Abstract
Purpose: According to the international guidelines, acute subdural hematomas (aSDH) with a thickness of >10 mm, or causing a midline shift of >5 mm, should be surgically evacuated. However, high mortality rates in older patients resulted in ongoing controversy whether elderly patients benefit from surgery. We identified predictors of outcome in a single-centre cohort of elderly patients undergoing surgical evacuation of aSDH or subacute subdural hematoma (saSDH).
Materials and methods: This retrospective study included all patients aged ≥65 years undergoing surgical evacuation of aSDH/saSDH from 2000 to 2015. One-year outcome was dichotomized into favourable (Glasgow Outcome Scale (GOS) 4–5) and unfavourable (GOS 1–3). Predictors of outcome were identified by analysing patient characteristics.
Results: Eighty-four patients aged ≥65 years underwent craniotomy for aSDH/saSDH during the 16 year time period. Twenty-five percent regained functional independence, 11% survived severely disabled, and 64% died. Most patients died of respiratory failure following withdrawal of artificial respiration or following restriction of treatment. Age of the SDH or Glasgow Coma Scores ≤8/intubation did not predict unfavourable outcome. All patients with bilaterally absent pupillary light reflexes died, also those who still exhibited one normal-sized pupil.
Conclusion: The low number of operated patients per year probably suggests that this cohort represents a selection of patients who were judged to have good chances of favouring from surgery. Functional independence at one-year follow-up was reached in 25% of patients, 64% died. Patients with bilaterally absent pupillary light reflexes did not benefit from surgery. The tendency to restrict treatment because of presumed poor prognosis may have acted as a self-fulfilling prophecy.
Introduction
According to the international guidelines for the management of acute subdural hematomas (aSDH), an aSDH with a thickness greater than 10 mm, or causing a midline shift greater than 5 mm, on computed tomography (CT) scan should be surgically evacuated by craniotomy.Citation1 However, reported mortality rates as high as 68–88% in patients older than 70 years undergoing craniotomy for aSDH in the 1970s-1990s, together with poor neurological outcome in those surviving, have resulted in an ongoing controversy whether elderly patients truly benefit from surgery.Citation2–5 A recent Swiss retrospective study by Taussky et al. reported a mortality rate of only 35% and favourable outcome in 41% among 37 patients aged 65 years and older who were operated for traumatic aSDH between 2002 and 2007.Citation6 The authors hypothesised that improvements in the treatment of traumatic brain injury since the 1970s and 1980s, the better health status of elderly individuals nowadays, the high percentage of low velocity traumas in their cohort, and the application of strict criteria for undertaking surgery were possible explanations for their good results.
In their chapter on the international guidelines for the management of aSDH, Bullock et al. stressed the importance of future studies identifying subgroups that do not benefit from surgery.Citation1 To address these issues, we analysed the outcome of patients aged 65 years and older who underwent craniotomy for aSDH or subacute SDH (saSDH) in our Level I trauma centre between 2000 and 2015.
Materials and methods
Patients
The Academic Medical Centre (AMC) is a university hospital in the Amsterdam metropolitan area with a total population of approximately 2.5 million people. This retrospective study was designed to include all patients aged 65 years and older, who underwent craniotomy for aSDH or saSDH in the AMC in the period 2000–2015. Patients were selected from a retrospectively created operative database that included all consecutive 173 patients older than 55 years who underwent craniotomy for subdural hematoma (SDH) between January 1, 2000 and October 19, 2015. We excluded 31 patients operated for chronic SDH, and 10 with aSDH from intracranial aneurysm or arteriovenous malformation rupture. Of the remaining 132 patients, 85 patients were 65 years or older. Because this was an observational study, formal approval was waived by the institutional ethical review board of our hospital and patient consent was not required.
Clinical management
Treatment in the field was provided by emergency physicians and paramedics, according to Advances Trauma Life Support standards. After admission, trauma patients were examined by a trauma surgeon and neurologist, and non-trauma patients by a neurologist. Patients then underwent CT-imaging of the brain. If a relevant aSDH was documented, AMC patients were brought to the operating room, whereas patients initially presented elsewhere were transferred to the AMC after telephone consultation between the referring neurologist and the AMC neurosurgeon. Any anticoagulation was reversed before surgery by a concentrate of four-factor prothrombin complex, administered in the referring hospital or in the AMC. Patients using acetylsalicylic acid received platelet transfusions pre- and postoperatively. The surgical technique consisted of a large craniotomy and hematoma evacuation. Based on the presence of hydrocephalus and brain oedema, the attending neurosurgeon decided whether to implant an intracranial pressure (ICP) monitoring catheter or an external ventricular drain (EVD), and whether to replace the bone flap, or not. After surgery, patients were admitted to the ICU for supportive therapy.
Data collection
Data sources included patients’ rescue and evacuation records, hospital records from the AMC, and the referring hospital when applicable, rehabilitation summaries, and correspondence of neurologists, nursing home physicians and general practitioners caring for the patients after discharge from the AMC. The records were analysed for demographic characteristics (age, gender, premorbid functioning), mechanism of injury and the use of anticoagulant drugs (including thrombocyte aggregation inhibitors). CT-scans were evaluated to determine the thickness of the hyperdens SDH and the extent of midline shift. When the injury occurred <72 h before diagnosis, the SDH was classified as acute. When the injury occurred 3–7 days before diagnosis, the SDH was classified as subacute. When the hyperdens SDH developed spontaneously, for example in patients taking oral anticoagulants, the SDH was classified as spontaneous. In many patients, no reliable time interval in hours from injury to surgery could be determined. We therefore chose to record the time interval from diagnosis of the (s)aSDH on the CT-scan to the start of surgery. For similar reasons, the Glasgow Coma Scale (GCS) score and pupillary abnormalities at the moment of injury could not be determined in all patients. We recorded GCS scores and pupillary abnormalities at the moment of diagnosis (CT scan) and, to document any preoperative deterioration, immediately prior to surgery. Clinician’s notes at one year postoperatively, or at the longest follow-up available, were used to assess outcome according to the Glasgow Outcome Scale (GOS): GOS 1 = death, GOS 2 = persistent vegetative state, GOS 3 = severe disability (conscious but disabled), GOS 4 = moderate disability (disabled but independent), and GOS 5 = good recovery (resumption of normal life even though there may be minor neurological and psychological deficits).Citation7 The STROBE guidelines were used in order to report this observational study accurately and completely.Citation8
Data analysis
We defined unfavourable outcome as GOS 1–3 and favourable outcome as GOS 4–5. Continuous variables were tested for normal distribution using the Shapiro-Wilk test. A variable was considered normally distributed if the Shapiro-Wilk test was >0.9, otherwise the variable was considered as not normally distributed. Means ( ± standard deviation, SD) are given for continuous variables with a normal distribution whereas median (interquartile range, IQR 25%-75%) are given for not normally distributed continuous variables. To compare demographic and baseline characteristics between favourable and unfavourable outcome groups, univariate statistical analysis was performed. The 2-tailed t-test (for comparisons of normally distributed continuous variables), Mann-Whitney U test (for comparisons of continuous variables without a normal distribution), Fisher exact test (for analysis of 2 × 2 tables), and chi-square test (for analysis of N x 2 contingency tables) were done as appropriate to identify differences between groups. IBM SPSS Statistics 24.0 was used for calculations. A p value ≤0.05 was considered statistically significant.
Results
Sixty-three of the 85 patients were classified as suffering from aSDH, six as suffering from saSDH, and 16 as suffering from spontaneous SDH (hyperdens on CT). One 66-year-old foreign patient with a spontaneous SDH was repatriated to his home country in the weeks following surgery and was lost to follow-up. He was excluded from further analysis. Mean age of the remaining 84 patients was 75 ± 6 years (range 65–88). Further characteristics are presented in . For 80 patients, CT-images were either digitally available, or retrieved from the archives; for two patients the thickness of the SDH was retrieved from the physician’s notes. For the two patients without radiological information on SDH thickness, surgical notes clearly described the hematoma as ‘acute’. Seven patients had a SDH <10 mm, but all had concomitant midline shift >5 mm. The types of initial surgery are listed in . A postoperative CT was performed in 75 patients at median postoperative day 1 (IQR 0–2). In nine patients, no postoperative CT was performed: in five the neurologic condition was judged as too poor to undergo further diagnostics and treatment (GCS 3 or 4), three patients were doing clinically so well that postoperative imaging was judged as unnecessary (GCS 15), and in one patient no reason could be found in the clinical notes. Eighteen (21%) patients underwent a total of 22 additional surgeries (). For the whole group, median length of stay in the ICU and hospital was 5 days (IQR 2–8) and 20 days (IQR 7–35), respectively.
Table 1. Characteristics of 84 patients aged ≥65 years undergoing craniotomy for (s)aSDH according to outcome.
Table 2. Types of surgery in 84 patients aged ≥65 years undergoing craniotomy for (s)aSDH.
Outcome
Fifty-four (64%) patients died: 44 in the hospital, 10 in a nursing home or hospice. Median length of survival was 15 days (IQR 5–43). Causes of death are listed in . Nine (11%) patients recovered to GOS 3, 10 (12%) to GOS 4, and 11 (13%) to GOS 5. Outcome in these surviving patients was assessed at one year after surgery for 29 patients, and 164 days after surgery for one GOS 4 survivor.
Table 3. Causes of death in 54 patients aged ≥65 years undergoing craniotomy for (s)aSDH.
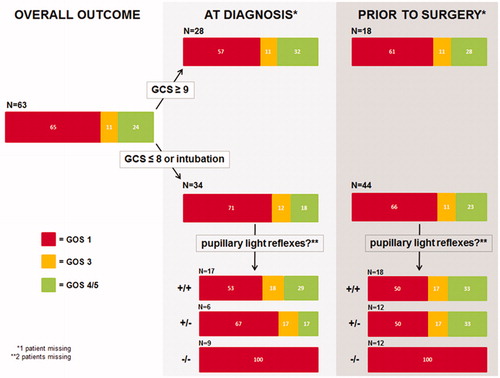
SDH at the dominant side, or the use of anticoagulants were not associated with higher chances of unfavourable outcome (). Clinical characteristics and outcome among patients harbouring an acute, subacute or spontaneous SDH were similar (). Patients who were non-comatose (GCS ≥9) upon admission had better chances of survival than comatose (GCS ≤8) or intubated patients (Odds ratio (OR) 2.48 [95% Confidence interval (CI) 0.99–6.20]; p = .04). All non-intubated patients with GCS M-scores 1 (N = 3) or 2 (N = 2) upon admission died. Chances of favourable outcome only tended to be better for patients who were non-comatose upon admission compared to comatose or intubated patients (OR 2.41 [95% CI 0.87–6.65]; p = .07). The time elapsed from admission to surgery did not differ between patients with favourable outcome and patients with unfavourable outcome (). Prior to surgery, chances of survival and chances of favourable outcome for non-comatose patients compared to comatose/intubated patients were not different (survival: OR 2.05 [95% CI 0.78–5.37]; p = .11, favourable outcome: OR 2.16 [95% CI 0.77–6.07]; p = .12). One of three non-intubated patients with a GCS M-score of 2 prior to surgery survived favourably. The 13 patients who deteriorated from non-comatose to comatose/intubated between the moment of diagnosis and surgery had similar outcomes as patients who were non-comatose upon admission: 50% survived, 33% survived favourably. Eight patients had GCS scores 13–14 prior to surgery: five died, three survived favourably. Their outcome was not different from patients with GCS scores ≤12 (survival: OR 1.09 [95% CI 0.24–4.91]; p = .60, favourable outcome: OR 1.9 [95% CI 0.42–8.89]; p = .32). Unilateral absence of pupillary light reflex was not a predictor of unfavourable outcome (at the moment of diagnosis: OR 1.15 [95% CI 0.27–4.92]; p = .58, prior to surgery: OR 1.2 [95% CI 0.35–4.08]; p = .51), but bilateral absence of pupillary light reflexes was: all died, even those who still exhibited one normal-sized pupil (). All 14 patients without pupillary light reflexes at the moment of diagnosis died of respiratory failure following withdrawal of artificial respiration (N = 9) or following restriction of treatment (N = 5) because of presumed poor prognosis. Seventeen of the 18 patients without pupillary light reflexes prior to surgery died of respiratory failure following withdrawal of artificial respiration (N = 11) or following restriction of treatment (N = 6) because of presumed poor prognosis. One patient, who still exhibited one pupillary light reflex at diagnosis but then lost it during the one and a half hour delay between diagnosis and surgery, initially survived with GOS 4 but died at follow-up day 246 of acute myeloid leukaemia. Chances of survival and chances of favourable outcome for patients undergoing craniotomy compared to patients undergoing decompressive craniectomy at initial surgery were not different (survival: OR 2.44 [95% CI 0.48–12.29]; p = .23, favourable outcome: OR 1.38 [95% CI 0.27–7.09]; p = .52).
Table 4. Characteristics of 84 patients aged ≥65 years undergoing craniotomy according to age of SDH.
Table 5. Literature review of elderly patients undergoing craniotomy for aSDH.
illustrates the outcome of different subpopulations of the 63 patients suffering from aSDH when assorted for GCS and presence or absence of pupillary light reflexes at the moment of diagnosis or prior to surgery. Chances of survival and chances of favourable outcome for non-comatose aSDH patients compared to comatose/intubated aSDH patients were not different (at diagnosis: survival OR 1.80 [95% CI 0.63–5.15]; p = .20, favourable outcome OR 2.21 [95% CI 0.68–7.24]; p = .15; prior to surgery: survival OR 1.23 [95% CI 0.40–3.83]; p = .47, favourable outcome OR 1.31 [95% CI 0.38–4.56]; p = .45). All patients without pupillary light reflexes died.
Discussion
We reviewed the clinical course of patients aged 65 years or older who underwent craniotomy for acute, or subacute, subdural hematoma from 2000 to 2015 to find predictors of clinical outcome. Eighty-four patients underwent surgery during this 16 year time period, which is less than six patients per year. Such low number suggests that many older patients suffering from acute subdural hematoma who were presented to our hospital during these years were not operated on. Unfortunately, we did not register such not-operated patients in our database. We therefore cannot indicate how representative our cohort is for the overall population of older patients presenting with an acute subdural hematoma. Our cohort probably represents a selection of patients who were judged to have good chances of favouring from surgery by the attending neurosurgeons.
Overall, mortality rate was high and only 25% of the patients of our cohort regained functional independence. Patients with GCS scores 9–15 at the moment of diagnosis had better chances of survival than patients who were comatose or intubated, but their favourable outcome rate was not significantly higher. All patients with bilaterally absent pupillary light reflexes died, those with still one normal-sized pupil included.
Outcome among patients harbouring an acute, subacute or spontaneous SDH was similar, contrary to the previous finding of Rosen⊘rn and Gjerris that patients with subacute SDH have better prognosis than patients with acute SDH.Citation9 Our finding can be explained by the fact that, in the current cohort, patients harbouring SDHs of different ages had comparable levels of unconsciousness whereas Rosen⊘rn and Gjerris reported considerably higher levels of consciousness for patients with subacute SDH compared to patients with acute SDH. The neurological condition of a patient thus seems to be more important for the prediction of prognosis than the age of the (hyperdens) SDH, although the small number of subacute and spontaneous SDH patients in our cohort limits drawing strong conclusion.
Literature review
The 75% unfavourable outcome rate (64% mortality) in our study is similar to most previously published series ().Citation2,Citation4,Citation5,Citation10–20 The high percentage of favourable outcome and concomitant low mortality rate in the recent Swiss series by Taussky et al. seems exceptional, but may be explained by their strict criteria for undertaking surgery: they excluded patients with Karnofsky scores <80, with dementia, or with bilaterally fixed and dilated pupils from surgery.Citation6 If we would have applied the same exclusion criteria in the current patient series, only 71 instead of 84 patients would have undergone surgery. Twelve of the 13 patients who would have been excluded from surgery according to the Taussky criteria died, whereas the single survivor remained severely disabled. It thus seems reasonable to apply such criteria to exclude subgroups of patients from surgery. If we would have excluded these 13 patients from surgery, the mortality rate in the remaining 71 patients would have been 59%, and the favourable outcome rate 30%.
Should we adhere to the international guidelines in older patients?
In their chapter on the international guidelines for the management of aSDH, Bullock et al. wrote that “there is a relationship between poor outcome and age, low GCS, and signs of cerebral herniation, but it is not possible to predict death on the basis of old age and poor GCS with certainty”.Citation1 In the current series of elderly patients, none of the patients with bilateral absence of pupillary light reflexes survived. Petridis et al. and Jamjoom also reported 100% mortality in 12 patients aged ≥75 years and 25 patients aged ≥65 years with bilaterally absent pupillary light reflexes prior to craniotomy for aSDH, respectively.Citation16,Citation21 Based on these results and our findings, it seems reasonable to conclude that patients aged ≥65 years with bilaterally non-reactive pupils at the moment of diagnosis will not benefit from surgery, and that withholding surgery in these cases is appropriate. When a patient loses pupillary light reflex(es) in the time interval between diagnosis and surgery, he/she may still have chances of surviving favourably. Of course, factors confounding proper evaluation of pupillary light reflexes such as narcotics, hypothermia and orbital injury should be carefully ruled out in all cases.Citation22
Limitations of the study
The present study has several limitations. First, data analysis was performed retrospectively. Second, there was large heterogeneity in patient characteristics. For example, the majority of patients harboured an aSDH but a significant subset of patients harboured a hyperdens SDH that occurred spontaneously or a saSDH. But since outcome among these groups was similar, we decided to analyse them together as one cohort. Moreover, such heterogeneous cohort may better reflect the daily neurosurgical practice where many patients are operated for hyperdens SDH at a stage when details about the exact cause or age of the SDH are still unknown. Similarly, there was no standardised treatment protocol with the attending neurosurgeon deciding whether to replace the bone flap at initial surgery, or not. But since outcome among patients undergoing craniotomy and patients undergoing decompressive craniectomy was not different, we decided to analyse them together as one cohort. Third, a well-known problem with studies identifying clinical parameters of poor prognosis is the so-called self-fulfilling prophecy, i.e. the tendency to restrict treatment selectively in patients with certain characteristics presumed to predict unfavourable outcome.Citation23,Citation24 Nine of the 14 patients with bilaterally non-reactive pupils at the moment of diagnoses died of respiratory failure following withdrawal of artificial respiration, in most cases because of presumed poor prognosis. The remaining five died of respiratory failure following restriction of treatment (“do not resuscitate”) because of presumed poor prognosis. Thus, post-surgical symptoms and signs “known” to be related to unfavourable outcome lead to treatment restriction, and the treatment restriction in itself lead to the unfavourable outcome. Maybe some would still be alive if active treatment had been continued, but whether any of them would have regained functional independence remains speculative. Fourth, we classified patients who did not regain physical independency as having an unfavourable outcome, but we recently reported normal mental quality of life (QoL) despite worse physical QoL in a cohort of 25 patients undergoing decompressive craniectomy for space-occupying middle cerebral artery infarction.Citation25 In the current cohort we did not determine mental QoL.
Conclusions
In 84 consecutive patients aged ≥65 years who underwent craniotomy for acute, or subacute, subdural hematoma during a 16 year time period, 64% died and 25% regained functional independence at one year follow-up. The low number of operated patients per year suggests that our cohort represents a selection of patients who were judged to have good chances of favouring from surgery. Patients without pupillary light reflexes at the moment of diagnosis had no chances of survival. One could therefore seriously question whether aSDH patients aged ≥65 years without pupillary light reflexes at the moment of diagnosis should be treated according to the international guidelines, although some uncertainty remains due to the small numbers in this and other case series, and the possibility of a self-fulfilling prophecy due to the tendency to restrict treatment because of presumed poor prognosis.
Ethical approval
For this type of study formal consent is not required.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, et al. Surgical Management of acute subdural hematomas. Neurosurgery 2006;58:S16–24
- Hernesniemi J. Outcome following head injuries in the aged. Acta Neurochir (Wien) 1979;49:67–79
- Howard MA 3rd, Gross AS, Dacey RJ Jr, Winn HR. Acute subdural hematomas: an age-dependent clinical entity. J Neurosurg 1989;71:858–63
- Cagetti B, Cossu M, Pau A, Rivano C, Viale G. The outcome from acute subdural and epidural intracranial haematomas in very elderly patients. Br J Neurosurg 1992;6:227–31
- Kotwica Z, Brzezinski J. Acute subdural hematoma in adults: an analysis of outcome in comatose patients. Acta Neurochir (Wien) 1993;121:95–99
- Taussky P, Hidalgo ET, Landolt H, Fandino J. Age and salvageability: analysis of outcome of patients older than 65 years undergoing craniotomy for acute traumatic subdural hematoma. World Neurosurg 2012;78:306–11
- Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet 1975;1:480–4
- Von Elm E, Altman DG, Egger M, Pocock SJ, Götzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573–7
- Rosenørn J, Gjerris F. Long-term follow-up review of patients with acute and subacute subdural hematomas. J Neurosurg 1978;48;345–9
- Fell DA, Fitzgerald S, Moiel RH, Caram P. Acute subdural hematomas. Review of 144 cases. J Neurosurg 1975;42:37–42
- Wilberger JE, Harris M, Diamond DL. Acute subdural hematoma: morbidity, mortality, and operative timing. J Neurosurg 1991;74:212–8
- Jamjoom A, Nelson R, Stranjalis G, et al. Outcome following surgical evacuation of traumatic intracranial haematomas in the elderly. Br J Neurosurg 1992;6:27–32
- Phuenpathom N, Choomuang M, Ratanalert S. Outcome and outcome prediction in acute subdural hematoma. Surg Neurol 1993;40:22–5
- Koç RK, Akdemir H, Öktem LS, Meral M, Menkü A. Acute subdural hematoma: outcome and outcome prediction. Neurosurg Rev 1997;20:239–44
- Hanif S, Abodunde O, Ali Z, Pidgeon C. Age related outcome in acute subdural haematoma following traumatic head injury. Ir Med J 2009;102:255–77
- Petridis AK, Dörner L, Doukas A, Eifrig S, Barth H, Mehdorn M. Acute subdural hematoma in the elderly: clinical and CT factors influencing the surgical treatment decision. Cent Eur Neurosurg 2009;70:73–8
- Leitgeb J, Mauritz W, Brazinova A, Janciak I, Majdan M, Wilbacher I, et al. Outcome after severe brain trauma due to acute subdural hematoma. J Neurosurg 2012;117:324–33
- Raj R, Mikkonen ED, Kivisaari R, Skrifvars MB, Korja M, Siironen J. Mortality in elderly patients operated for an acute subdural hematoma: a single case series. World Neurosurg 2016;88:592–7
- Benedetto N, Gambacciani C, Montemurro N, Morganti R, Perrini P. Surgical management of acute subdural haematomas in elderly: report of a single center experience. Br J Neurosurg 2017;31:244–8
- McGinity MJ, Michalek JE, Rodriguez JS, Floyd JR. Surgical evacuation of acute subdural hematoma in octogenarians: a ten-year experience from a single trauma center. Br J Neurosurg 2017;31:714–717 DOI: 10.1080/02688697.2017.1341041
- Jamjoom A. Justification for evacuating acute subdural haematomas in patients above the age of 75 years. Injury 1992;23:518–20
- Meyer S, Gibb T, Jurkovich GJ. Evaluation and significance of the pupillary light reflex in trauma patients. Ann Emerg Med 1993;22:1052–7
- Kirkman MA, Jenks T, Bouamra O, Edwards A, Yates D, Wilson MH. Increased mortality associated with cerebral contusions following trauma in the elderly: bad patients or bad management? J Neurotrauma 2013;30:1385–90
- Zahuranec DB, Brown DL, Lisabeth LD, Gonzales NR, Longwell PJ, Smith MA, et al. Early care limitations independently predict mortality after intracerebral haemorrhage. Neurology 2007;68:1651–7
- Van Middelaar T, Richard E, van der Worp HB, Van den Munckhof P, Nieuwkerk PT, Visser MC, et al. Quality of life after surgical decompression for a space-occupying middle cerebral artery infarct: A cohort study. BMC Neurology 2015;15:156