Abstract
Purpose
To analyze the current practice of regional hyperthermia (RHT) for soft tissue sarcoma (STS) at 12 European centers to provide an overview, find consensuses and identify controversies necessary for future guidelines and clinical trials.
Methods
In this cross-sectional survey study, a 27-item questionnaire assessing clinical subjects and procedural details on RHT for STS was distributed to 12 European cancer centers for RHT.
Results
We have identified seven controversies and five consensus points. Of 12 centers, 6 offer both, RHT with chemotherapy (CTX) or with radiotherapy (RT). Two centers only offer RHT with CTX and four centers only offer RHT with RT. All 12 centers apply RHT for localized, high-risk STS of the extremities, trunk wall and retroperitoneum. However, eight centers also use RHT in metastatic STS, five in palliative STS, eight for superficial STS and six for low-grade STS. Pretherapeutic imaging for RHT treatment planning is used by 10 centers, 9 centers set 40–43 °C as the intratumoral target temperature, and all centers use skin detectors or probes in body orifices for thermometry.
Discussion
There is disagreement regarding the integration of RHT in contemporary interdisciplinary care of STS patients. Many clinical controversies exist that require a standardized consensus guideline and innovative study ideas. At the same time, our data has shown that existing guidelines and decades of experience with the technique of RHT have mostly standardized procedural aspects.
Conclusions
The provided results may serve as a basis for future guidelines and inform future clinical trials for RHT in STS patients.
1. Introduction
The addition of regional hyperthermia (RHT) to anthracycline-based chemotherapy (CTX) has improved local progression-free survival and overall survival in patients with localized soft tissue sarcoma (STS) carrying high-risk features (>5 cm, deep to the fascia and grade 2 or 3 according to the Fédération Nationale des Centres de Lutte Contre le Cancer classification) in the European Organization for Research and Treatment of Cancer (EORTC) 62961 - European Society for Hyperthermic Oncology (ESHO) 95 phase-III study [Citation1–3]. The EORTC 62961-ESHO 95 study applied four cycles of preoperative CTX (doxorubicin, ifosfamide and etoposide) with or without RHT, surgical resection and postoperative RT (whenever feasible) followed by another 4 cycles of CTX with or without RHT [Citation1]. Inclusion criteria were localized STS of the extremities, trunk wall, head and neck and intraabdominal STS with high-risk features.
According to current German and European guidelines, perioperative combination therapy of RHT + CTX can be considered as an individualized, alternative therapy for resectable and unresectable localized, high-grade STS [Citation4,Citation5]. While there is still some skepticism regarding RHT among European sarcoma experts, more than half of all centers that are currently not equipped with a RHT facility would recommend its use for STS patients [Citation6]. Still, RHT is not widely adopted in sarcoma centers. The underlying reason may be the necessary technical and personal investments and expertise to incorporate RHT in sarcoma centers, lack of reimbursement and the nonstandard CTX regimen applied in the EORTC 62961-ESHO 95 study, which led the majority of international sarcoma experts call for a confirmatory study to establish the role of RHT for STS treatment [Citation6–8]. Questions remain regarding the optimal integration of RHT into state-of-the-art contemporary treatment schedules for STS patients, and additional supportive literature is scarce [Citation9]. While guidelines on RHT quality assurance and clinical application exist, there are no current guidelines for the clinical use of RHT in STS patients [Citation10,Citation11].
This study aims to analyze the current practice of RHT for STS in different sarcoma centers with access to RHT to provide an overview, find consensus and identify controversies necessary for future guidelines and clinical trials.
2. Material and methods
In this cross-sectional survey study, we distributed a written 27-item questionnaire to 12 European cancer centers for RHT from Germany (n = 7), Switzerland (n = 4) and Poland (n = 1). Participating centers were determined by literature and online search for centers reporting the use of RHT for STS and recruited through email invitations. The questionnaire was written in English and designed to assess clinical topics (indications and contraindications for RHT, dosing, cytotoxic agents) as well as procedural details (RHT planning, instruments, quality assurance). The survey contains 12 closed-ended single-choice, 10 closed-ended multiple-choice and 5 open-ended question items and was sent to the contributing centers on 14th June 2023. The responses to closed single- or multiple-choice questions were analyzed and displayed descriptively in relation to the total number of responses. The responses to open-ended questions were analyzed through the following multi-step systematic approach: compilation of all responses into one dataset, categorization of responses, analysis of the frequency of response categories and interpretation in relation to the topic of the question. Ambiguous answers or answers not addressing the topic of the questions were removed from the analysis. The results were displayed descriptively in relation to the total number of responses. Abstentions were reported and removed from the descriptive statistical analysis. GraphPad Prism v.9.3.1 (GraphPad Software, San Diego, CA, USA) was used for statistical analysis and figure design.
3. Results
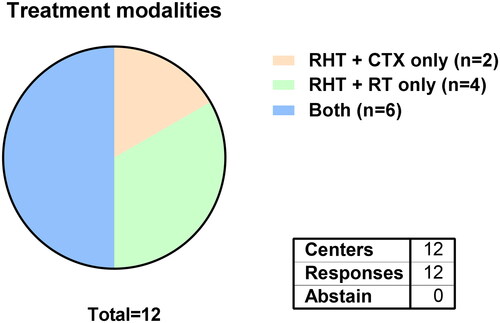
From 12 participating centers, 6 offer both, RHT with CTX and RHT with RT, while 2 centers only offer RHT with CTX and all 4 Swiss centers only offer RHT with RT, due to local reimbursement policies ().
Figure 1. Available treatment modalities for STS patients at all participating hyperthermia centers.
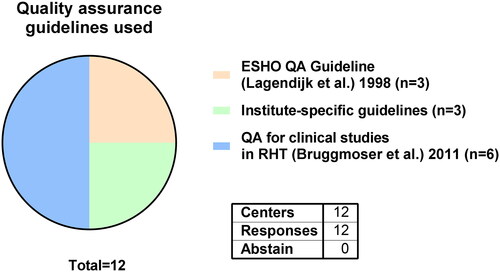
The quality assurance guideline by Bruggmoser et al. was used by 6 out of 12 centers [Citation10]. Three centers act in accordance to the ESHO quality assurance guideline by Lagendijk et al. while 3 centers apply institute-specific guidelines () [Citation12].
3.1. Clinical application and indications for RHT for STS
3.1.1. Treatment aims of RHT
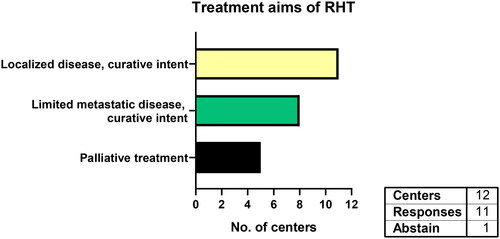
All responding centers (n = 11) use RHT for localized STS with curative intent (). Eight centers also treat the primary tumor in limited metastatic disease with local curative intent. Five centers stated using RHT in palliative STS patients as well.
3.1.2. Indications for RHT: tumor site, location, grading, size and resectability
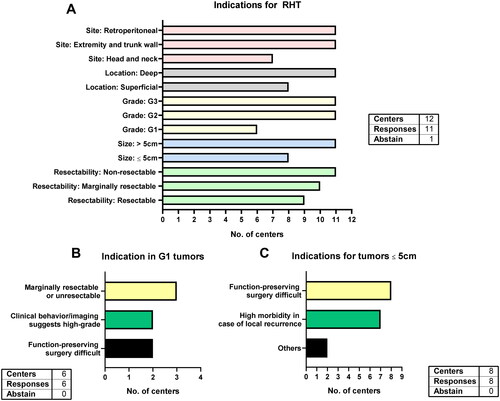
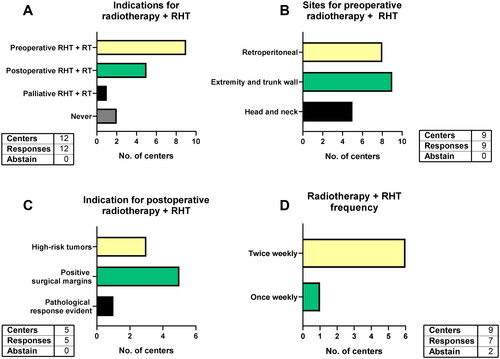
All 11 responding centers use RHT for retroperitoneal as well as extremity and trunk wall tumors while 7 centers include head and neck tumors (). All centers apply RHT in high-risk STS (deeply located, G2/G3 and >5cm). However, the majority of centers also uses RHT in non-high-risk STS: eight centers apply it in superficial STS, six in grade 1 STS and eight in tumors ≤5cm in size. The indications for the six centers using RHT in grade 1 STS were marginally resectable or non-resectable STS (n = 3), in situations where function-preserving surgery is challenging (n = 2) and when clinical behavior and imaging suggest a high-grade STS (n = 2, ). The difficulty of function-preserving surgery is also an indication for RHT among all 8 centers using RHT for STS ≤5cm (). Additionally, high morbidity in case of local recurrence (n = 7) were stated. Two centers name other indications (head and neck STS, deep-seated and high grade STS etc.). All responding centers (n = 11) apply RHT in unresectable STS, whereas 10 centers use it in marginally resectable tumors and even less (n = 9) consider RHT when the tumor is resectable.
3.1.3. RHT with CTX: regimen and treatment schedule
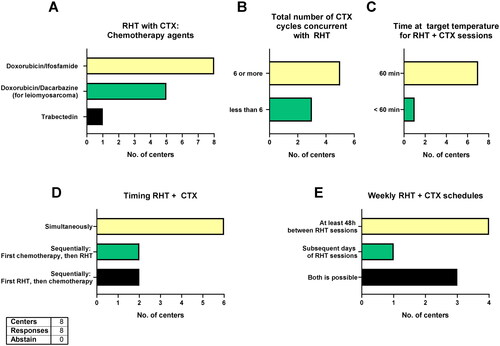
All eight centers applying RHT with CTX use doxorubicin and ifosfamide and five centers switch to doxorubicin and dacarbazine for leiomyosarcomas (). One center combines ifosfamide, carboplatin and etoposide in case of disease recurrence after previous anthracycline-based CTX and one center combines RHT with trabectedin. Moreover, five centers use six or more CTX cycles while three centers give less than six cycles (). All centers schedule RHT in combination with CTX twice per cycle within one week and almost all centers treat for 60 min at target temperature (except one center treating for 31 to < 60 min, ). Most commonly (n = 6), the CTX is administered simultaneously to RHT, although two centers also give CTX first then RHT or vice versa (). Half of all eight centers wait at least 48 h between RHT sessions, while one center regularly applies RHT on subsequent days and three use both options ().
Figure 5. Chemotherapy and RHT.
Chemotherapy agents combined with RHT (A), total number of chemotherapy cycles concurrent with RHT (B), duration of each RHT with chemotherapy session (C), chemotherapy and RHT timing (C) and weekly chemotherapy with RHT schedule (E). All eight centers applying RHT with chemotherapy responded. CTX = chemotherapy; RHT = regional hyperthermia
3.1.4. Timing of RHT + CTX
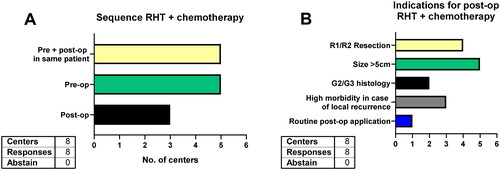
In this multiple-choice question eight centers stated they offer RHT with CTX, five in the preoperative setting and three in the postoperative setting. Moreover, five centers also apply pre- and postoperative RHT + CTX in the same patient (). The indications for postoperative RHT + CTX were tumor size >5cm (n = 5), positive surgical margins (n = 4), high morbidity in case of local recurrence (n = 3) and high-grade histology (n = 2, ). One center routinely uses pre- and postoperative RHT + CTX.
3.1.5. RHT with RT
Ten centers combine RHT with RT for STS. Nine of them preoperatively and one for palliative treatments only (). For preoperative RHT with RT, all nine centers use it for STS of the extremities and trunk wall, while eight also apply it for retroperitoneal STS and five for head and neck STS (). After preoperative RHT with CTX, five centers would offer postoperative RHT with RT in cases with positive surgical margins (n = 5), high-risk tumors (n = 3) and when a pathological response of the preoperative therapy was evident (n = 1, ). Most centers give preoperative RHT twice weekly in combination with 25–28 RT fractions, to a total number of 10–12 RHT with RT sessions. One center combines twice-weekly RHT with hypofractionated RT (10 times 3.25 Gy once daily) in two weeks to a total number of four RHT sessions. In contrast, one center uses RHT once weekly ().
3.1.6. Contraindications for RHT
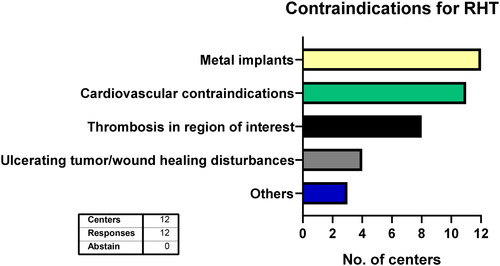
All 12 centers uniformly withhold RHT treatment when metal implants other than small surgical clips are in the region of interest (). Cardiovascular diseases (e.g., advanced heart failure) are considered contraindications in almost all centers (n = 11) while one center requires individual case-by-case evaluation. Similarly, eight centers see thrombosis in the region of interest as a contraindication, while one other center asks for individual assessments of the thromboembolism risks. Furthermore, impaired local wound healing and ulcerating tumors are considered contraindications at four centers. Three centers additionally name other contraindications such as pregnancy, breastfeeding, active infections, claustrophobia and large seromas or hematomas.
3.2. Treatment planning, target temperature and quality assurance for RHT
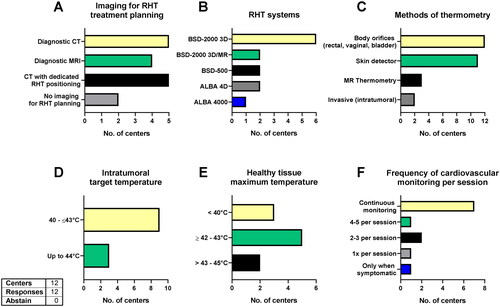
Ten out of 12 centers use imaging for treatment planning, while 2 centers do not use imaging (). Computed tomography (CT) with dedicated RHT positioning is equally often used to diagnostic CTs (n = 5). Four centers also integrate diagnostic magnetic resonance imaging (MRI) for the treatment planning. Most centers have the BSD-2000 3D system (Dr. Sennewald Medizintechnik GmbH, Munich, Germany, n = 6). MR-guided hyperthermia with the BSD-2000 3D/MR (Dr. Sennewald Medizintechnik GmbH, Munich, Germany) is available in three centers and the ALBA 4D system (Med-Logix srl, Rome, Italy) in two other centers. For superficial hyperthermia, two centers use the BSD-500 (Dr. Sennewald Medizintechnik GmbH, Munich, Germany) and one the ALBA 4000 (Med-Logix srl, Rome, Italy, ). For STS of the head and neck region 9 out of 12 centers use superficial hyperthermia, 1 uses capacitive hyperthermia and 2 do not use superficial hyperthermia for head and neck STS. For thermometry, all 12 centers insert detectors in body orifices (e.g., rectal, vaginal) and almost all (n = 11) add skin detectors depending on tumor location to measure normal tissue temperature as close to the tumors as possible. Two centers directly position intratumoral detectors, whenever possible, and three centers with available hybrid systems use MR thermometry (). The target temperature for most centers is between 42 and 43 °C, although three centers aim for 40–42 °C and three other centers accept temperatures up to 44 °C (). Similarly, most centers (n = 5) accept maximum temperatures in the surrounding healthy tissue of between 42 and 43 °C, while three centers aim for less than 40 °C and two accept temperatures of 43–45 °C (). Nine out of 12 centers monitor the cardiovascular vital signs continuously during treatment, while others do it less frequently and 1 center only in symptomatic patients ().
Figure 9. RHT planning and settings.
Imaging modalities for RHT planning (A), RHT systems in use (B), methods of thermometry (C), intratumoral target temperature (D), maximum temperature accepted in the surrounding healthy tissue (E) and frequency of cardiovascular monitoring during each RHT session (F). CT: computed tomography; MRI: magnetic resonance imaging; RHT: regional hyperthermia.
4. Discussion
To our knowledge, this is the first analysis on the clinical practice of RHT for STS at 12 large European RHT centers. Striking differences were found between centers with regard to indications and the question of which STS patients benefit from RHT in addition to perioperative RT or CTX. These variances ultimately reflect the lack of solid, prospectively collected evidence [Citation9]. The low incidence of STS compared to other tumor entities such as breast or cervical cancer and the rarity of experienced RHT centers to conduct such studies certainly contribute to this lack of evidence and the differences in indication setting presented herein [Citation13,Citation14]. In contrast, there is a much higher level of agreement between the centers with regard to the planning, technical execution and quality assurance of RHT. This reflects the decades of previous experience with the therapy modality of RHT for tumors of various locations and extent and the existence of guidelines on RHT quality assurance [Citation10,Citation11,Citation15–17].
In the following, a list of controversies and consensuses in the treatment of STS with RHT is compiled based on the results of the survey conducted herein. This list does not claim completeness, but should rather serve as a thought-provoking stimulus and a basis for discussion for future guidelines and clinical research.
Clinical application and indication for RHT for STS
RHT with CTX
Controversy 1: therapy sequence of RHT-CTX
Despite the evidence from two phase II studies and the subsequent EORTC 62961-ESHO 95 study proving the benefits of RHT-CTX for STS, the widespread availability of this combinatorial treatment is scarce and the clinical indication setting varies greatly among European sarcoma centers [Citation1,Citation3,Citation18,Citation19]. For instance, until the date the present survey has been distributed the 4 participating Swiss centers did not offer RHT-CTX due to a lack of reimbursement in Switzerland which emphasized the missing acceptance or lack of awareness of the scientific evidence in the past [Citation20]. Recently, the Swiss Hyperthermia Network submitted a proposal for reimbursement to the Ministry of Health in Switzerland finally achieving a timely restricted reimbursement for two years followed by a reevaluation of the evidence. In July 2023, the reimbursement for RHT-CTX has officially been accepted in Switzerland. The phase II RHT-95 study used RHT-CTX preoperatively only and showed inferior local failure-free survival compared to the phase II RHT-91 study, in which RHT-CTX was given both pre- and postoperative [Citation18,Citation19]. Still, from eight centers using RHT-CTX only one center regularly applies RHT pre- and postoperatively. All others either give RHT-CTX pre- or postoperatively only or decide based on other criteria such as resection margin status and morbidity in case of local recurrence. Moreover, more than half of centers also apply RHT in oligometastatic or palliative situations, probably related to the HyperTET trial even though its results were not published yet [Citation21].
Controversy 2: chemotherapeutic agents
When selecting chemotherapeutic agents, centers are guided by the data available on CTX alone, instead of the EORTC 62961-ESHO 95 study regimen. Etoposide for STS treatment has been abandoned due to poor efficacy and its leukemogenic potential and is therefore not used by any center in combination with RHT, despite its application in the EORTC 62961-ESHO 95 study [Citation1,Citation22,Citation23]. Instead, all centers use doxorubicin and ifosfamide for the majority of STS subtypes as the combination has proven its superiority to histology-tailored regimens in a prospective randomized trial [Citation24]. Additionally, preclinical data suggests a strong thermos-enhancement for ifosfamide [Citation25]. For leiomyosarcomas, however, a large proportion of centers uses doxorubicin and dacarbazine due to its improved survival compared to doxorubicin and ifosfamide in a multicenter propensity-matched retrospective analysis [Citation26]. Although preclinical data suggest synergistic cell-killing effects of dacarbazine combined with RHT, clinical data on application for STS is lacking [Citation27].
Controversy 3: RHT for superficial STS
Similarly, the majority of centers use RHT for the treatment of superficial STS, i.e., tumors located above the muscle fascia, although no data exists specifically for STS. The rationale stems from the positive impact of hyperthermia in combination with RT for other malignancies such as melanoma and various malignancies with metastases to cutaneous or subcutaneous locations [Citation28,Citation29]. Moreover, 9 out of 12 centers use superficial hyperthermia devices (BSD 500, ALBA 4000, etc.) for STS of the head and neck region (deep or superficially located STS), since common ring applicators cannot be used in this location. One safe and feasible alternative method specifically designed for the use of deep RHT for tumors in the head and neck region is the HYPERcollar system [Citation30]. However, currently superficial RHT devices are used for possible deeply located STS of the head and neck region, an application without previous data for this indication which constitutes another important controversy point.
Controversy 4: RHT for low-grade STS
Some centers use RHT for G1 tumors with difficult resectability when function-preserving surgery is challenging or when the clinical behavior and imaging suggests a high-grade tumor (suspecting sampling error). RT is an alternative option for low-grade STS of the extremities and trunk wall and more recent data from the post-hoc STREXIT analysis on retroperitoneal STS suggest a benefit in low-grade dedifferentiated liposarcoma as well [Citation31,Citation32]. In contrast, CTX is not recommended for low-grade tumors [Citation31]. Thus, RHT as a therapy enhancer for RT or CTX should generally be reserved for RHT with RT, if a low-grade tumor is clearly confirmed. Despite this rationale, the role of RHT with RT for low-grade STS needs to be investigated in clinical studies.
Consensus 1: RHT-CTX for unresectable or marginally resectable STS
In the EORTC 62961-ESHO 95 study, the combination of RHT and CTX significantly downsized STS compared to CTX alone [Citation1]. Consequently, there is consistency among centers to use RHT-CTX for preoperative tumor reduction in unresectable and marginally resectable STS.
RHT with RT
Controversy 5: synergistic effect of trimodal therapy: chemoradiation with RHT
Ten out of 12 centers regularly combine RHT with RT. The most common indications among centers are preoperative therapy of extremity and trunk wall STS, for which also the largest proportion of prospective evidence with favorable toxicity profiles and acceptable oncological outcomes exist [Citation33–36]. Importantly, postoperative RT was also used in the EORTC 62961-ESHO 95 study, and the number of patients were balanced among both treatment arms [Citation1]. To what extent the effects may have been synergistically enhanced if RT would have been given concomitantly to RHT-CTX (doxorubicin, ifosfamide and etoposide) is unclear. More recent, retrospective data suggests an increased rate of high pathological response after preoperative trimodal therapy (RHT-RT-CTX) with doxorubicin and ifosfamide [Citation37]. Despite its limitations as a retrospective study, pathological complete remission after preoperative therapy in STS is an important prognostic factor associated with survival and therefore warrants further investigation of the trimodal therapy [Citation38].
Controversy 6: hypofractionated RT combined with RHT
One center uses preoperative hypofractionated RT with RHT twice a week. There is increasing prospective clinical data showing promising outcomes with hypofractionated preoperative RT for extremity and trunk wall STS [Citation39,Citation40]. First results have proven feasibility of a moderate hypofractionation (10 × 3.25 Gy) in combination with RHT as well [Citation34]. To what extent preoperative RHT can enhance hypofractionated RT and possibly have a downsizing effect of tumors remains to be elucidated.
Controversy 7: RHT-RT for retroperitoneal STS
Retroperitoneal STS are the second most common disease site for which centers apply RHT-RT. For all histological subtypes of STS taken together, the randomized STRASS study of the EORTC has shown no survival advantages by preoperative RT [Citation41]. However, for selected subtypes such as G1-G2 dedifferentiated liposarcomas and primary well-differentiated liposarcomas, the post-hoc propensity-matched STREXIT analyses indicate a survival benefit by preoperative RT [Citation32]. Additionally, very recent unpublished data from the Transatlantic Australasian Retroperitoneal Working Group presented in the Society of Surgical Oncology meeting in 2024 has revealed a benefit of neoadjuvant RT for primary well-differentiated liposarcomas of the retroperitoneum. This data corroborates the STREXIT analysis until the longer follow-up data from the STRASS trial will finally elucidate this topic. Until then, adding RHT to RT as a therapy-enhancing measure for retroperitoneal G1-G2 dedifferentiated liposarcomas and primary well-differentiated liposarcomas appears justifiable, even though no prospective data on RHT with RT for retroperitoneal STS exists. Another clinically relevant indication for the combination of RHT with RT is the situation of re-irradiation in local recurrences. The synergistic effect of RHT-RT allows a reduction in RT dose in re-irradiation and has shown promising results in recurrent breast cancer [Citation42–44]. Local recurrences are a frequent clinical problem, particularly in some subtypes of retroperitoneal STS and is associated with increased morbidity [Citation45–47]. Thus, another point of controversy is the role of RHT with RT among histological subtypes of retroperitoneal STS in light of the STRASS/STREXIT data and its potential for re-irradiation.
RHT treatment planning, execution and quality assurance
RHT treatment scheduling and contraindications
Consensus 2: twice weekly RHT to adjust for thermotolerance
There is a high degree of uniformity among centers about the number of two RHT sessions per week and a minimum time interval of 48 h between sessions. Although only described preclinically, thermotolerance is a widely accepted phenomenon which most centers and clinical trials tackle by applying one to two RHT sessions weekly [Citation48,Citation49].
Consensus 3: contraindications
Contraindications as mentioned in the guidelines by Bruggmoser et al. are widely accepted among centers [Citation10,Citation11]. All 12 centers consider large metal implants as a contraindication with reasonable rationale. Moreover, RHT can induce vasodilation with subsequent hypotension and tachycardia which may be challenging for older, anorectic or frail patients [Citation50]. The majority therefore continuously monitors cardiovascular vital signs during therapy as recommended [Citation12]. Although cardiovascular contraindications are accepted by 11 centers, a more precise specification is preferable to not unnecessarily withhold therapy for patients fit enough for RHT. Similarly, the contraindication criteria for thrombosis in the target region for RHT need to be defined. According to Bruggmoser et al. thrombosis in the target region detected less than 3 months before therapy may constitute a contraindication due to the risk of embolism [Citation11].
Consensus 4: imaging for treatment planning
Except for two centers, all others adhere to the guidelines recommending pretherapeutic cross-sectional imaging such as CT with or without dedicated RHT positioning or MRI for treatment planning [Citation11]. Three centers equipped with MR-guided hyperthermia devices (BSD-2000 3D/MR) use MRI images obtained by the hybrid system for treatment planning. A variety of devices, applicators and planning software are in use at different centers. While the majority uses the BSD-2000 3D, the specific device and applicator are chosen based on tumor location and patient body size and are all valid alternatives depending on the clinical scenario [Citation11,Citation15,Citation51].
Consensus 5: target temperatures and thermometry
Nearly all centers agree on the conventional 60 min time at target temperature for each session as recommended in the guidelines [Citation10,Citation11]. Intratumoral target temperatures of 40–43 °C are also widely accepted, although three centers also accept up to 44 °C, if delivery to the intratumoral region is safely confirmed. Solid underlying preclinical data support the synergistic effect of ionizing radiation and target temperatures of around 43.5 °C particularly for large tumors [Citation8,Citation52–54]. Moreover, higher intratumoral temperatures correlate with increased pathological response in STS of the lower extremities [Citation55]. Therefore, target temperatures around 40–43 °C are implemented in the guidelines and complied with by most centers [Citation10–12]. Most centers agree on thermometry for extremity and trunk wall STS with skin detectors or use probes in body orifices (rectum, vagina) for thermometry in intraabdominal STS [Citation56]. In three centers with available hybrid MR-guided hyperthermia devices, MR thermometry is applied according to guidelines [Citation11].
Limitations and future perspectives
This study has several limitations. Firstly, the developed and distributed questionnaire is not a validated tool for assessing quality assurance in RHT but aims to cover essential points in clinical indications and technical implementation for STS treatment. Secondly, the generated list does not cover all relevant controversies and consensuses. Moreover, the centers surveyed represent only a portion of all hyperthermia centers. Still, the list of controversies and consensuses provides a basis for future guidelines and studies. One controversy described herein is currently being tackled: based on promising results showing favorable pathological tumor response rates by combined preoperative chemoradiotherapy with RHT, the study group around Lindner and Semrau et al. is planning a multicenter single-arm trial for marginally or borderline-resectable STS of the extremities or trunk wall [Citation37]. Furthermore, to investigate the synergistic effect of the second line CTX trabectedin with RHT compared to trabectedin alone in localized, not resectable or metastasized STS progressive under first line CTX, the randomized multicenter phase II HyperTET study is being conducted and estimated to have completed enrollment by August 2023 [Citation21]. The results are eagerly awaited. Based on the STRASS and STREXIT data the EORTC has recently launched the STRASS II randomized study comparing preoperative chemotherapy to surgery alone for high-grade dedifferentiated liposarcoma and leiomyosarcoma of the retroperitoneum [Citation57]. A randomized controlled study combining the preoperative chemotherapy regimens in the STRASS II trial with RHT would finally clarify the position of RHT for retroperitoneal STS and additionally serve as the confirmatory study that the sarcoma community requires before RHT can be adopted as a standard treatment modality for STS [Citation6].
5. Conclusion
The EORTC 62691-ESHO-95 trial has shown the survival benefit of combining RHT with perioperative CTX and sequential RT [Citation3,Citation18]. Since the first results were published more than a decade ago, many innovations have emerged with regard to systemic therapies and RT. In European and German guidelines, RHT still holds the status of an optional recommendation [Citation4,Citation5]. The majority of interdisciplinary sarcoma experts call for a new, confirmatory study before RHT becomes a permanent pillar of STS therapy [Citation6]. As shown in this study, there is a high level of disagreement regarding the detailed use of RHT for STS patients due to lack of additional evidence. There are many controversies that require both, a standardized consensus guideline and innovative clinical trials for RHT in STS patients. At the same time, our data has shown that the existing guidelines and decades of experience with RHT have mostly standardized procedural aspects. The provided results may serve as a basis for future guidelines and can inform future clinical trials for RHT in STS patients.
Ethics statement
Due to the nature of this paper and its methodology, no institutional review board approval was required.
Author contributions
Study concept and design: SR, DK, PG.
Acquisition of data: All authors.
Analysis and interpretation of data: SR, FE, MB, DK, PG.
Drafting of the manuscript: SR.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: SR.
Administrative, technical, or material support: None.
Supervision: DK, MB and PG.
Supplemental Material
Download MS Word (18.1 KB)Acknowledgements
Siyer Roohani is a participant in the BIH Charité Junior Clinician Scientist Program funded by the Charité – Universitätsmedizin Berlin and the Berlin Institute of Health (BIH).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data are available on request from the corresponding author.
Additional information
Funding
References
- Issels RD, Lindner LH, Verweij J, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol. 2010;18(11):e630–e570. doi: 10.1016/S1470-2045(10)70071-1.
- Angele MK, Albertsmeier M, Prix NJ, et al. Effectiveness of regional hyperthermia with chemotherapy for high-risk retroperitoneal and abdominal soft-tissue sarcoma after complete surgical resection: a subgroup analysis of a randomized phase-III multicenter study. Ann Surg. 2014;260(5):749–756; discussion 54–56. doi: 10.1097/SLA.0000000000000978.
- Issels RD, Lindner LH, Verweij J, et al. Effect of neoadjuvant chemotherapy plus regional hyperthermia on long-term outcomes among patients with localized high-risk soft tissue sarcoma: the EORTC 62961-ESHO 95 randomized clinical trial. JAMA Oncol. 2018;4(4):483–492. doi: 10.1001/jamaoncol.2017.4996.
- Gronchi A, Miah AB, Dei Tos AP, et al. Soft tissue and visceral sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(11):1348–1365. doi: 10.1016/j.annonc.2021.07.006.
- Deutsche Krebsgesellschaft DK. AWMF. S3-Leitlinie adulte weichgewebesarkome, langversion version 1.0. 2021. Leitlinienprogramm Onkologie. 2021;1.1:119–121.
- Rothermundt C, Andreou D, Blay J-Y, et al. Controversies in the management of patients with soft tissue sarcoma: recommendations of the conference on state of science in sarcoma 2022. Eur J Cancer. 2023;180:158–179. doi: 10.1016/j.ejca.2022.11.008.
- van der Zee J, Vujaskovic Z, Kondo M, et al. The kadota fund international forum 2004–clinical group consensus. Int J Hyperthermia. 2008;24(2):111–122. doi: 10.1080/02656730801895058.
- Overgaard J. The heat is (still) on–the past and future of hyperthermic radiation oncology. Radiother Oncol. 2013;109(2):185–187. doi: 10.1016/j.radonc.2013.11.004.
- Veltsista PD, Oberacker E, Ademaj A, et al. Hyperthermia in the treatment of high-risk soft tissue sarcomas: a systematic review. Int J Hyperthermia. 2023;40(1):2236337.
- Bruggmoser G, Bauchowitz S, Canters R, et al. Quality assurance for clinical studies in regional deep hyperthermia. Strahlenther Onkol. 2011;187(10):605–610. doi: 10.1007/s00066-011-1145-x.
- Bruggmoser G, Bauchowitz S, Canters R, et al. Guideline for the clinical application, documentation and analysis of clinical studies for regional deep hyperthermia: quality management in regional deep hyperthermia. Strahlenther Onkol. 2012;188(S2):198–211. doi: 10.1007/s00066-012-0176-2.
- Lagendijk JJW, Van Rhoon GC, Hornsleth SN, et al. ESHO quality assurance guidelines for regional hyperthermia. Int J Hyperthermia. 1998;14(2):125–133. doi: 10.3109/02656739809018219.
- Datta NR, Puric E, Klingbiel D, et al. Hyperthermia and radiation therapy in locoregional recurrent breast cancers: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys. 2016;94(5):1073–1087. doi: 10.1016/j.ijrobp.2015.12.361.
- Datta NR, Stutz E, Gomez S, et al. Efficacy and safety evaluation of the various therapeutic options in locally advanced cervix cancer: a systematic review and network meta-analysis of randomized clinical trials. Int J Radiat Oncol Biol Phys. 2019;103(2):411–437. doi: 10.1016/j.ijrobp.2018.09.037.
- Paulides MM, Dobsicek Trefna H, Curto S, et al. Recent technological advancements in radiofrequency- andmicrowave-mediated hyperthermia for enhancing drug delivery. Adv Drug Deliv Rev. 2020;163-164:3–18. doi: 10.1016/j.addr.2020.03.004.
- Datta NR, Kok HP, Crezee H, et al. Integrating loco-regional hyperthermia Into the current oncology practice: SWOT and TOWS analyses. Front Oncol. 2020;10:819. doi: 10.3389/fonc.2020.00819.
- Fiorentini G, Sarti D, Gadaleta CD, et al. A narrative review of regional hyperthermia: updates From 2010 to 2019. Integr Cancer Ther. 2020;19:153473542093264–1534735420932648. doi: 10.1177/1534735420932648.
- Issels RD, Abdel-Rahman S, Wendtner C-M, et al. Neoadjuvant chemotherapy combined with regional hyperthermia (RHT) for locally advanced primary or recurrent high-risk adult soft-tissue sarcomas (STS) of adults: long-term results of a phase II study. Eur J Cancer. 2001;37(13):1599–1608. doi: 10.1016/S0959-8049(01)00183-6.
- Wendtner C-M, Abdel-Rahman S, Baumert J, et al. Treatment of primary, recurrent or inadequately resected high-risk soft-tissue sarcomas (STS) of adults: results of a phase II pilot study (RHT-95) of neoadjuvant chemotherapy combined with regional hyperthermia. Eur J Cancer. 2001;37(13):1609–1616. doi: 10.1016/S0959-8049(01)00191-5.
- Stutz E, Puric E, Ademaj A, et al. Present practice of radiative deep hyperthermia in combination with radiotherapy in Switzerland. Cancers. 2022;14(5):1175. doi: 10.3390/cancers14051175.
- Hyper-Thermia. Enhanced anti-tumor efficacy of trabectedin; [cited 2024 Feb 08]. Available from: https://classic.clinicaltrials.gov/show/NCT02359474
- Keizer HJ, Crowther D, Nielsen OS, et al. EORTC group phase II study of oral etoposide for pretreated soft tissue sarcoma. Sarcoma. 1997;1(2):99–101. doi: 10.1080/13577149778371.
- Zhang W, Gou P, Dupret JM, et al. Etoposide, an anticancer drug involved in therapy-related secondary leukemia: enzymes at play. Transl Oncol. 2021;14(10):101169. doi: 10.1016/j.tranon.2021.101169.
- Gronchi A, Ferrari S, Quagliuolo V, et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 2017;18(6):812–822. doi: 10.1016/S1470-2045(17)30334-0.
- Wiedemann G, Roszinski S, Biersack A, et al. Local hyperthermia enhances cyclophosphamide, ifosfamide andcis-diamminedichloroplatinum cytotoxicity on human-derived breast carcinoma and sarcoma xenografts in nude mice. J Cancer Res Clin Oncol. 1992;118(2):129–135. doi: 10.1007/BF01187501.
- D’Ambrosio L, Touati N, Blay J-Y, et al. Doxorubicin plus dacarbazine, doxorubicin plus ifosfamide, or doxorubicin alone as a first-line treatment for advanced leiomyosarcoma: a propensity score matching analysis from the european organization for research and treatment of cancer soft tissue and bone sarcoma group. Cancer. 2020;126(11):2637–2647. doi: 10.1002/cncr.32795.
- Salvador D, Bastos V, Oliveira H. Combined therapy with dacarbazine and hyperthermia induces cytotoxicity in A375 and MNT-1 melanoma cells. Int J Mol Sci. 2022;23(7):5–9.
- Jones EL, Oleson JR, Prosnitz LR, et al. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol. 2005;23(13):3079–3085. doi: 10.1200/JCO.2005.05.520.
- Overgaard J, Bentzen SM, Overgaard J, et al. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. Lancet. 1995;345(8949):540–543. doi: 10.1016/S0140-6736(95)90463-8.
- Verduijn GM, de Wee EM, Rijnen Z, et al. Deep hyperthermia with the HYPERcollar system combined with irradiation for advanced head and neck carcinoma – a feasibility study. Int J Hyperthermia. 2018;34(7):994–1001. doi: 10.1080/02656736.2018.1454610.
- Network NCC. Soft tissue sarcoma (Version 2.2021). 2021 [2024 February 08]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf
- Callegaro D, Raut CP, Ajayi T, et al. Preoperative radiotherapy in patients with primary retroperitoneal sarcoma: EORTC-62092 trial (STRASS) versus off-trial (STREXIT) results. Ann Surg. 2023;278(1):127–134. doi: 10.1097/SLA.0000000000005492.
- Prosnitz LR, Maguire P, Anderson JM, et al. The treatment of high-grade soft tissue sarcomas with preoperative thermoradiotherapy. Int J Radiat Oncol Biol Phys. 1999;45(4):941–949. doi: 10.1016/S0360-3016(99)00272-2.
- Spałek MJ, Borkowska AM, Telejko M, et al. The feasibility study of hypofractionated radiotherapy with regional hyperthermia in soft tissue sarcomas. Cancers. 2021;13(6):1332. doi: 10.3390/cancers13061332.
- Eckert F, Braun LH, Traub F, et al. Radiotherapy and hyperthermia with curative intent in recurrent high risk soft tissue sarcomas. Int J Hyperthermia. 2018;34(7):980–987. doi: 10.1080/02656736.2017.1369174.
- Eckert F, Gani C, Kluba T, et al. Effect of concurrent chemotherapy and hyperthermia on outcome of preoperative radiotherapy of high-risk soft tissue sarcomas. Strahlenther Onkol. 2013;189(6):482–485. doi: 10.1007/s00066-013-0312-7.
- Willner A, Agaimy A, Fechner K, et al. Chemoradiotherapy plus hyperthermia (CRTH) versus chemoradiotherapy (CRT) alone in neoadjuvant treatment of soft tissue sarcoma: tumor response, treatment toxicity and disease control. Int J Hyperthermia. 2023;40(1):2248424. doi: 10.1080/02656736.2023.2248424.
- Wang D, Harris J, Kraybill WG, et al. Pathologic complete response and clinical outcomes in patients with localized soft tissue sarcoma treated with neoadjuvant chemoradiotherapy or radiotherapy: the NRG/RTOG 9514 and 0630 nonrandomized clinical trials. JAMA Oncol. 2023;9(5):646–655. doi: 10.1001/jamaoncol.2023.0042.
- Cury FL, Viani GA, Gouveia AG, et al. Meta-analysis of 5-day preoperative radiotherapy for soft tissue sarcoma (5D-PREORTS). Radiother Oncol. 2023;190:109935. doi: 10.1016/j.radonc.2023.109935.
- Roohani S, Ehret F, Kobus M, et al. Preoperative hypofractionated radiotherapy for soft tissue sarcomas: a systematic review. Radiat Oncol. 2022;17(1):159. doi: 10.1186/s13014-022-02072-9.
- Bonvalot S, Gronchi A, Le Péchoux C, et al. Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: STRASS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020;21(10):1366–1377. doi: 10.1016/S1470-2045(20)30446-0.
- Linthorst M, van Geel AN, Baaijens M, et al. Re-irradiation and hyperthermia after surgery for recurrent breast cancer. Radiother Oncol. 2013;109(2):188–193. doi: 10.1016/j.radonc.2013.05.010.
- Oldenborg S, Hannoun-Levi J-M. Re-irradiation combined with hyperthermia. In: Kaidar-Person O, Meattini I, Poortmans P, editors. Breast cancer radiation therapy: a practical guide for technical applications. Cham: Springer International Publishing; 2022. p. 413–419.
- Parzen JS, Siddiqui ZA, Jawad MS, et al. Reirradiation with concurrent hyperthermia for recurrent breast cancer is safe and effective. Int J Radiat Oncol Biol Phys. 2019;105(1):E45. doi: 10.1016/j.ijrobp.2019.06.2366.
- Eilber FC, Rosen G, Nelson SD, et al. High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003;237(2):218–226. doi: 10.1097/01.SLA.0000048448.56448.70.
- Indelicato DJ, Meadows K, Gibbs CPJr, et al. Effectiveness and morbidity associated with reirradiation in conservative salvage management of recurrent soft-tissue sarcoma. Int J Radiat Oncol Biol Phys. 2009;73(1):267–272. doi: 10.1016/j.ijrobp.2008.04.032.
- Gronchi A, Strauss DC, Miceli R, et al. Variability in patterns of recurrence after resection of primary retroperitoneal sarcoma (RPS): a report on 1007 patients from the multi-institutional collaborative RPS working group. Ann Surg. 2016;263(5):1002–1009. doi: 10.1097/SLA.0000000000001447.
- Gerner EW, Schneider MJ. Induced thermal resistance in HeLa cells. Nature. 1975;256(5517):500–502. doi: 10.1038/256500a0.
- Overgaard J, Nielsen OS. The importance of thermotolerance for the clinical treatment with hyperthermia. Radiother Oncol. 1983;1(2):167–178. doi: 10.1016/S0167-8140(83)80019-X.
- Izukura R, Imada H, Hashiguchi N, et al. Cardiac and respiratory effects of deep regional hyperthermia using an 8 MHz radiofrequency-capacitive device on patients with cancer. Int J Hyperthermia. 2017;33(4):428–434. doi: 10.1080/02656736.2017.1283064.
- Adibzadeh F, Sumser K, Curto S, et al. Systematic review of pre-clinical and clinical devices for magnetic resonance-guided radiofrequency hyperthermia. Int J Hyperthermia. 2020;37(1):15–27. doi: 10.1080/02656736.2019.1705404.
- Overgaard J. Hyperthermic oncology, 1984: proceedings of the 4th international symposium on hyperthermic oncology, held at Aarhus, Denmark, 2–6 July 1984. Aarhus, Denmark: Taylor & Francis; 1984.
- Overgaard K, Overcaard J. Radiation sensitizing effect of heat. Acta Radiol Ther Phys Biol. 1974;13(6):501–511.
- Overgaard K, Overgaard J. Hyperthermic tumour-cell devitalization in vivo. Acta Radiol Ther Phys Biol. 1977;16(1):1–16.
- Unsoeld M, Lamprecht U, Traub F, et al. MR thermometry data correlate with pathological response for soft tissue sarcoma of the lower extremity in a single center analysis of prospectively registered patients. Cancers. 2020;12(4):959. doi: 10.3390/cancers12040959.
- Wust P, Gellermann J, Harder C, et al. Rationale for using invasive thermometry for regional hyperthermia of pelvic tumors. Int J Radiat Oncol Biol Phys. 1998;41(5):1129–1137. doi: 10.1016/S0360-3016(98)00165-5.
- Surgery with or without neoadjuvant chemotherapy in high risk retroperitoneal sarcoma; [2024 Feb 08]. Available from: https://classic.clinicaltrials.gov/show/NCT04031677