cells. Int J Hyperthermia. 2023;40(1):2265590. doi: 10.1080/02656736.2023.2265590.
- Hader M, Streit S, Rosin A, et al. In vitro examinations of cell death induction and the immune phenotype of cancer cells following radiative-based hyperthermia with 915 MHz in combination with radiotherapy. Cells. 2021;10(6):1436. doi: 10.3390/cells10061436.
- Wang J, Han Y, Li Y, et al. Targeting tumor physical microenvironment for improved radiotherapy. Small Methods. 2022;6(11):e2200570. doi: 10.1002/smtd.202200570.
- Rodríguez-Ruiz ME, Vanpouille-Box C, Melero I, et al. Immunological mechanisms responsible for radiation-induced abscopal effect. Trends Immunol. 2018;39(8):644–655. doi: 10.1016/j.it.2018.06.001.
- Ui A, Chiba N, Yasui A. Relationship among DNA double-strand break (DSB), DSB repair, and transcription prevents genome instability and cancer. Cancer Sci. 2020;111(5):1443–1451. doi: 10.1111/cas.14404.
- Toulany M. Targeting DNA double-strand break repair pathways to improve radiotherapy response. Genes (Basel). 2019;10(1):25. doi: 10.3390/genes10010025.
- De Ruysscher Dirk N, Gabriele B, Neil G, et al. Radiotherapy toxicity. Nat Rev, Dis Primers. 2019;5(1):13. doi: 10.1038/s41572-019-0064-5.
- Shibata A, Jeggo PA. Canonical DNA non-homologous end-joining; capacity versus fidelity. Br J Radiol. 2020;93(1115):20190966. doi: 10.1259/bjr.20190966.
- Nickoloff JA. Toward greater precision in cancer radiotherapy. Cancer Res. 2021;81(12):3156–3157. doi: 10.1158/0008-5472.CAN-21-0664.
- Falck J, Coates J, Jackson SP. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature. 2005;434(7033):605–611. doi: 10.1038/nature03442.
- Menolfi D, Zha S. ATM, ATR and DNA-PKcs kinases-the lessons from the mouse models: inhibition ≠ deletion. Cell Biosci. 2020;10(1):8. doi: 10.1186/s13578-020-0376-x.
- Gomes LR, Menck CFM, Leandro GS. Autophagy roles in the modulation of DNA repair pathways. Int J Mol Sci. 2017;18(11):2351. (11):23. doi: 10.3390/ijms18112351.
- Gulliver C, Hoffmann R, Baillie GS. Ataxia-telangiectasia mutated and ataxia telangiectasia and Rad3-related kinases as therapeutic targets and stratification indicators for prostate cancer. Int J Biochem Cell Biol. 2022;147:106230. doi: 10.1016/j.biocel.2022.106230.
- Kantidze OL, Velichko AK, Luzhin AV, et al. Synthetically lethal interactions of ATM, ATR, and DNA-PKcs. Trends Cancer. 2018;4(11):755–768. doi: 10.1016/j.trecan.2018.09.007.
- Ashley AK, Kemp CJ. DNA-PK, ATM, and ATR: PIKKing on p53. Cell Cycle. 2018;17(3):275–276. doi: 10.1080/15384101.2017.1412147.
- Baek SY, Hwang UW, Suk HY, et al. Hemistepsin a inhibits cell proliferation and induces G0/G1-phase arrest, cellular senescence and apoptosis via the AMPK and p53/p21 signals in human hepatocellular carcinoma. Biomolecules. 2020;10(5):713. doi: 10.3390/biom10050713.
- Kabakov AE, Yakimova AO. Hypoxia-Induced cancer cell responses driving radioresistance of hypoxic tumors: approaches to targeting and radiosensitizing. Cancers (Basel). 2021;13(5):1102. doi: 10.3390/cancers13051102.

