ABSTRACT
Background: A minority of patients with mild traumatic brain injury (mTBI) experience a persistent symptom complex also known as post-concussion syndrome. Explanations for this syndrome are still lacking.
Objective: To investigate if the fear avoidance model, including catastrophizing thoughts and fear avoidance behaviour, poses a possible biopsychosocial explanation for lingering symptoms and delay in recovery after traumatic brain injury (TBI) with special focus on mTBI.
Design: Cross-sectional study.
Participants: 48 patients with TBI, of which 31 patients with mTBI, had persistent symptoms (mean time since injury 48.2 months); 92% of the entire sample fulfilled the criteria for post-concussion syndrome.
Outcome variables: catastrophizing, fear-avoidance, depression and post-concussion symptoms.
Results: High levels of catastrophizing were found in 10% and high levels of fear avoidance behaviour were found in 35%. Catastrophizing, fear avoidance behaviour, depressive symptoms and post-concussion symptoms correlated significantly with each other (p < 0.05).
Conclusion: The fear-avoidance model proposes a possible explanation for persistent symptoms. Validation and normative data are needed for suitable measures of catastrophizing and fear avoidance of post-concussion symptoms after TBI. Longitudinal prospective cohort studies are needed to establish its causal and explanatory nature.
Traumatic brain injury (TBI) poses a major global health issue with its high prevalence and subsequently high costs in western society (Citation1). The annual costs due to traumatic brain injury are estimated around €33 billion in Europe (Citation2). Approximately 80–90% of TBIs are considered to be mild (Citation3). The vast majority of patients with mild traumatic brain injury (mTBI) across different populations (e.g. civilian, military or sports) show a rapid recovery within the first months and do not report any symptoms at three months post injury (Citation4). A minority of the patient group reports persistent symptoms and experience long term interfering consequences of their mTBI, also known as post-concussion syndrome. The exact size of this minority remains debatable, with reported percentages ranging between 15 and 47% due to methodological variations across studies and inconsistencies regarding its definition (Citation5–Citation7). Finding a possible explanation for the persistence of symptoms has been of interest to many researchers in the last three decades, but no uniform explanation has been found (Citation8,Citation9).
New and recent advances in brain imaging techniques reveal brain tissue damage in mTBI, mostly vascular microstructural damage, that could not be visualized before (Citation10). However, these parameters or other biological explanations do not predict the persistence of symptoms or occurrence of post-concussion syndrome (Citation11). Although its name suggests that this symptom complex is specifically seen after concussion, post-concussion syndrome seems not specifically related to mTBI (Citation6,Citation12,Citation13). The nature and extent of this symptom complex is similar in other patient populations such as those with chronic pain syndromes (Citation14) or following traumas not involving the brain (e.g. after orthopaedic injuries) (Citation6,Citation13). Moreover, healthy controls report post-concussion like symptoms such as cognitive problems, fatigue and headache. When removing the criterion of a history of mTBI, a comparable prevalence of post-concussion syndrome can be found in healthy controls (Citation15,Citation16). These findings suggest that post-concussion syndrome is not brain injury specific; therefore psychological models should also be considered in explaining the development and nature of the post-concussion syndrome.
Regarding psychological causes, the most consistent finding has been that pre-injury mental health status predicts post-concussion syndrome (Citation9). Furthermore, early post-injury stress and anxiety levels after mTBI are also indicated as predictors of post-concussion syndrome (Citation9,Citation17). A multi modal explanation including both biological and psychosocial factors has also been suggested, but appears to explain not more than 40% variance of clinically relevant long term outcomes, such as post-concussion syndrome (Citation9). Despite many efforts, an unequivocal explanation as to why this minority of patients experiences persistent symptoms indicative of post-concussion syndrome is still lacking. Silverberg, et al. (Citation9) have suggested an integrated biopsychosocial approach for future studies on the basis of their systematic review of prognostic models.
A possible biopsychosocial explanation may be found in the fear avoidance model. This model is well-validated in patients with several bodily distress syndromes including chronic pain (Citation18), tinnitus (Citation19,Citation20), cancer survivors (Citation21), chronic fatigue (Citation22), fibromyalgia (Citation22) and fatigue in multiple sclerosis (Citation23,Citation24). Furthermore, it provides the theoretical underpinnings of effective treatment options in these patient groups, such as graded exposure therapy (Citation25) or newer generations of cognitive behavioural therapy (CBT) such as mindfulness based CBT (Citation23).
Applying this model to post-concussion syndrome, patients with mTBI may (mis)interpret information regarding the damage to their brain and its immediate consequences in a catastrophic way, which results in increasing anxiety and avoidance behaviour over time. According to the fear avoidance model, symptoms are wrongly interpreted as a sign of serious injury or disease over which one experiences little or no control. It is proposed that such misinterpretation of symptoms typically leads to a disproportional fear of symptoms and injury that develops over time into a disabling fear of experiencing symptoms such that people will avoid those activities that are presumed to worsen their problem (Citation26). Although avoidance behaviour may be adaptive in the acute phase, it can contribute to disuse, disability and depression which paradoxically worsen the symptoms in later stages (Citation27). In sum, this model suggests that it is not necessarily the severity of the injury, but rather a disease process of extended catastrophic thinking about the initial symptoms and fear avoidance behaviour initiated by a biological injury that will explain in a time dependent manner the persistence of symptoms and the level of disability.
The fear avoidance model has been postulated as a possible explanation for persistent symptoms in mTBI already over two decades ago by Kay, et al. (Citation28). They proposed the combination of two fear-avoidance cycles - one regarding the pain (i.e. headache) experience as previously validated in patients with pain and one regarding the cognitive symptoms. According to the cognitive fear avoidance cycle, the cognitive symptoms are erroneously interpreted as a sign of pathology over which one has little or no control. Such catastrophizing could extend to fear and avoidance of mental activities, also known as cogniphobia23,24, which subsequently decreases activity levels and may result in disuse, disability and depression. This could then increase the amount of cognitive failures, concluding its cyclic pattern, as suggested by Todd, et al. (Citation29) and Martelli, et al. (Citation30).
This combined pain/cognition-related fear avoidance model has not been tested empirically, although it is consistent with the current literature in several ways. Dean, et al. (Citation13) stated that headache and cognitive complaints are the most specific symptoms of the post-concussion symptoms following mTBI. The combined fear avoidance model targets these symptoms specifically. In support of this, Khoury, et al. (Citation31) and Broomhall, et al. (Citation5) found respectively that catastrophizing about pain and fear avoidance symptoms was significantly greater in patients with TBI in comparison to healthy or trauma controls. Moreover, multiple studies have shown that the fear avoidance model explained the pain experience of patients with whiplash disorders (Citation32,Citation33). Furthermore, Schmidt (Citation34) showed higher levels of fear avoidance regarding mental work in adults with chronic work-related stress compared to actively working employees. Despite these findings, to our knowledge the fear avoidance model has never been empirically examined as a possible explanation for post-concussion syndrome in patients with mTBI.
Therefore this study examined the prevalence of catastrophizing thoughts and fear avoidance behaviour in patients with persistent symptoms after TBI. In order to investigate whether the fear avoidance model provides a possible explanation for post-concussion syndrome, the relationships between post-concussion symptoms, catastrophizing thoughts and fear avoidance beliefs after TBI were examined. Separate analyses were performed on a subgroup of patients with mTBI because a biopsychosocial approach is proposed to be of special importance in explaining persistent symptoms after mTBI (Citation9,Citation12).
Methods
Participants
Participants were recruited at the Zuyderland Medical Centre, Sittard-Geleen, the Netherlands. Inclusion criteria were incidence of TBI and fluent in Dutch. TBI was defined according to WHO criteria as an acute brain injury resulting from external mechanical force to the head (Citation35). Patients with TBI who had received multidisciplinary treatment at the rehabilitation centre Zuyderland, Sittard-Geleen (NL) in the period 2009 till 2012 were asked to participate. If they were willing to participate, they were sent an information letter, an informed consent form and questionnaires in 2013. In the period 2013–2016 patients with TBI who were receiving a multidisciplinary neuropsychological rehabilitation treatment for persistent symptoms were approached by their treating neuropsychologist for inclusion. These patients received the questionnaires as part of regular care and were asked for their permission to use this data for research purposes. Depending on the complaints, patients were referred by their rehabilitation physician to one or more of the following disciplines; physiotherapy, social work, medical psychology or occupational therapy. Recruitment into the study was carried out at least 3 months after the injury to ensure presence of persistent symptoms.
Compliance with ethical standards
All procedures were approved by and in accordance with the ethical standard of the medical ethics committee of Zuyderland Medical Centre, the Medical Review Ethics Committee of Maastricht University and the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all participants included in the study. Patients did not receive any financial compensation for their participation.
Measures
Basic demographic information
Personal characteristics including age, gender, level of education and current and premorbid employment status were retrieved from the hospital database. The level of education was based on the highest completed level of education and divided according to the 7 point Verhage classification (Citation36). Medical data such as time since injury, cause of injury, severity of injury, presence of abnormal findings on available CT and/or MRI scans, total duration of multidisciplinary treatment received and presence of cognitive disorders when a neuropsychological assessment was available, were also retrieved from the hospital database. Causes of injury were categorized into ‘traffic accidents’, ‘sport’, ‘violence’ and ‘falls’. Severity of TBI was classified as ’mild’ or ‘moderate to severe’ and based on criteria developed by the WHO collaboration centre task force on mTBI. These stated that mTBI is identified by at least one of the following characteristics: a Glasgow Coma Scale (GCS) score of 13 to 15, a maximum duration of post-traumatic amnesia of 24 hours and a loss of consciousness up to 30 minutes (Citation37). These variables were extracted from the hospital database. If none of these variables were available, the severity rating based on clinical judgement of the treating neurologist or rehabilitation physician was used.
Catastrophizing
Catastrophizing about post-concussion symptoms was assessed with the Post-Concussion Symptoms Catastrophizing Scale (PCS-CS), which is an adaptation of the Dutch translation of the Pain Catastrophizing Scale (PCS) (Citation38,Citation39).The PCS has adequate psychometric properties (Citation40) and is validated in acute and chronic whiplash disorders (Citation41–Citation43) and used in patients with mTBI by Khoury, et al. (Citation31). It consists of 13 items measuring the self-reported frequency of catastrophizing thoughts about the experienced pain with a 5 point Likert scale. The PCS was adapted by replacing the word ‘pain’ with common post-concussion symptoms in all items: ‘headaches, dizziness, fatigue, memory and concentration problems’. The score ranges from 0 and 52, with higher scores indicating a higher intensity of catastrophizing. A score of 30 or higher can be used for identifying a high level of catastrophizing thoughts based on patients with pain and represents the 75th percentile according to the manual (Citation39). According to Severeijns, et al. (Citation44) this score represents a Z-score of at least 1.5 in several pain populations within a community setting. A score of 23 or higher corresponds to the 50th percentile and has also been used in the literature to indicate an above average level of catastrophizing (Citation45). To our knowledge, no cut-off scores for patients with TBI specifically are available. Internal consistency of the PCS-CS was excellent in this sample (Cronbach’s alpha = 0.94).
Fear avoidance
Concussion-related fear avoidance behaviour was assessed with an adapted version of the valid and reliable Dutch version of Tampa Scale for Kinesiophobia (TSK) (Citation46,Citation47), called the Fear of Mental Activity scale (FMA). The TSK was adapted by replacing the word ‘pain’ with common post-concussion symptoms in all items: ‘headaches, dizziness, fatigue, memory and concentration problems’. Additionally, items were adjusted to make them suitable for mTBI, e.g. ‘My head tells me there is something dangerously wrong’, instead of ‘My body tells me there is something dangerously wrong’. It consists of 17 items and the score ranges from 17 to 68 with a score higher than 37 indicating an above average and according to the manual a high level of fear-avoidance behaviour in patients with pain (Citation48,Citation49). Using a more conservative cut-off equivalent to the 75th percentile, a score higher than 48 indicates a high level of fear avoidance behaviour (Citation50). To our knowledge, no cut-off scores for patients with TBI specifically are available. Internal consistency of the FMA was good in this sample (Cronbach’s alpha = 0.80).
Depressive symptoms
Depressive symptoms were assessed with the subscale depression of the Dutch version of the Hospital Anxiety and Depression Scale (HADS) (Citation51). It is a valid and reliable measure for screening depression in patients with TBI (Citation52). The score ranges from 0 to 21 with a higher score indicating a higher intensity of depressive symptoms. Whelan-Goodinson, et al. (Citation52) showed that a score of 8 or higher is an indication for depression in patients with TBI, which is in line with findings of a large review in the general population of Crawford, et al. (Citation53).
Post-concussion symptoms
Post-concussion symptoms were assessed with the Dutch version of the Rivermead Post-Concussion Symptoms Questionnaire (RPQ) developed by King, et al. (Citation54). The RPQ is commonly used to assess the severity of symptoms after mild or moderate TBI (Citation55). It consists of 16 items assessing severity of symptoms in the last 24 hours in comparison to premorbid levels. It is a valid and reliable measure in TBI (Citation55). The total score ranges from 0 to 64. Report of three or more remaining symptoms, indicated by an item score of two or higher, was used as criterion for post-concussion syndrome. The same criterion has been used in previous research (Citation56–Citation60).
Statistical analyses
Data analyses were performed using SPSS Statistics 22.0 for Windows (IBM Corp., Armonk, NY). If ≤ 25% of the items of the questionnaires were missing, the mean of the remaining non-missing items of the scale were imputed. If more than 25% of the items were missing, no imputation took place and the total score was included as missing value in subsequent analyses. Sample characteristics are described by descriptive statistics. No outcome variable was significantly skewed nor were there any significant outliers (the confidence interval of skewness and kurtosis included zero). Pearson’s χ2 tests or independent sample t tests were performed to analyse differences for all patient characteristics and within the RPQ between the two severity groups; ‘mild’ and ‘moderate to severe’. Pearson correlation coefficients were calculated to show relationships between the variables constituting the fear avoidance model; PCS-CS score, HADS score, FMA score and RPQ total score. These correlations were compared between the two severity groups: ‘mild’ and ‘moderate to severe’ after Fisher’s Z transformation. For all statistical tests an alpha level of 0.05 was used.
Results
Patient sample
A total of 93 Dutch-speaking patients with TBI were approached and 48 patients were willing to participate (52%). Our sample included 23 men and 25 women with a mean age of 45.5 years (SD = 15.6, range 16–78). In most cases TBI was caused by traffic accidents (45.8%). The sample consisted of 31 mTBI cases and 17 moderate to severe TBI cases. The mean time since injury was 48.2 months (SD = 60.9, range 2–373) and the patients received on average a total of 7.4 months multidisciplinary treatment for persistent symptoms. One patient with mTBI was assessed in the third month after his/her injury. All other patients were assessed after at least 3 months. Regarding work status, the percentage of participants working more than 24 hours/week before their TBI decreased from 59.4 to 24.3 at the moment of inclusion. See for patient characteristics of the entire sample and the mTBI cases. Sample size deviations are the result of missing data in the hospital database. Except for gender, there were no significant differences found between the two severity groups on patient characteristics. The mTBI group had significantly more women than the more severe TBI group (χ = 5.42; p < 0.02).
Table 1. Patient characteristics.
Frequency of post-concussion syndrome, depression, catastrophizing and fear-avoidance behaviour
shows the prevalence of post-concussion symptoms in the entire sample and in mTBI cases specifically. Significant differences between the ‘mild’ and ‘moderate to severe’ group are also indicated. On the items where significant differences were found, the proportion of patients who were experiencing the symptom was higher in the mild group compared to the more severely injured group. Independent of severity, cognitive problems (memory/concentration/mental slowness) and fatigue were part of the most reported symptoms. Respectively 85.4% of the entire sample and 93.5% of the mTBI sample reported fatigue. Frequencies of cognitive problems varied for the entire and mTBI sample in the range of 81.3% - 91.7% and 87.1% – 93.5% respectively. shows the scores on the PCS-CS, FMA, RPQ and HADS. The mean of the RPQ was 30.6. A total of 92% of the patients fulfilled the criterion of post-concussion syndrome (having three or more post-concussion symptoms). The mean HADS score was 6.5. A total of 42% reported depressive symptoms at a clinical significant level. Furthermore, the mean PCS-CS score was 16.0. Using 23 as cut-off, 29% of the entire sample reported above average levels of catastrophizing. Using the more conservative cut-off of 30 as suggested by the scoring manual, 10% of the entire sample reported heightened levels of catastrophizing. The mean FMA score was 35.5. Using 37 as cut-off as suggested by the scoring manual, 35% of the entire sample reported above average levels of catastrophizing or fear avoidance behaviour. Using the more conservative criterion of 48 as cut-off, 4% of the entire sample reported heightened levels of fear avoidance behaviour. The frequencies of heightened levels of catastrophizing and fear avoidance behaviour in the mTBI subsample, using the cut-off scores suggested by the scoring manuals, are shown in (%I).
Table 2. Prevalence of post-concussion symptoms (%).
Table 3. Correlations among and descriptive statistics of all measures.
Relationships between the variables in the FA model
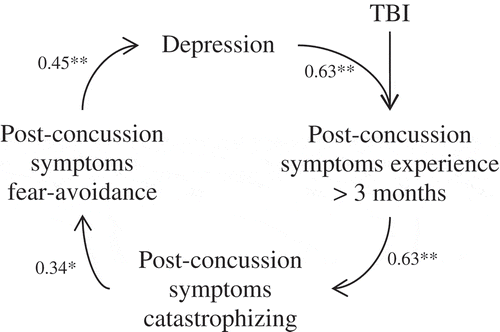
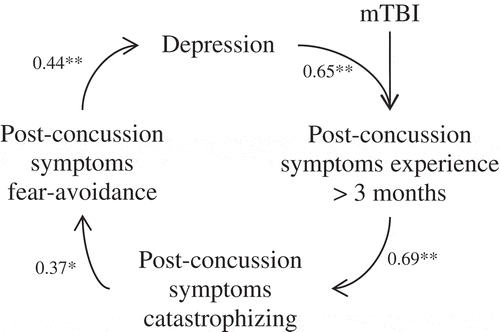
All measures were significantly correlated with each other (p < 0.05). The strongest association was seen between post-concussion symptoms and catastrophizing about these symptoms (r = 0.63 in the entire sample and r = 0.69 in the mTBI sample). See and for a graphical presentation of the fear avoidance model in the entire sample and mTBI subsample respectively. As can been seen in , the correlations between post-concussion symptoms versus depression and catastrophizing are slightly higher in the mTBI subsample than in the entire sample. However, no significant differences were found between the ‘mild’ and ‘moderate to severe’ TBI group (p > 0.05).
Discussion
This study investigated in a group of TBI out-patients whether the fear avoidance model is able to explain persistent symptoms in patients with TBI. It was hypothesized that this model would be of special importance for mTBI due to its integrative 360 biopsychosocial nature whereas unimodal biological and/or psychological explanations lack the explanatory value needed for this ‘miserable minority’ (Citation61).
The results showed low levels of catastrophizing and fear avoidance behaviour regarding post-concussion symptoms in comparison to pain experiences in several bodily distress syndromes, such as chronic pain and fibromyalgia (Citation39,Citation45,Citation47,Citation50). Despite these low levels, all correlations suggested by the fear avoidance model regarding post-concussion symptom experience were significant. The correlations between post-concussion symptoms versus catastrophizing and depression were slightly higher in the mTBI subgroup. These findings provide a preliminary indication that the fear avoidance model has explanatory value in accounting for persistency of symptoms.
Despite the promising significant correlations within the fear avoidance model, the levels of catastrophizing and fear avoidance were relatively low. This discrepancy could suggest that the cut-off values used to classify patients with TBI as ‘high catastrophizing’ or ‘highly fear avoidant’ in this study lacked sensitivity due to the scale adaptations made for this study. The PCS-CS was adapted by replacing the word ‘pain’ with ‘headaches, dizziness, fatigue, memory and concentration problems’. Although unlikely given the high frequency of symptom reports, these post-concussion symptoms may not represent the symptom complex experienced by the patient resulting in lower levels reported. Another more plausible explanation could be that the question format, whereby multiple symptoms were included in a single item, makes it difficult to understand and interpret the question. The FMA was adapted in two different ways. We have changed the experienced symptom of pain into post-concussion symptoms. Furthermore, we asked about fear avoidance behaviour regarding ‘cogniphobia’ in contrast to its original construct ‘kinesiophobia’. These changes may have influenced levels of reporting and the use of the cut-off validated with the original measures in different populations may therefore have limited validity in our sample. Furthermore, our sample already received on average more than 7 months of neuropsychological rehabilitation treatment, which may have lowered their levels of catastrophizing or fear avoidance. Moreover, lower frequency reports could also be the result of applicability of the fear avoidance model to only a subgroup of clinically relevant size of the miserable minority and aiding individual tailored care.
To our knowledge, this is the first study of catastrophizing and fear avoidance behaviour with regard to post-concussion symptoms, which makes it impossible to compare levels of catastrophizing or fear avoidance behaviour with other studies. Some studies, including other patient populations or non-patient populations, have found confirmatory results for the fear avoidance model or its components with regard to pain and kinesiophobia or cogniphobia (Citation5,Citation31,Citation34,Citation62,Citation63). Other patient studies have looked at catastrophizing about pain in mTBI (Citation31,Citation62), the presence of a symptom of fear avoidance in mTBI (Citation5) and the levels of catastrophizing and fear avoidance with regard to pain and kinesiophobia in patients with whiplash disorders (Citation33,Citation64). In non-patient populations the level of fear avoidance regarding chronic stress symptoms and cogniphobia (Citation34) and the level of catastrophizing and fear avoidance regarding headache and cogniphobia have been studied (Citation63).
Another finding that may seem remarkable is that the prevalence of post-concussion symptoms is equal or higher in the mTBI group compared to the moderate to severe TBI group. Previous studies have reported mixed findings regarding the number of complaints and injury severity. Some studies found similar results demonstrating equal or higher levels of complaints in patients with mTBI compared to patients with moderate to severe TBI on the one hand (Citation65,Citation66). On the other hand, van der Horn, et al. (Citation67) found increasing symptom reporting with increasing brain injury severity. Belanger, et al. (Citation65) mentioned that their group differences disappeared when controlling for post-traumatic stress complaints. These inconsistent results show that a biological explanation on its own, such as injury severity, is not sufficiently able to explain persistence of symptoms (Citation11) and highlight the need for a biopsychosocial explanation (Citation9), such as the currently investigated fear avoidance model.
Our findings have to be interpreted with caution as this was a Cross-sectional exploratory study with a relatively small sample size and a heterogeneous cohort. We have included one patient who was assessed in the third month after the injury. However, on average participants were assessed 48.2 months after their injury. Because we were interested in whether the fear avoidance model would apply to patients with post-concussion syndrome specifically, the current sample, which consisted almost entirely of patients with post-concussion syndrome, was highly suitable for evaluating this specific research question. A selection bias and inclusion of a heterogeneous group of participants in different stages of their recovery or disease process should also be taken into account. A large longitudinal cohort study is needed to establish the evolution of catastrophizing thoughts and fear avoidance behaviour and their time dependent role regarding post-concussion syndrome.
Despite these considerations and the exploratory nature of this study, the results do provide preliminary evidence that the fear avoidance model may have added value in explaining persistency of symptoms, especially after mTBI. Prospective longitudinal studies are needed to confirm this preliminary evidence. Furthermore, to assess catastrophizing thoughts and fear avoidance behaviour with the existing questionnaires in the mTBI patient population, we suggest a validation study for these measurements with a simplified administration which matches the capabilities of and symptoms experienced by the individual patient. Moreover, normative data is needed to establish correct cut-off scores for catastrophizing thoughts and fear avoidance behaviour regarding post-concussion symptoms. These future directions will unravel the relevance of the biopsychosocial fear-avoidance model in patients with mTBI.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Corrigan JD, Selassie AW, Orman JAL. The epidemiology of traumatic brain injury. J Head Trauma Rehabil. 2010;25(2):72–80. doi:10.1097/HTR.0b013e3181ccc8b4.
- Olesen J, Gustavsson A, Svensson M, Wittchen HU, Jönsson B. The economic cost of brain disorders in Europe. Eur J Neurol. 2012;19(1):55–62. doi:10.1111/j.1468-1331.2011.03590.x.
- Faul M, Xu L, Wald M, Coronado V, Dellinger AM. Traumatic brain injury in the United States: national estimates of prevalence and incidence, 2002–2006. Inj Prev. 2010;16(Suppl 1):A268–A268. doi:10.1136/ip.2010.029215.951.
- Levin HS, Diaz-Arrastia RR. Diagnosis, prognosis, and clinical management of mild traumatic brain injury. Lancet Neurol. 2015;14(5):506–17. doi:10.1016/S1474-4422(15)00002-2.
- Broomhall LG, Clark CR, McFarlane AC, O’Donnell M, Bryant R, Creamer M, Silove D. Early stage assessment and course of acute stress disorder after mild traumatic brain injury. J Nerv Ment Dis. 2009;197(3):178–81. doi:10.1097/NMD.0b013e318199fe7f.
- Meares S, Shores EA, Taylor AJ, Batchelor J, Bryant RA, Baguley IJ, Chapman J, Gurka J, Marosszeky JE. The prospective course of postconcussion syndrome: the role of mild traumatic brain injury. Neuropsychology. 2011;25(4):454. doi:10.1037/a0022580.
- Wood RL. Post concussional syndrome: all in the minds eye! J Neurol Neurosurg Psychiatry. 2007;78(6):552. doi:10.1136/jnnp.2006.113845.
- Ruff R. Two decades of advances in understanding of mild traumatic brain injury. J Head Trauma Rehabil. 2005;20(1):5–18. doi:10.1097/00001199-200501000-00003.
- Silverberg ND, Gardner AJ, Brubacher JR, Panenka WJ, Li JJ, Iverson GL. Systematic review of multivariable prognostic models for mild traumatic brain injury. J Neurotrauma. 2015;32(8):517–26. doi:10.1089/neu.2014.3600.
- Yuh EL, Cooper SR, Mukherjee P, Yue JK, Lingsma HF, Gordon WA, Valadka AB, Okonkwo DO, Schnyer DM, Vassar MJ. Diffusion tensor imaging for outcome prediction in mild traumatic brain injury: a TRACK-TBI study. J Neurotrauma. 2014;31(17):1457–77. doi:10.1089/neu.2013.3171.
- Wäljas M, Iverson GL, Lange RT, Hakulinen U, Dastidar P, Huhtala H, Liimatainen S, Hartikainen K, Öhman J. A prospective biopsychosocial study of the persistent post-concussion symptoms following mild traumatic brain injury. J Neurotrauma. 2015;32(8):534–47. doi:10.1089/neu.2014.3339.
- Broshek DK, De Marco AP, Freeman JR. A review of post-concussion syndrome and psychological factors associated with concussion. Brain Inj. 2015;29(2):228–37. doi:10.3109/02699052.2014.974674.
- Dean PJ, O’Neill D, Sterr A. Post-concussion syndrome: prevalence after mild traumatic brain injury in comparison with a sample without head injury. Brain Inj. 2012;26(1):14–26. doi:10.3109/02699052.2011.635354.
- Iverson GL, McCracken LM. ‘Postconcussive’symptoms in persons with chronic pain. Brain Inj. 1997;11(11):783–90. doi:10.1080/026990597122990.
- Clarke LA, Genat RC, Anderson JF. Long-term cognitive complaint and post-concussive symptoms following mild traumatic brain injury: the role of cognitive and affective factors. Brain Inj. 2012;26(3):298–307. doi:10.3109/02699052.2012.654588.
- Iverson GL, Lange RT. Examination of” postconcussion-like” symptoms in a healthy sample. Appl Neuropsychol. 2003;10(3):137–44. doi:10.1207/S15324826AN1003_02.
- Ponsford J, Cameron P, Fitzgerald M, Grant M, Mikocka-Walus A. Long-term outcomes after uncomplicated mild traumatic brain injury: a comparison with trauma controls. J Neurotrauma. 2011;28(6):937–46. doi:10.1089/neu.2010.1516.
- Vlaeyen JW, Linton SJ. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain. 2012;153(6):1144–47. doi:10.1016/j.pain.2011.12.009.
- Cima RF, Crombez G, Vlaeyen JW. Catastrophizing and fear of tinnitus predict quality of life in patients with chronic tinnitus. Ear Hear. 2011;32(5):634–41. doi:10.1097/AUD.0b013e31821106dd.
- Kleinstäuber M, Jasper K, Schweda I, Hiller W, Andersson G, Weise C. The role of fear-avoidance cognitions and behaviors in patients with chronic tinnitus. Cogn Behav Ther. 2013;42(2):84–99. doi:10.1080/16506073.2012.717301.
- Velthuis MJ, Peeters PH, Gijsen BC, Van Den Berg J-P, Koppejan-Rensenbrink RA, Vlaeyen JW, May AM. Role of fear of movement in cancer survivors participating in a rehabilitation program: a longitudinal cohort study. Arch Phys Med Rehabil. 2012;93(2):332–38. doi:10.1016/j.apmr.2011.08.014.
- Nijs J, Roussel N, Van Oosterwijck J, De Kooning M, Ickmans K, Struyf F, Meeus M, Lundberg M. Fear of movement and avoidance behaviour toward physical activity in chronic-fatigue syndrome and fibromyalgia: state of the art and implications for clinical practice. Clin Rheumatol. 2013;32(8):1121–29. doi:10.1007/s10067-013-2277-4.
- Wijenberg M. L., Stapert S. Z., Köhler S., & Bol Y. (2016). Explaining fatigue in multiple sclerosis: cross-validation of a biopsychosocial model. Journal of behavioral medicine, 39(5);815–822.
- Bol Y, Duits AA, Lousberg R, Hupperts RM, Lacroix MH, Verhey FR, Vlaeyen JW. Fatigue and physical disability in patients with multiple sclerosis: a structural equation modeling approach. J Behav Med. 2010;33(5):355–63. doi:10.1007/s10865-010-9266-8.
- Volders S, Boddez Y, De Peuter S, Meulders A, Vlaeyen JW. Avoidance behavior in chronic pain research: A cold case revisited. Behav Res Ther. 2015;64:31–37. doi:10.1016/j.brat.2014.11.003.
- Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JW. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med. 2007;30(1):77–94. doi:10.1007/s10865-006-9085-0.
- Crombez G, Eccleston C, Van Damme S, Vlaeyen JW, Karoly P. Fear-avoidance model of chronic pain: the next generation. Clin J Pain. 2012;28(6):475–83. doi:10.1097/AJP.0b013e3182385392.
- Kay T, Newman B, Cavallo M, Ezrachi O, Resnick M. Toward a neuropsychological model of functional disability after mild traumatic brain injury. Neuropsychology. 1992;6(4):371. doi:10.1037/0894-4105.6.4.371.
- Todd DD, Martelli MF, Grayson RL. The Cogniphobia Scale (C-Scale): A Measure of Headache Impact. Glen Allen, VA: Concussion Care Centre of Virginia (Test in the public domain); 1998.
- Martelli M, MacMillan P, Grayson R. Kinesiophobia and cogniphobia: avoidance-conditioned pain-related disability (ACPRD). Arch Clin Neuropsychol. 1999;14(8):804–804. doi:10.1093/arclin/14.8.804.
- Khoury S, Chouchou F, Amzica F, Giguère J-F, Denis R, Rouleau GA, Lavigne GJ. Rapid EEG activity during sleep dominates in mild traumatic brain injury patients with acute pain. J Neurotrauma. 2013;30(8):633–41. doi:10.1089/neu.2012.2519.
- Kamper SJ, Maher CG, Costa L, McAuley JH, Hush JM, Sterling M. Does fear of movement mediate the relationship between pain intensity and disability in patients following whiplash injury? A prospective longitudinal study. Pain. 2012;153(1):113–19. doi:10.1016/j.pain.2011.09.023.
- Nieto R, Miró J, Huguet A. The fear-avoidance model in whiplash injuries. Eur J Pain. 2009;13(5):518–23. doi:10.1016/j.ejpain.2008.06.005.
- Schmidt AJ. Does ‘mental kinesiophobia’exist? Behav Res Ther. 2003;41(10):1243–49. doi:10.1016/S0005-7967(03)00155-4.
- Cassidy J, Carroll L, Peloso P, Borg J, Von Holst H, Holm L, Kraus J, Coronado V. WHO collaborating centre task force on mild traumatic brain, injury, incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO collaborating centre task force on mild traumatic brain injury. J Rehab Med. 2004;43:28–60. doi:10.1080/16501960410023732.
- Verhage F. Het coderen van het opleidingsniveau voor researchdoeleinden. Groningen, The Netherlands: Academic Hospital Groningen, State University Groningen-internal publication; 1983.
- Carroll L, Cassidy J, Holm L, Kraus J, Coronado V. Methodological issues and research recommendations for mild traumatic brain injury: the WHO collaborating centre task force on mild traumatic brain injury. J Rehab Med. 2004;43:113–25. doi:10.1080/16501960410023877.
- Crombez G, Vlaeyen JWS The Pain Catastrophizing Scale. Unpublished authorized Dutch/Flemish translation. 1996.
- Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524. doi:10.1037/1040-3590.7.4.524.
- Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The pain catastrophizing scale: further psychometric evaluation with adult samples. J Behav Med. 2000;23(4):351–65. doi:10.1023/A:1005548801037.
- Miró J, Nieto R, Huguet A. The catalan version of the pain catastrophizing scale: a useful instrument to assess catastrophic thinking in whiplash patients. J Pain. 2008;9(5):397–406. doi:10.1016/j.jpain.2007.12.004.
- Sterling M, Hodkinson E, Pettiford C, Souvlis T, Curatolo M. Psychologic factors are related to some sensory pain thresholds but not nociceptive flexion reflex threshold in chronic whiplash. Clin J Pain. 2008;24(2):124–30. doi:10.1097/AJP.0b013e31815ca293.
- Rivest K, Côté JN, Dumas J-P, Sterling M, De Serres SJ. Relationships between pain thresholds, catastrophizing and gender in acute whiplash injury. Man Ther. 2010;15(2):154–59. doi:10.1016/j.math.2009.10.001.
- Severeijns R, Van Den Hout MA, Vlaeyen JW, Picavet HSJ. Pain catastrophizing and general health status in a large Dutch community sample. Pain. 2002;99(1):367–76. doi:10.1016/S0304-3959(02)00219-1.
- Van Damme S, Crombez G, Vlaeyen J, Goubet L, Van Den Broeck A, Van Houdenhove B. De pain catastrophizing scale: psychometrische karakteristieken en normering. Gedragstherapie. 2000;33(3):209–20.
- Goubert L, Crombez G, Vlaeyen JW, Van Damme S, Van Den Broeck A, Van Houdenhove B. De Tampa schaal voor kinesiofobie: psychometrische karakteristieken en normering. Gedrag En Gezondheid. 2000;28:54–62.
- Miller R, Kori S, Todd D The tampa scale. Unpublished report. Tampa, FL 1991.
- Houben RM, Leeuw M, Vlaeyen JW, Goubert L, Picavet HSJ. Fear of movement/injury in the general population: factor structure and psychometric properties of an adapted version of the Tampa Scale for Kinesiophobia. J Behav Med. 2005;28(5):415–24. doi:10.1007/s10865-005-9011-x.
- Vlaeyen JW, Kole-Snijders AM, Rotteveel AM, Ruesink R, Heuts PH. The role of fear of movement/(re) injury in pain disability. J Occup Rehabil. 1995;5(4):235–52. doi:10.1007/BF02109988.
- Roelofs J, Van Breukelen G, Sluiter J, Frings-Dresen MH, Goossens M, Thibault P, Boersma K, Vlaeyen JW. Norming of the Tampa Scale for Kinesiophobia across pain diagnoses and various countries. Pain. 2011;152(5):1090–95. doi:10.1016/j.pain.2011.01.028.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. doi:10.1111/acp.1983.67.issue-6.
- Whelan-Goodinson R, Ponsford J, Schönberger M. Validity of the hospital anxiety and depression scale to assess depression and anxiety following traumatic brain injury as compared with the structured clinical interview for DSM-IV. J Affect Disord. 2009;114(1):94–102. doi:10.1016/j.jad.2008.06.007.
- Crawford J, Henry J, Crombie C, Taylor E. Normative data for the HADS from a large non-clinical sample. Br J Clin Psychol. 2001;40(4):429–34. doi:10.1348/014466501163904.
- King N, Crawford S, Wenden F, Moss N, Wade D. The rivermead post concussion symptoms questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995;242(9):587–92. doi:10.1007/BF00868811.
- Eyres S, Carey A, Gilworth G, Neumann V, Tennant A. Construct validity and reliability of the Rivermead post-concussion symptoms questionnaire. Clin Rehabil. 2005;19(8):878–87. doi:10.1191/0269215505cr905oa.
- Oldenburg, C., Lundin, A., Edman, G., Nygren-de Boussard, C., & Bartfai, A. (2016). Cognitive reserve and persistent post-concussion symptoms—A prospective mild traumatic brain injury (mTBI) cohort study. Brain injury, 30(2), 146–155.
- McLean SA, Kirsch NL, Tan-Schriner CU, Sen A, Frederiksen S, Harris RE, Maixner W, Maio RF. Health status, not head injury, predicts concussion symptoms after minor injury. Am J Emerg Med. 2009;27(2):182–90. doi:10.1016/j.ajem.2008.01.054.
- Ingebrigtsen T, Waterloo K, Marup-Jensen S, Attner E, Romner B. Quantification of post-concussion symptoms 3 months after minor head injury in 100 consecutive patients. J Neurol. 1998;245(9):609–12. doi:10.1007/s004150050254.
- Sheedy J, Harvey E, Faux S, Geffen G, Shores EA. Emergency department assessment of mild traumatic brain injury and the prediction of postconcussive symptoms: A 3-month prospective study. J Head Trauma Rehabil. 2009;24(5):333–43. doi:10.1097/HTR.0b013e3181aea51f.
- Faux S, Sheedy J, Delaney R, Riopelle R. Emergency department prediction of post-concussive syndrome following mild traumatic brain injury—an international cross-validation study. Brain Inj. 2011;25(1):14–22. doi:10.3109/02699052.2010.531686.
- Ruff RM, Camenzuli L, Mueller J. Miserable minority: emotional risk factors that influence the outcome of a mild traumatic brain injury. Brain Inj. 1996;10(8):551–66. doi:10.1080/026990596124124.
- Geneviève Chaput, Susanne P. Lajoie, Laura M. Naismith, and Gilles Lavigne. “Pain Catastrophizing Correlates with Early Mild Traumatic Brain Injury Outcome,” Pain Research and Management, vol. 2016, Article ID 2825856, 7 pages, 2016. doi:10.1155/2016/2825856.
- Suhr J, Spickard B. Pain-related fear is associated with cognitive task avoidance: exploration of the cogniphobia construct in a recurrent headache sample. Clin Neuropsychol. 2012;26(7):1128–41. doi:10.1080/13854046.2012.713121.
- Nieto R, Miró J, Huguet A. Pain-related fear of movement and catastrophizing in whiplash-associated disorders. Rehabil Psychol. 2013;58:4 361. doi:10.1037/a0034267.
- Belanger HG, Kretzmer T, Vanderploeg RD, French LM. Symptom complaints following combat-related traumatic brain injury: relationship to traumatic brain injury severity and posttraumatic stress disorder. J Int Neuropsych Soc. 2010;16(01):194–99. doi:10.1017/S1355617709990841.
- Dikmen S, Machamer J, Fann JR, Temkin NR. Rates of symptom reporting following traumatic brain injury. J Int Neuropsych Soc. 2010;16(03):401–11. doi:10.1017/S1355617710000196.
- Van Der Horn HJ, Spikman JM, Jacobs B, Van Der Naalt J. Postconcussive complaints, anxiety, and depression related to vocational outcome in minor to severe traumatic brain injury. Arch Phys Med Rehabil. 2013;94(5):867–74. doi:10.1016/j.apmr.2012.11.039.