Abstract
Introduction
Pharmacological prophylaxis of venous thromboembolism (VTE) requires nuanced decision-making to balance the risk of VTE against haemorrhage. This problem is compounded in neurosurgical patients, in whom postoperative intracranial haemorrhage (ICH) may be catastrophic, compared to non-neuraxial bleeding in other types of surgery. Current major guidelines recommend caution when using pharmacological prophylaxis in elective cranial surgery, but incorporate low-quality evidence and lack precise guidance on timing and duration of anticoagulation.
Methods
We aimed to answer the following questions for patients undergoing elective cranial surgery: (1) when is the optimal time to initiate postoperative anticoagulation, and (2) how long should postoperative anticoagulation be continued for? In this systematic review, we screened randomised and non-randomised studies reporting original data on pharmacological VTE prophylaxis in elective cranial surgery. Outcomes of interest were VTE and ICH.
Results
Three retrospective, single-centre observational studies met eligibility criteria, with a total of 923 participants. Meta-analysis was not performed due to a high risk of bias across all studies. Through narrative synthesis, we found that patients who developed VTE were significantly more likely to receive their first postoperative dose at a later time (mean: 144 vs. 29 h, p = .04). Shorter courses of anticoagulation (<7 days) were associated with significantly lower ICH rates (p = .03) compared to longer courses (>21 days).
Conclusion
The limited evidence favours earlier initiation and shorter courses of thromboprophylactic anticoagulation. These findings are specific to patients undergoing surgery for meningioma or glioma and may not apply to other populations. Randomised controlled trials or robustly designed observational studies are necessary to establish a clearer evidence base.
Introduction
Venous thromboembolism (VTE) remains one of the most common surgical complications. The increased risk of VTE in surgical patients can be explained by Virchow’s triad of stasis, hypercoagulability and vessel wall injury. When deep vein thrombosis (DVT) occurs, the resulting thrombus may migrate to the pulmonary vasculature and cause pulmonary embolism (PE). The 12-month mortality rate of otherwise healthy patients with DVT and PE is 1.7% and 1.8%, respectively; in patients with comorbidities mortality rates can be as high as 30%.Citation1
DVT can develop in up to 40% of neurosurgical patients without any prophylaxis.Citation2,Citation3 Neurosurgical patients are at a higher risk of VTE, particularly those with stroke, spinal cord injury or neuraxial tumours who typically undergo lengthy operations and are immobilised for extended periods of time.Citation4 Unless contraindicated, VTE prophylaxis is crucial in all surgical patients to reduce morbidity and mortality. Intracranial haemorrhage (ICH) is a feared complication following cranial surgery. Indeed, the effects of ICH are frequently seen as more catastrophic than a bleed at another surgical site. Two large retrospective observational studies found that patients experiencing ICH following cranial surgery had a mortality rate of 15%Citation5 and 27.5%Citation6 respectively, as well as high rates of neurological disability. The incidence of clinically apparent postoperative ICH has been reported to range from 0.8% to 6.9%, whereas the incidence of postoperative ICH identified on routine imaging surveillance can reach 50%.Citation7 Pharmacological prophylaxis of VTE is likely to increase the risk of ICH, which is something neurosurgeons must routinely consider when deciding whether to start anticoagulation.
A substantial body of evidence supports the use of mechanical VTE prophylaxis for neurosurgical procedures but less so for pharmacological prophylaxis (). Pharmacological prophylaxis is more effective than placebo alone at reducing VTE risk,Citation25–27 but is similar in efficacy when compared with intermittent pneumatic compression (IPC).Citation19,Citation28 Furthermore, the safety of pharmacological prophylaxis remains inconclusive: whereas some meta-analyses have revealed an increased bleeding risk compared to placeboCitation25 and IPC,Citation19 others found no such difference.Citation26–28 Reflecting this uncertainty, guidelines from the National Institute for Health and Care Excellence (NICE) in the United Kingdom recommend using mechanical methods as first-line prophylaxis and only adding pharmacological prophylaxis if the risk of VTE exceeds the risk of bleeding.Citation8 On the other hand, the American Society of Hematology and Thrombosis Canada do not recommend pharmacological prophylaxis,Citation14,Citation29 whilst the Royal Australasian College of Physicians advocate ‘extreme caution’ if using pharmacological prophylaxis in neurosurgical patients.Citation24
Table 1. Summary of major national guidelines for pharmacological VTE prophylaxis in cranial neurosurgery.
The evidence concerning the timing and duration of VTE prophylaxis in cranial surgery is even more limited. The 2018 NICE guidance recommends considering prophylactic low-molecular-weight heparin (LMWH) starting 24 to 48 h after surgery in patients at higher risk of VTE than of bleeding and continuing for a minimum of seven days.Citation8 These recommendations are based on a mix of expert opinion and equivocal trial data. There have been no direct comparisons regarding the effect of starting prophylaxis at different times on rates of postoperative bleeding in the trials cited in these guidelines.
Current practice concerning decisions to commence prophylactic anticoagulation, as well as its time of initiation, dose and duration differs across patient populations and clinical settings. In a survey of Canadian neurosurgeons and intensivists,Citation4 approximately 80% of respondents stated they would prescribe prophylactic anticoagulation in patients who had undergone elective craniotomy. Unfractionated heparin was slightly preferred over LMWH. The preferred time of initiation was distributed similarly across the immediate period, at one day, at one to two days and at two to four days. A similar 2008 survey in the UK showed high variability between willingness to use pharmacological prophylaxis across neurosurgical units, as well as the time of initiating anticoagulation.Citation30
Aims
In this systematic review, we aimed to synthesise the evidence among adult patients undergoing elective cranial surgery to determine: (I) the optimal time to initiate pharmacological VTE prophylaxis postoperatively and (II) the optimal duration of pharmacological VTE prophylaxis.
Materials and methods
Search strategy
Guidelines from the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statementCitation31 were adhered to throughout this review. Articles from electronic databases including MEDLINE (PubMed), Embase and the Cochrane Central Register of Controlled Trials (CENTRAL) were sought from database inception until 8 November 2020. All results, including reviews, that passed the initial screening were hand searched for relevant citations. A repeat search was conducted on 28 May 2021 to identify any newly added records.
Study designs of interest included any randomised trial or observational study that reported original data. Studies investigating the incidence of VTE after cranial neurosurgery in patients who received pharmacological prophylaxis with unfractionated or low-molecular-weight heparin were identified using a search strategy comprising combinations of free-text and MeSH terms describing venous thromboembolism (e.g. pulmonary embolism, clot, deep vein thrombosis), intracranial haemorrhage (e.g. haemorrhage, bleed), cranial surgery (e.g. craniotomy, craniectomy, cranial surgery) and anticoagulation (e.g. heparin, anticoagulation, antithrombotic, LMWH). Our search strategy was in line with Cochrane recommendationsCitation32 and is included in the Online Appendix.
Other sources such as OpenSIGLE, OpenGrey, NICE Evidence Search, ClinicalTrials.gov, WHO International Trials Registry Platform, EU Clinical Trials Register, OpenTrials were also searched for ongoing or unpublished trials.
Initial screen of articles for eligibility
Abstracts were independently evaluated by two reviewers (I.T. and S.J.) using RayyanCitation33 to identify eligible articles for full-text review. Abstracts that did not report original data or include specific mention of prophylaxis of VTE, DVT or PE in patients undergoing neurosurgery were excluded. Conflicts were resolved by consensus among the reviewers.
Second screen of articles for eligibility issues
Inclusion
Full-text articles were reviewed for eligibility based on these inclusion criteria: (1) the study reported on original data; (2) the study participants were aged 16 and older and underwent elective cranial neurosurgery; (3) the study participants were given LMWH or unfractionated heparin (UFH) for VTE prophylaxis; (4) the study compared any of the following: time of initiation, regimen or duration of prophylaxis and (5) reported outcomes included objectively identified VTE or ICH or mortality rate.
Exclusion
Studies including patients who received mechanical prophylaxis only were excluded. Studies were also excluded if they did not report on patients undergoing cranial procedures or if the indication for surgery was trauma-related. Eligible studies were discussed with a senior author (A. S. P.) for final inclusion.
Risk of bias assessment
We planned to assess risk of bias using the Cochrane ROB-2 toolCitation34 for randomised studies and the Cochrane ROBINS-I toolCitation35 for non-randomised studies. Two reviewers (I.T. and S.J.) independently assessed the studies and resolved any differences in judgement through discussion. The quality of evidence was rated according to the GRADE guidelines using the GRADEpro tool.Citation36
Data extraction
Data extraction was performed independently by two reviewers (S.J. and M.K.) using a standardised web form. Discrepancies were resolved by discussion with a third reviewer (I.T.).
Statistical analysis
We intended to pool risk ratios from individual randomised controlled trials (RCTs) using the Mantel-Haenszel test for sparse data and estimate inter-study heterogeneity using the I2 statistic. A p value of .05 was regarded as the threshold for statistical significance in this review and in all included studies. For each comparison, a minimum of two studies with low risk of bias were required for meta-analysis.Citation32
Results
Identification of eligible studies
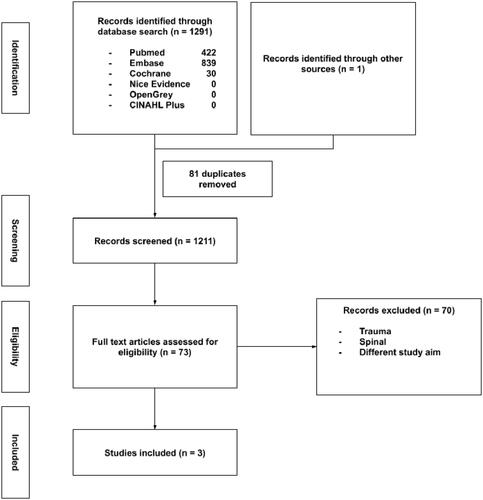
A flow chart of our search process is presented in . We identified 422 records from PubMed, 839 from Embase and 30 from CENTRAL. No relevant records were available on NICE Evidence search, OpenGrey or CINAHL Plus. One additional record was identified by hand-searching the references of key papers. After removing 81 duplicate records and screening the remaining 1211 abstracts, 73 articles were assessed in full. Three studies satisfied our inclusion criteria. Reverse citation search using Scopus yielded no additional records. A repeat search yielded no new records.
Characteristics of eligible studies
Study characteristics are shown in . Three studies totalling 923 participants who underwent elective cranial surgery were identified.Citation37–39 These were all retrospective, observational, single-centre cohort studies conducted in the USA, Germany, and the Netherlands. Collectively, the study duration ranged from 6 to 11 years with a mean patient age ranging from 55 to 61 years. The studies by Smith et al.Citation37 and Wilhelmy et al.Citation38 assessed the time of initiating anticoagulation, whereas the study by Senders et al.Citation39 assessed the duration of anticoagulation.
Table 2. Characteristics of eligible studies included in this systematic review.
According to Smith et al.Citation37 and Wilhelmy et al.,Citation38 the first dose was usually initiated on the first postoperative day unless bleeding risk factors were present. It is unclear when patients were started on anticoagulation in the study by Senders et al.Citation39 The LMWH used for prophylaxis in the three studies included enoxaparin, fraxiparin, tinzaparin and dalteparin whilst Smith et al.Citation37 also administered unfractionated heparin. Notably, only a small proportion of patients received chemical prophylaxis in the study by Smith et al.,Citation37 and the selection criteria for these patients were not clearly defined.
Forty-one patients in the study by Wilhelmy et al.Citation38 were on long-term anticoagulation prior to surgery, but the number of patients on prior anticoagulants was not stated in the other two studies. None of the studies specified whether patients received simultaneous mechanical thromboprophylaxis or assessed differences in anticoagulation dose regimen.
All studies included VTE and ICH as outcomes of interest. Wilhelmy et al.Citation38 performed routine surveillance to detect postoperative ICH using computed tomography (CT) imaging, whereas the confirmation of ICH in all other studies either relied on the identification of clinical symptoms or were not specified. VTE was detected using clinical symptoms alone,Citation37 or with further confirmation by imagingCitation39; Wilhelmy et al.Citation38 did not specify their method of measuring VTE occurrence. Whilst our review is focused on venous thromboembolism in the form of deep vein thrombosis and pulmonary embolism, Wilhelmy et al.Citation38 also included patients who experienced non-venous thromboembolic events, including two patients who developed ischaemic stroke. None of the studies investigated the association between the interventions and mortality.
Risk of bias
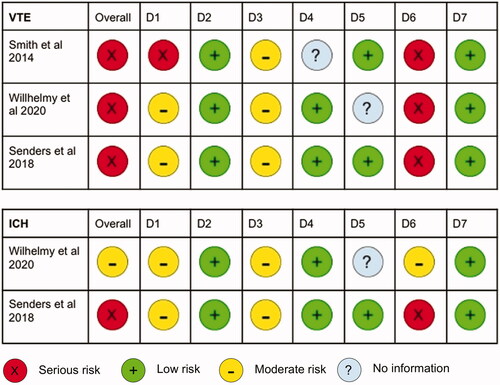
illustrates the risk of bias in seven domains across each study. Although the studies demonstrated a low risk of bias in participant selection and outcome reporting, they remained at high risk of overall bias stemming from issues related to confounding, intervention classification and outcome measurement. Wilhelmy et al.Citation38 and Senders et al.Citation39 attempted to control for confounders by performing multiple logistic regression. On the other hand, Smith et al.Citation37 did not address the issue of confounders. Furthermore, the small group of patients who received postoperative anticoagulation were likely to have a higher baseline VTE risk. In all studies, it was unclear whether information used to classify intervention groups were recorded before the interventions were administered, resulting in a moderate risk of bias in intervention classification. There was a serious risk of ascertainment bias in all studies due to inconsistent postoperative screening of VTE and ICH, as well as a lack of blinding. Furthermore, the method of outcome measurement varied across the studies. A meta-analysis was deemed unsuitable due to the high overall risk of bias.
Figure 2. Risk of bias, determined according to the Cochrane ROBINS-I tool. Risk of bias in each domain is classed as serious, moderate, low or no information as seen in the legend. VTE: venous thromboembolism; ICH: intracranial haemorrhage; D1: bias due to confounding; D2: bias in selection of participants into study; D3: bias in classification of interventions; D4: bias due to deviations from intended intervention; D5: bias due to missing data; D6: bias in measurement of outcomes, D7: bias in selection of reported result.
Findings
Time of initiation of pharmacological prophylaxis and risks of VTE and ICH
Two studies investigated the association between the risk of VTE and ICH and the time of initiating prophylactic anticoagulation (); 25 and 281 patients received anticoagulation in the studies by Smith et al.Citation37 and Wilhelmy et al.Citation38 respectively. In both studies, patients who developed VTE received their first dose of anticoagulation at later postoperative time points as compared to those that did not. This was statistically significant in Smith et al.’sCitation37 study, where patients who developed VTE were anticoagulated with a mean starting time of 144 h after surgery, compared to 28.8 h in those who did not develop VTE (p = .042). In Wilhelmy et al.’sCitation38 study, patients who developed VTE commenced anticoagulation at a median time of 54 h after surgery, compared to 29 h in those who did not develop VTE; this difference was not statistically significant.
Table 3. VTE and ICH Rates and time of initiating prophylactic anticoagulation after surgery.
In addition, Wilhelmy et al.Citation38 performed a multivariate logistic regression with VTE as the response variable and explanatory variables that included time of initiating anticoagulation, maximum systolic blood pressure and haematinic laboratory results. This model showed that delayed anticoagulation was associated with an increased risk of VTE, independent of other predictor variables (OR = 1.029, 95% CI 1.007–1.051, p = .008). Other significant predictors of VTE included higher white cell count on admission, lower maximum white cell count and lower minimum haematocrit.
For postoperative ICH, Wilhelmy et al.Citation38 found that among the 17 (5.9%) patients who experienced this complication, early anticoagulation was associated with a lower rate of ICH. However, of the 15 who were administered anticoagulation, 13 developed ICH prior to the first anticoagulation dose being administered. Four patients with ICH were on antiplatelet therapy prior to surgery. A multivariate logistic regression that included the time of initiating anticoagulation, maximum systolic blood pressure and steroid use as features showed that later time of initiation was associated with increased risk of postoperative ICH (OR = 1.016, 95% CI 1.001–1.032, p = .042).
None of the patients who received prophylactic anticoagulation developed bleeding complications in Smith et al.’sCitation37 study.
Duration of pharmacological prophylaxis and risks of VTE and ICH
One study evaluated the duration of anticoagulation and its association with VTE and ICH. Senders et al.Citation39 discovered that the rate of return to theatre for ICH was significantly higher (p = .03) in patients who received dalteparin for 21 days (n = 8, 5.9%) as compared to 7 days or less (n = 1, 0.6%). In a multivariate logistic regression with age as a covariate, the 21-day group was associated with a higher risk of ICH (OR 9.67, CI 1.73–180.95, p = .03). At 90 days, the VTE rate was higher in the 21-day group, but this was not statistically significant. High body-mass index (BMI) (OR 1.66 per 5 kg/m2 increase, CI 1.08–1.83, p = .02) and postoperative immobility (OR 4.15, CI 1.15–13.03, p = .03) were significant predictors of VTE within 90 days independent of LMWH duration.
Discussion
Summary of results
In this systematic review, we found three observational studies that address the time of initiation and duration of pharmacological prophylaxis of VTE in patients undergoing elective cranial surgery. No published RCTs were identified among the eligible studies. Due to heterogeneity and high risk of bias, we were unable to perform a meta-analysis but have instead provided a narrative synthesis. Two studies showed an association between later initiation of pharmacological prophylaxis and increased VTE rates.Citation37,Citation38 One study found that a longer duration of anticoagulation was associated with a higher ICH rate without a significant change in VTE rate.Citation39 None evaluated the effect of different interventions on mortality.
Quality of evidence
There is a low certainty of evidence across all outcomes (summarized in Supplemental Tables 1 and 2) owing to a high risk of bias in all included studies. Overall, there were no statistically significant differences in age and sex at baseline in all study groups. Where such differences were present in other characteristics such as BMI, comorbidities and medication, the authors used regression methods to adjust for confounders. However, this approach does not account for the myriad unmeasured clinical variables which may exert a confounding effect such as previous history of VTE and anticoagulant use. In addition, there was a high risk of ascertainment bias across all studies.
Interpretation of findings
In both included studies that compared the time of initiation of anticoagulation, participants who developed postoperative VTE received their first dose at a later time point.Citation37,Citation38 In Smith et al.’sCitation37 study, the difference in the mean initiation time after surgery between VTE (144 h) and non-VTE groups (28 h) was statistically significant, suggesting that postoperative anticoagulation may be more effective at reducing VTE risk if given earlier. However, the true sample size in this paper was much smaller than suggested, since only 25 of the total cohort of 336 patients were given postoperative anticoagulation. In addition, the authors did not control for postoperative length of stay and history of VTE.
Using a multivariate logistic analysis, Wilhelmy et al.Citation38 found a statistically significant association between delayed initiation of postoperative anticoagulation and increased VTE risk. For every hour that the first dose of anticoagulation was delayed, the odds of VTE increased by 2.9%. Using the Hosmer–Lemeshow test,Citation40 Wilhelmy et al.Citation38 demonstrated a good fit between the modelled probabilities and the observed outcomes for VTE and ICH. However, the model fit for both VTE and ICH was later reported as suboptimal due to the small number of outcome events (11 thromboembolic events, including 2 ischaemic stroke and 17 ICH). Furthermore, variables such as length of surgery were excluded despite their statistical significance in univariate tests.
Observational data from Senders et al.Citation39 suggests that longer periods of postoperative anticoagulation beyond seven days may confer little benefit whilst significantly increasing the risk of ICH, albeit with large precision estimates. Current NICE guidelines recommend a minimum duration of seven days but do not specify an upper limit.Citation8
Both Wilhelmy et al.Citation38 and Senders et al.Citation39 attempted to elucidate patient-related risk factors associated with occurrence of VTE. Wilhelmy et al.Citation38 found that some haematological parameters such as higher white cell count and lower minimum haematocrit predicted VTE risk. Their clinical significance is unclear and may in fact be spurious since there was no correction for multiple comparisons. On the other hand, Senders et al.Citation39 found that VTE was associated with high BMI and postoperative immobility, which are both well recognized VTE risk factors.Citation41,Citation42 No other predictive features were identified for ICH in the models described above.
Among patients with cancer, which is itself a strong risk factor for VTE, those with brain tumours are at a particularly high risk of VTE, especially if they undergo surgery.Citation41,Citation42,Citation43 This is reflected in the fact that the included studies were conducted on patients undergoing cranial surgery for meningioma or high-grade glioma. Thus, it may not be possible to generalise these findings to other neurosurgical cohorts. Moreover, the extent to which mechanical prophylaxis – which is widely used in the form of IPCs and graduated compression stockings – influenced the findings is unclear. Any generalisability is further hampered by the lack of validation of the statistical models produced in the included studies.
Limitations and strengths of this review
Incomplete data is a significant limitation of this review, further exacerbating the uncertainty of our findings. All included studies were lacking in published raw data, details of analysis and the reporting of outcome measurement. We are unable to offer clear recommendations for clinical practice owing to the low quality of evidence found in this review.
We have attempted to minimise bias in our review process by requiring at least two reviewers to independently perform screening, risk of bias assessments and data extraction. Our scoping search at the beginning of this review suggested a scarcity of original reports on the topics we intended to address. We therefore maximised the chances of identifying suitable studies by using a permissive approach to our initial search, before applying stricter inclusion criteria to abstracts included for full-text screening. Furthermore, we extended our search to trial registries, conference abstracts and other grey literature.
Future directions for research
RCTs are clearly needed in this area. It is perhaps unsurprising that no such trials have been identified in this review, given the ethical and logistical challenges. The numerous known risk factors for VTE and ICH suggest a lack of clinical equipoise necessary to randomise patients to different prophylactic regimens. Nevertheless, the preliminary and indirect nature of the evidence we have presented here provides a sound argument for randomising patients to different times of initiation, doses, and durations. Since this would no doubt require large sample sizes, RCTs would need to be coordinated across multiple institutions.
Whilst ICH can be detected relatively easily in the early postoperative period with imaging, following up participants to detect VTE would require intensive resources, as many cases of VTE may be asymptomatic or occur after hospital discharge. Patients would need to be followed up either in person or remotely, and duplex ultrasound imaging would be required to detect asymptomatic DVT.
It is important for future studies to compare the clinical consequences of VTE and ICH among neurosurgical patients. In Wilhelmy et al.’sCitation38 study, VTE and ICH both resulted in worse functional status and higher in-hospital mortality rates, but VTE was associated with worse outcomes than ICH (median Glasgow Outcome Scale 3 vs. 4, median modified Rankin Scale 4 vs. 2, mortality 18.2% vs. 11.8%). In contrast, there was no significant difference in five-year mortality between patients who experienced VTE and patients who did not in Smith et al.’sCitation37 study. Good evidence in this area would aid pragmatic decision-making.
Conclusion
Our systematic review highlights a lack of evidence around the optimal time of initiation and duration of prophylactic anticoagulation in patients undergoing elective cranial surgery. To the best of our knowledge, this is the first systematic review addressing these topics.
Early anticoagulation may be associated with a lower risk of VTE. Shorter regimens of up to seven days may be associated with a lower bleeding risk without a concurrent increase in VTE risk. High BMI and postoperative immobility are risk factors for VTE independent of the time of initiation of anticoagulation. These findings are specific to patients undergoing surgery for meningioma or glioma and may not apply to other neurosurgical populations. The existing evidence base is poor and would benefit from head-to-head trials that directly compare timing, dose and duration of anticoagulation.
Supplemental Material
Download MS Word (5.9 KB)Supplemental Material
Download MS Word (5.5 KB)Supplemental Material
Download MS Word (5.9 KB)Disclosure statement
No potential conflicts of interest were reported by the author(s).
Additional information
Funding
References
- Kroep S, Chuang L, Cohen A, et al. The impact of co-morbidity on the disease burden of VTE. J Thromb Thrombolysis 2018;46:507–15.
- Geerts W, Pineo G, Heit J, et al. Prevention of venous thromboembolism. Chest 2004;126:338S–400S.
- Shaikhouni A, Baum J, Lonser R. Deep vein thrombosis prophylaxis in the neurosurgical patient. Neurosurg Clin N Am 2018;29:567–74.
- Scales D, Riva-Cambrin J, Le T, et al. Prophylaxis against venous thromboembolism in neurointensive care patients: survey of Canadian practice. J Crit Care 2009;24:176–84.
- Desai V, Grossman R, Sparrow H. Incidence of intracranial hemorrhage after a cranial operation. Cureus 2016;8:e616.
- Kalfas I, Little J. Postoperative hemorrhage: a survey of 4992 intracranial procedures. Neurosurgery 1988;23:343–7.
- Seifman M, Lewis P, Rosenfeld J, Hwang P. Postoperative intracranial haemorrhage: a review. Neurosurg Rev 2011;34:393–407.
- National Guideline Centre (UK). Venous thromboembolism in over 16s: Reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism. London: National Institute for Health and Care Excellence (UK); 2018.
- Cerrato D, Ariano C, Fiacchino F. Deep vein thrombosis and low-dose heparin prophylaxis in neurosurgical patients. J Neurosurg 1978;49:378–81.
- Wautrkcht JC, Macquaire V, Vandksteene A, et al. Prevention of deep vein thrombosis in neurosurgical patients with brain tumors: a controlled, randomized study comparing graded compression stockings alone and with intermittent sequential compression. Correlation with pre-and postoperative fibrinolysis. Int Angiol 1996;15:5–10.
- Dickinson L, Miller L, Patel C, Gupta S. Enoxaparin increases the incidence of postoperative intracranial hemorrhage when initiated preoperatively for deep venous thrombosis prophylaxis in patients with brain tumors. Neurosurgery 1998;43:1074–9.
- Goldhaber S, Dunn K, Gerhard-Herman M, Park J, Black P. Low rate of venous thromboembolism after craniotomy for brain tumor using multimodality prophylaxis. Chest 2002;122:1933–7.
- Macdonald R, Amidei C, Baron J, et al. Randomized, pilot study of intermittent pneumatic compression devices plus dalteparin versus intermittent pneumatic compression devices plus heparin for prevention of venous thromboembolism in patients undergoing craniotomy. Surg Neurol 2003;59:362–71.
- Anderson D, Morgano G, Bennett C, et al. American Society of Hematology 2019 guidelines for management of venous thromboembolism: prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv 2019;3:3898–944.
- Gruber U, Rem J, Meisner C, Gratzl O. Prevention of thromboembolic complications with miniheparin-dihydroergotamine in patients undergoing lumbar disc operations. Eur Arch Psychiatry Neurol Sci 1984;234:157–61.
- Rokito S, Schwartz M, Neuwirth M. Deep vein thrombosis after major reconstructive spinal surgery. Spine (Phila Pa 1976) 1996;21:853–8.
- Agnelli G, Piovella F, Buoncristiani P, et al. Enoxaparin plus compression stockings compared with compression stockings alone in the prevention of venous thromboembolism after elective neurosurgery. N Engl J Med 1998;339:80–5.
- Constantini S, Kanner A, Friedman A, et al. Safety of perioperative minidose heparin in patients undergoing brain tumor surgery: a prospective, randomized, double-blind study. J Neurosurg 2001;94:918–21.
- Collen J, Jackson J, Shorr A, Moores L. Prevention of venous thromboembolism in neurosurgery: a metaanalysis. Chest 2008;134:237–49.
- Bauman J, Church E, Halpern C, et al. Subcutaneous heparin for prophylaxis of venous thromboembolism in deep brain stimulation surgery: evidence from a decision analysis. Neurosurgery 2009;65:276–80.
- Glotzbecker M, Bono C, Wood K, Harris M. Thromboembolic disease in spinal surgery: a systematic review. Spine (Phila Pa 1976) 2009;34:291–303.
- Dermody M, Alessi-Chinetti J, Iafrati M, Estes J. The utility of screening for deep venous thrombosis in asymptomatic, non-ambulatory neurosurgical patients. J Vasc Surg 2011;53:1309–15.
- Hamidi S, Riazi M. Incidence of venous thromboembolic complications in instrumental spinal surgeries with preoperative chemoprophylaxis. J Korean Neurosurg Soc 2015;57:114.
- Wickham N, Gallus A, Walters B, et al. Prevention of venous thromboembolism in patients admitted to Australian hospitals: summary of National Health and Medical Research Council clinical practice guideline. Intern Med J 2012;42:698–708.
- Hamilton M, Yee W, Hull R, Ghali W. Venous thromboembolism prophylaxis in patients undergoing cranial neurosurgery: a systematic review and meta-analysis. Neurosurgery 2011;68:571–81. [21311292]
- Iorio A, Agnelli G. Low-molecular-weight and unfractionated heparin for prevention of venous thromboembolism in neurosurgery. Arch Intern Med 2000;160:2327.
- Khan N, Patel P, Sharpe J, Lee S, Sorenson J. Chemical venous thromboembolism prophylaxis in neurosurgical patients: an updated systematic review and meta-analysis. J Neurosurg 2018;129:906–15.
- Wang X, Zhang Y, Fang F, et al. Comparative efficacy and safety of pharmacological prophylaxis and intermittent pneumatic compression for prevention of venous thromboembolism in adult undergoing neurosurgery: a systematic review and network meta-analysis. Neurosurg Rev 2021;44:721–9.
- Thrombosis Canada. Thromboprophylaxis: non-orthopedic surgery [online]; 2020. Available from: https://thrombosiscanada.ca/wp-uploads/uploads/2021/01/8-Thromboprophylaxis-Non-Ortho_17November2020.pdf [Accessed 24 Jul 2021].
- Gudipati S, Varma M, 2008. Practice of perioperative thromboembolic prophylaxis in elective intracranial surgery: a survey of current practice in the United Kingdom. Annual Scientific Meeting of the Neuroanaethesia Society of Great Britain and Ireland. J Neurosurg Anesthesiol 2008;20(:218.
- Page M, McKenzie J, Bossuyt P, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71
- Lefebvre C, Glanville J, Briscoe S, et al.. Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ and Welch VA, eds. Cochrane handbook for systematic reviews of interventions. 2nd Edition. Chichester (UK): John Wiley & Sons; 2019;67–107.
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev 2016;5:210.
- Sterne J, Savović J, Page M, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898.
- Sterne J, Hernán M, Reeves B, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919.
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime, 2021. Available from gradepro.org. [last accessed 11 Apr 2022]
- Smith T, Lall R, Graham R, et al. Venous thromboembolism in high grade glioma among surgical patients: results from a single center over a 10 year period. J Neurooncol 2014;120:347–52.
- Wilhelmy F, Hantsche A, Wende T, et al. Perioperative anticoagulation in patients with intracranial meningioma: no increased risk of intracranial hemorrhage? PLos One 2020;15:e0238387.
- Senders J, Snijders T, van Essen M, et al. Length of thromboprophylaxis in patients operated on for a high-grade glioma: a retrospective study. World Neurosurg 2018;115:e723–e730.
- Hosmer D, Lemesbow S. Goodness of fit tests for the multiple logistic regression model. Comm in Stats Theory Methods 1980;9:1043–69.
- Anderson F, Spencer F. Risk factors for venous thromboembolism. Circulation 2003;107:I-9–I-16.
- Heit J, Spencer F, White R. The epidemiology of venous thromboembolism. J Thromb Thrombolysis 2016;41:3–14.
- Schneck M. Venous thromboembolism in neurologic disease. In: Biller J and Ferro J, eds. Handbook of clinical neurology. Amsterdam: Elsevier; 2014:289–304.