ABSTRACT
Objective: Previous research suggests that saccadic eye movements can be uniquely sensitive to impairment in chronic traumatic brain injury (TBI). This study was conducted to examine saccadic eye movements across varying levels of cognitive load and TBI history/severity. We hypothesized that saccadic impairment in chronic mild and moderate-severe TBI would be most pronounced under conditions of high cognitive load.
Methods: In total, 61 participants (including n = 20 with chronic mild TBI, n = 15 with chronic moderate-severe TBI, and 26 uninjured controls) completed a battery of conventional neuropsychological tests and the Fusion n-Back Test, which measures manual and saccadic response time (RT) across varying cognitive load and cueing conditions.
Results: Consistent with our hypotheses, chronic mild and moderate-severe TBI were associated with substantial saccadic impairment under conditions of high cognitive load. Participants with moderate-severe TBI also demonstrated saccadic impairment at low levels of cognitive load. TBI groups and uninjured controls did not differ significantly on manual metrics or conventional neuropsychological measures.
Conclusions: This study provides additional support for the value of eye tracking for enhanced assessment of TBI. Additionally, findings suggest that TBI is associated with greatest susceptibility to oculomotor interference under high levels of cognitive load.
Introduction
After traumatic brain injury (TBI), many experience a positive recovery and resume regular activities without the need for long-term care; however, a substantial proportion continues to experience clinically significant difficulties into the chronic phase of recovery (Citation1–Citation6). Considering the wide range of potential outcomes, cognitive assessment can be invaluable to the process of developing plans for treatment and graded return to activity. In mild TBI, a number of cognitive deficits are detectable in the acute stage, but findings on common clinical tests typically resolve within a week to 3 months (Citation7,Citation8). In moderate-severe TBI, cognitive deficits are more likely to persist long-term (Citation9).
These general trends, however, obscure important forms of heterogeneity within the TBI population, and cognitive performance can be impacted by many factors (Citation10–Citation16). Using conventional assessment tools, it can be very difficult to objectively distinguish between neurological and non-neurological sources of impairment. For example, button presses, verbal responses, and other forms of somatomotor output can be strongly influenced by factors unrelated to injury—such as intelligence or level of education—that can complicate interpretation of clinical results (Citation17–Citation19). Because post-injury cognitive performance is typically interpreted in comparison with ‘best estimates’ of pre-injury functioning, it can be especially challenging to identify cognitive changes among those patients whose pre-injury intellectual functioning falls outside of the average range (Citation20,Citation21). In particular, patients who were functioning at a high intellectual level prior to injury may demonstrate ‘average’ or ‘normal’ cognitive performance after injury, despite having experienced a significant decline from pre-injury levels of function.
Eye tracking technology may provide a means to circumvent some of the limitations of conventional assessment methods. Our group has shown that saccadic metrics—variables representing the speed and accuracy of eye movements in response to targets and cues—can serve as reliable and unique measures of cognitive and neuromotor function (Citation19). Unlike many conventional metrics, saccadic response time (RT) appears to be minimally impacted by education or intelligence (Citation19,Citation22,Citation23). In terms of clinical applications, eye tracking has demonstrated particular value for TBI assessment, with a growing body of evidence indicating that acute and chronic TBI are associated with slower and less accurate acquisition (e.g. planning and execution of saccadic eye movements) and tracking (e.g. smooth pursuit) of visual targets (Citation24–Citation38). For example, in a recent study using a cued saccadic RT paradigm to compare participants with remote mild TBI to uninjured controls, we found evidence for persistent saccadic impairment among those with multiple previous injuries and/or chronic symptoms, whereas asymptomatic participants with a single mild TBI performed similarly to controls (Citation35). Impaired eye movements have also been found to relate to TBI clinical characteristics such as severity of symptoms and extent of diffuse axonal injury (as measured by diffusion tensor imaging) (Citation22,Citation36–Citation38).
However, not all eye movements are equally sensitive to the effects of TBI. In a survey of the literature on oculomotor assessment of TBI, including studies of smooth pursuit, accommodation, pupillary light reflex, saccades and vergence, Barker et al. (Citation39) suggested that saccadic metrics demonstrate the greatest sensitivity to impairment—particularly those that require significant cognitive processing. While saccadic responses are routinely influenced by attentional processing (Citation40,Citation41), the neurocognitive contributions to saccadic responses can also be modulated or magnified through the design of the cognitive task in which they are embedded (Citation19). In a recent demonstration of the potential value of this approach for clinical assessment, our group found that sensitivity of saccadic RT to remote mild TBI was strongest in the presence of cognitive cues that increase executive and attentional processing demands (Citation35).
These findings are consistent with an established body of research showing that performance is most likely to be compromised when cognitive demand exceeds available cognitive resources (Citation42,Citation43). A number of previous studies have found that increased cognitive load can disproportionately affect cognitive and motor performance in those with a history of TBI (Citation44–Citation48). A ‘threshold’ model focusing on availability of cognitive resources in low- versus high-load situations may help to explain the experiences of those individuals with TBI who are able to perform normally on highly structured or overlearned tasks (including many forms of cognitive testing), but who are easily flustered or overwhelmed by the multiplexed demands of everyday life. Potentially, this model of cognitive load interference might also be leveraged to further enhance the sensitivity of eye tracking for assessment of TBI.
In summary, previous research suggests that saccadic eye movements may provide unique value in assessment of TBI-related impairment and that cognitive and motor deficits in TBI may be most apparent under conditions of high cognitive load. Little is known, however, about how cognitive load may impact saccadic performance after TBI. The current study was conducted to investigate methods for further enhancement of eye tracking for TBI assessment by probing performance across multiple levels of cognitive load. We hypothesized that chronic TBI would be associated with impaired saccadic RT and that these effects would be most pronounced under conditions of high cognitive load.
Methods
Participants and procedures
A community sample of participants with and without a history of TBI was recruited via advertisements posted within the Washington, D.C. metro area. The TBI group consisted of adults (> 18 years old) with a history of mild, moderate or severe TBI (defined according to American Congress of Rehabilitation Medicine criteria) sustained 3 months to 10 years prior to enrollment, whereas the control group consisted of adults without a history of concussion/TBI or other neurological conditions. Participants with medical conditions other than TBI that would be expected to impact performance, uncorrected visual impairment, failure on two or more measures of response validity/effort, or failure to follow task instructions were excluded from analysis. Out of 67 participants enrolled based upon initial screening, 61 (n = 26 Control; n = 35 TBI) met full eligibility requirements and were included in the study. All procedures were approved by the local Institutional Review Board. Training and supervision for all study procedures was provided by a licensed clinical neuropsychologist. Testing took place in a non-clinical setting, and participants were informed that data would be used only for research purposes. After written informed consent, participants provided demographic information and medical history. TBI history, including mechanism of injury, loss of consciousness (LOC), post-traumatic amnesia (PTA) and alteration of consciousness (AOC), was assessed using the Ohio State University TBI Identification Method (OSU TBI-ID (Citation49,Citation50)) and confirmed using available medical records. Participants then completed a fixed battery of standardized neuropsychological measures and the Fusion n-Back Test, described below.
Measures
Neuropsychological assessment
Standardized neuropsychological tests included the Wechsler Test of Adult Reading (WTAR), a measure of premorbid IQ (Citation51); Trail Making Test (TMT) Parts A and B (Citation52), measures of psychomotor speed and executive functions; Hopkins Verbal Learning Test-Revised (HVLT-R (Citation53)), a measure of learning and memory; and the following subtests of the Wechsler Adult Intelligence Scale-IV (WAIS-IV (Citation54,Citation55)): Digit Span, a measure of working memory; Symbol Search, a measure of visual scanning and processing speed; and Coding, a measure of processing speed and working memory. Additionally, embedded metrics from WAIS-IV Digit Span and TMT were used to evaluate performance validity (i.e. test-taking effort) using previously validated cut-offs for each measure (Citation56,Citation57). The Glasgow Outcome Scale-Extended (GOS-E (Citation58)) was administered as an index of functional outcome. Post-concussive symptoms were measured using the Neurobehavioral Symptom Inventory (NSI) (Citation59).
Fusion n-Back Test
The Fusion n-Back Test was designed to systematically measure the effects of cognitive load (attentional demands and memory) and predictive cues (valid or invalid) on saccadic and manual RT (Citation60) (see ). This test adds the working memory demands of the classic ‘n-back’ continuous performance task (Citation61) to the alerting, orienting and interference cues used to modulate saccadic and manual RT in the Bethesda Eye and Attention Measure (BEAM) (Citation19) (please see (Citation62) for previous use of similar cues with manual RT in the Attention Network Task [ANT]). Across each of three separate cognitive load conditions, participants completed a series of test trials in which circular targets appeared to the left or the right of a central fixation cross. Participants were instructed to respond as quickly as possible by moving their eyes towards each target and pressing the appropriate button. After each target, participants returned their gaze to the central fixation cross and awaited the next target. To increase the attentional demands of the task, directional (valid) and misdirectional (invalid) visual cues (left- or rightward facing arrows) or no cue were presented centrally for 200 ms immediately prior to the appearance of the target. Whereas directional cues pointed towards the impending target, providing an opportunity to reduce RT latency through anticipatory spatial orienting, misdirectional cues pointed away from the impending target, diverting spatial orientation and provoking attentional conflict. At the ‘Low’ level of cognitive load (‘Simple RT’), participants were instructed to press a single button as quickly as possible in response to all targets (white circles). At the ‘Moderate’ level of cognitive load (‘0-Back’/colour discrimination), participants were instructed to press buttons labelled ‘Green’ or ‘Blue’ in response to green or blue targets. Relative to the ‘Simple RT’ condition, the ‘0-Back’ condition added the momentary cognitive demand of discriminating between green and blue targets and selecting the correct versus incorrect response. At the ‘High’ level of cognitive load (‘1-Back’/working memory), participants were instructed to press buttons labelled ‘Same’ or ‘Different’, depending on whether the target circle was the same colour or a different colour relative to the previous target. Compared with the ‘0-Back’ condition, the ‘1-Back’ condition added the continuous cognitive demand of maintaining the representation of the previous target colour in working memory. Cognitive load condition was randomized across test blocks, and cue type was randomized on a trial-by-trial basis within each test block.
Eye tracking
Saccadic eye movement data were acquired at 120 Hz using an Applied Science Laboratories (ASL) D6 High-Speed Desktop Eye Tracker. Eye tracking calibration was performed at the beginning of each testing session using a 9-point rectangular calibration screen. Manual responses were recorded with a Cedrus RB-530 response pad. While completing the Fusion n-Back Test, participants wore a Lycra cap for EEG acquisition; these data will be reported elsewhere. Stimuli were presented using Presentation software (Neurobehavioral Systems) at 1920 × 1080 resolution on a 19” LCD monitor with 60 Hz refresh rate. Head movements were minimized with a chin rest. Participants were seated with eyes positioned 24” from the stimulus display. Gaze data and manual responses were synchronized with task event markers during data acquisition.
Analysis
Gaze data were processed using ASL Results (Version 2.4.3; ASL, 2011) to identify fixations and saccades. Custom scoring software was then used to remove invalid trials and aggregate RTs across trials as median scores. Raw scores from the conventional neuropsychological battery were converted to Z-scores based on published age-corrected normative data and aggregated to represent ‘Global Cognition’, with better performance yielding higher scores. Statistical analyses were conducted using the SPSS 24.0 statistical package. Missing data (4.9% of all primary metrics) were imputed using expectation maximization. A one-way ANOVA was used to compare neuropsychological test results between groups. Primary analyses of Fusion n-Back results were performed using 3(load) x 3(cue) x 3(group) mixed model ANCOVAs for manual and saccadic RT, controlling for age.
Results
Participant characteristics
presents participant characteristics. There was a non-significant trend towards a higher proportion of females in the control (73.1%) versus mild TBI (50.0%) and moderate-severe TBI (40.0%) groups, p = 0.09. However, gender was not significantly associated with primary outcomes in any of the participant groups; therefore, results from male and female participants were pooled for analyses. Participant groups did not differ significantly in age (p = 0.43), education (p = 0.15), estimated premorbid IQ (p = 0.28), or race/ethnicity (p = 0.11). Participants in the control group reported significantly fewer post-concussive symptoms (NSI total M = 8.81, SD = 10.06) than those in the mild TBI (M = 28.35, SD = 14.82) and the moderate-severe TBI (M = 27.47, SD = 11.48) groups, p < 0.001. Participants in the control group also had significantly higher functional outcome scores on the GOS-E (M = 7.81, SD = 0.49) than those in the mild TBI (M = 6.65, SD = 1.27) and moderate-severe TBI (M = 6.20, SD = 1.42) groups, p < 0.001. Time since injury did not differ significantly between the mild TBI (M = 39.30 months, SD = 33.24) and moderate-severe TBI (M = 57.27 months, SD = 42.44) groups, p = 0.17. Primary injury mechanisms related to TBI included motor vehicle accidents, sports participation and accidental falls; mechanism did not differ between mild and moderate-severe TBI groups, p = 0.58.
Table 1. Participant characteristics.
Neuropsychological tests
Neuropsychological tests results are presented in . As shown, individual neuropsychological tests yielded no significant differences between the control, mild TBI and moderate-severe TBI groups. Global cognition, a composite measure of these neuropsychological tests, also did not differ significantly across groups, F(2,58) = 0.521, p = 0.60. However, a non-significant trend was observed for poorer performance among the TBI groups relative to the control group on the WAIS-IV Coding test, p = 0.09.
Table 2. Neuropsychological test performance in control and TBI groups.
Fusion n-Back: saccadic responses
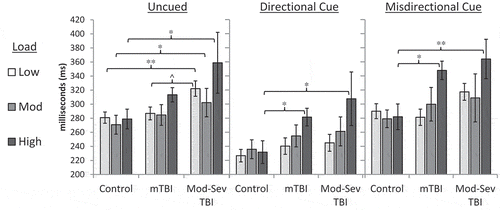
A mixed model ANCOVA of load*cue*group (controlling for age) on saccadic RT demonstrated main effects for group, F(2, 57) = 3.51, p = 0.037, ηp2 = 0.11, cue (F(1.62, 92.16) = 3.98, p = 0.03, ηp2 = 0.07) and cognitive load (F(1.55, 88.31) = 7.70, p = 0.002, ηp2 = 0.12), as well as an interaction for load*group, f(3.10, 88.31) = 2.72, p = 0.048, ηp2 = 0.09. The main effect of age was non-significant, F(1, 57) = 0.396, p = 0.53, ηp2 = 0.01. Pairwise comparisons for the load*group interaction demonstrated that saccadic RT in the high load (1-back) condition was significantly slower than in the low (simple RT) and moderate (0-back) load conditions for both the mild TBI and moderate-severe TBI groups (p < 0.03 for all); the low and moderate load conditions did not differ significantly (p > .27 for all). There were no significant pairwise load comparisons for the control group (p > .52 for all).
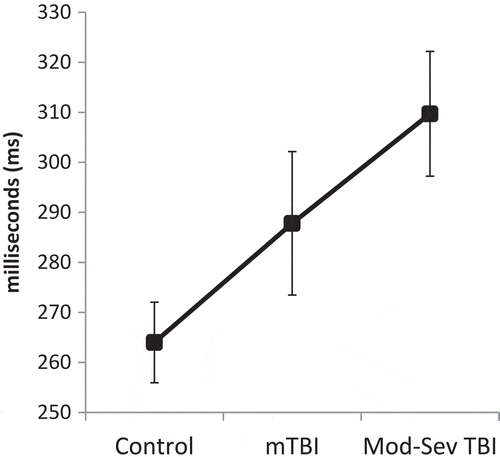
Pairwise analyses controlling for age revealed that saccadic RT was slower in the mild TBI group than the control group for 1-Back/Directional Cue, F(1, 43) = 4.84, p = 0.03, and 1-Back/Misdirectional Cue, F(1,43) = 4.72, p = 0.04. Comparison of moderate-severe TBI and control groups demonstrated significant differences for simple RT/Uncued, F(1, 43) = 6.24, p = 0.02; 0-Back/Uncued, F(1, 43) = 4.21, p = 0.047; 1-Back/Uncued, F(1, 43) = 5.39, p = 0.03; 1-Back/Directional Cue, F(1, 43) = 5.33, p = 0.03; and 1-Back/Misdirectional Cue, F(1, 43) = 8.97, p = 0.005. An additional non-significant trend was noted for slower performance in the moderate-severe TBI group relative to the mild TBI group for simple RT/Uncued, F(1, 43) = 3.35, p = 0.08. Saccadic RT by group, load, and cue is presented in . Overall saccadic RT by group is presented in . Follow-up correlations demonstrated that WAIS-IV Coding test performance was not significantly related to overall Fusion saccadic RT (r = 0.03, p = 0.82).
Figure 1. Saccadic target detection in the Fusion n-Back Test. Task was performed across multiple conditions: Low Load (Simple Response Time; press a single button [all target circles were white]), Moderate Load (0-Back/Colour Discrimination; press a button representing green vs. blue current target circle) and High Load (1-Back/Working Memory; press a button representing same vs. different colour of current target circle relative to the previous target circle).
![Figure 1. Saccadic target detection in the Fusion n-Back Test. Task was performed across multiple conditions: Low Load (Simple Response Time; press a single button [all target circles were white]), Moderate Load (0-Back/Colour Discrimination; press a button representing green vs. blue current target circle) and High Load (1-Back/Working Memory; press a button representing same vs. different colour of current target circle relative to the previous target circle).](/cms/asset/b8d54ba1-6290-48d2-9e18-49fe5057faea/ibij_a_1511067_f0001_b.gif)
Figure 2. Saccadic RT on the Fusion n-Back Test by group, cue type and cognitive load. mTBI = Mild TBI; Mod-Sev TBI = Moderate-Severe TBI. Error bars represent SE. Main effect of Group was significant, p = 0.04, ηp2 = 0.11; pairwise between-group differences are noted with * for p < .05 and ^ for p < 0.10. Significant effects were also present for Cue (ηp2 = 0.07, p = 0.03), Load (ηp2 = 0.12, p = 0.002); and a Group*Load interaction (ηp2 = 0.09, p = 0.048); pairwise comparisons not shown. For the Mild TBI and Moderate-Severe TBI groups only, saccadic RT was significantly slower in the high load (1-Back) condition than in the low (Simple RT) and moderate (0-Back) load conditions (p ≤ 0.03 for all).
Fusion n-Back: manual responses
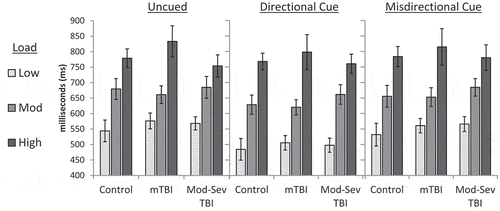
A mixed model ANCOVA of load*cue*group (controlling for age) on manual RT demonstrated main effects of cue (F(2, 114) = 8.32, p < 0.001, ηp2 = 0.13), cognitive load (F(1.95, 110.92) = 11.80, p < 0.001, ηp2 = 0.17), and age F(1, 57) = 8.60, p = 0.005, ηp2 = 0.13. No effect of group was evident, F(2, 57) = 0.02, p = 0.98, and all interactions were non-significant (p > .05). Manual RT by group, load, and cue is presented in . Follow-up correlations demonstrated that coding test performance was strongly related to overall Fusion manual RT (r = −0.33, p < 0.001).
Figure 4. Manual RT on the Fusion n-Back Test by group, cue type and cognitive load. mTBI = Mild TBI; Mod-Sev TBI = Moderate-Severe TBI. Error bars represent SE. No significant main effect or interaction was demonstrated for group. Significant main effects were present for cue (ηp2 = 0.13, p < .001) and load (ηp2 = 0.17, p < .001); pairwise comparisons not shown.
Discussion
This study evaluated effects of cognitive load and TBI on saccadic and manual performance using a multimodal cognitive task, the Fusion n-Back Test. Consistent with our hypotheses, the chronic TBI groups demonstrated substantial saccadic impairment, particularly under conditions of high cognitive load. In contrast, cognitive load did not significantly impact saccadic performance among uninjured controls. Performance on conventional neuropsychological measures and manual metrics from the Fusion n-Back Test did not differ between those with chronic TBI and uninjured controls. These findings have a number of implications for improved methods of clinical assessment as well as for our understanding of situations or task demands that are most likely to pose problems for those with chronic TBI.
Manual and saccadic responses were obtained concurrently in response to the same Fusion n-Back stimuli; however, consistent with previous findings (Citation19,Citation35), these two response modalities represent different forms of cognitive processing, particularly under the moderate and high cognitive load conditions. Across load conditions, manual response demands escalate from simple target detection (low load/Simple RT) to discriminating green versus blue targets (moderate load/0-back) to comparing the current target colour to the previous target colour held in working memory (high load/1-back). In this manner, manual RT provides a fairly direct measure of the added cognitive demands across load conditions. In contrast, the saccadic response is constant across cognitive load conditions and does not directly depend upon completion of load-specific cognitive operations—the same saccade is always required regardless of the colour of the current or previous target. Therefore, any saccadic slowing resulting from increased cognitive load on this task represents a shortage of available cognitive resources, or potentially another form of interference between cognitive and oculomotor processes.
Speed of response in this study differed according to group, cognitive load condition, cognitive cue and response modality. As expected, for manual responses, participants across all groups demonstrated increased RT as cognitive load demands increased. For saccadic responses, uninjured controls in this study were able to maintain comparable performance across all cognitive load conditions. However, TBI group participants demonstrated saccadic slowing when under high levels of cognitive load. Specifically, both the mild and moderate-severe TBI groups exhibited substantial saccadic slowing associated with the addition of working memory demands (1-back); the saccadic slowing associated with colour discrimination (0-back) relative to simple RT was minimal and non-significant. These findings are sensible in light of the fact that the 0-back colour discrimination relies upon relatively automatic and transient processes that do not necessarily overlap with the time frame in which saccades are executed. In contrast, the 1-back condition requires continuous effort to maintain information in working memory, producing cognitive interference during the preparation and execution of saccadic responses.
This pattern of results is consistent with previous findings suggesting that eye movements provide greater sensitivity to TBI-related impairment under conditions of increased cognitive demand (Citation35,Citation37,Citation63,Citation64). Aside from delivering insights into methods for increasing sensitivity of tools used for assessment of TBI through modulation of cognitive load, these results provide additional evidence for a capacity-constrained model of cognitive processing in TBI. According to such a model, multiple forms of cognitive and motor performance depend upon a more general set of cognitive resources (Citation42,Citation43). Despite reduced cognitive resources, functioning of those with chronic TBI may be intact in many situations due to surplus processing capacity when overall cognitive load is low. However, as shown by previous studies, performance is likely to suffer when cumulative task demands exceed the amount of available cognitive resources (Citation65,Citation66). In the Fusion n-Back Test used in this study, the saccadic response appears to provide an especially valuable means to measure this form of cognitive load interference. A wide range of factors may influence availability of cognitive resources relevant to saccadic performance, including damage to distributed neural systems supporting both working memory and executive control of eye movements (Citation67–Citation69) and individual differences in working memory capacity (Citation70).
The effect of TBI on saccadic performance also appears to vary depending upon cognitive cues. For example, the mild TBI group was only impaired relative to controls in the presence of a valid (directional) or invalid (misdirectional) cue, and not on uncued trials. This finding could be related to difficulties with spatial and executive processing in TBI, or to a more general increase in cognitive demands associated with processing these cues. In either case, this pattern of results highlights the value of pursuing an approach to assessment that systematically manipulates neurocognitive factors influencing eye movements, as opposed to focusing solely on neuromotor forms of impairment.
Whereas the mild TBI group demonstrated saccadic impairment only in the presence of cognitive cues at high cognitive load, the moderate-severe TBI group showed a broader pattern of impairment relative to healthy controls. At high levels of load, the moderate-severe TBI group was impaired across all cue types, whereas at low and moderate levels of load, the moderate-severe TBI group was significantly impaired only on uncued trials. This pattern may reflect a problem with arousal or sustained attention; whereas participants with moderate-severe TBI may have been ‘caught off guard’ when targets appeared without warning during less demanding (low load) task conditions, the presence of cognitive cues appeared to mitigate this deficit by providing an early warning or by increasing the momentary task demands/stimulation into a range that was more optimal for these individuals. Alternately, consistent with previous research examining saccadic performance after cerebrovascular accident (Citation71), participants with moderate-severe TBI may have experienced a form of disinhibition that accelerated saccadic responses to cues in low-load conditions.
Similar to previous research (Citation8,Citation9,Citation35,Citation72,Citation73), conventional cognitive measures in this study failed to discriminate between participants with and without chronic mild TBI. In the current study, these measures also failed to identify impairment in the moderate-severe TBI group. However, it is important to consider that our participants may be better-recovered, more resilient and generally higher functioning than the typical patient being evaluated for moderate-severe TBI in real-world clinical settings. Individuals with moderate-severe TBI may experience substantial cognitive recovery over time (Citation9), and our average moderate-severe TBI participant was greater than 57 months post-injury. Additionally, our sample was recruited in a non-clinical setting, and although our control and TBI groups did not differ significantly on demographic characteristics, the overall sample had higher-than-average intelligence and level of education, and the moderate-severe TBI group had a greater than five point estimated IQ advantage over the control group.
Despite a potential sampling bias towards higher functioning individuals, both mild and moderate-severe TBI groups demonstrated impairments on saccadic measures. This finding provides a powerful illustration of the relative sensitivity of different approaches to assessment of TBI-related impairment. The relative underperformance of conventional tests may also be related to many factors known to affect performance other than neural injury. Factors such as age, intelligence and education are known to impact many conventional cognitive tests (Citation17–Citation19), whereas saccadic measures appear to be resistant to these confounds (Citation19,Citation22,Citation23). This relative independence from common confounds may facilitate the detection of useful neural ‘signals’ with less interference from measurement ‘noise’. The one conventional cognitive measure that approached significance in these group comparisons was the Coding test. This test exhibits some similarities to the cognitive and oculomotor demands of the Fusion n-Back Test, in that it requires examinees to quickly execute saccades between test stimuli while retaining information in working memory. However, follow-up analyses demonstrated that Coding test performance was related only to manual RT, and not saccadic RT on the Fusion n-Back Test. These results are consistent with the psychometric divergence that has previously been noted between saccadic and manual approaches to assessment of neurocognitive performance (Citation19), highlighting important differences between direct and indirect measurements of oculomotor function for assessment of chronic TBI.
In summary, this study provides additional evidence that chronic TBI is associated with impaired saccadic performance, even among many individuals who perform normally on conventional neuropsychological tests. Our findings demonstrated that participants with mild and moderate-severe TBI were especially vulnerable to saccadic impairment when placed under conditions of high cognitive load. Participants with moderate-severe TBI also had difficulty with saccadic responses to unexpected (uncued) stimuli at low levels of cognitive load. These results provide additional support for the use of eye tracking in assessment of TBI—particularly for approaches that evaluate eye movements across multiple cognitive load conditions. Additionally, current findings provide additional insights about potential reductions in cognitive bandwidth associated with TBI. However, a number of study limitations must be considered in interpretation of these results. Similar to many previous studies of chronic TBI, this study relied upon structured interviews and review of medical records to identify and characterize injuries retrospectively, without the benefit of standardized evaluations during the acute phase of injury or direct measurements of neural injury. Additionally, sample size in this study was modest, limiting statistical power to detect small effects. Larger scale, prospective studies and additional assessment modalities (such as pupillometry, electroencephalography and magnetic resonance imaging) are needed to replicate and extend current findings.
Declaration of Interests
Support for this research was provided by Congressionally Directed Medical Research Program (CDMRP) Award #W81XWH-13-1-0095 and institutional support from the Defense and Veterans Brain Injury Center (DVBIC) and the Uniformed Services University of the Health Sciences (USUHS). The authors report no conflicts of interest. The technology described in this manuscript is included in US Patent Application No. 61/779,801, US Patent Application No. 14/773,987, European Patent Application No. 14780396.9, and International Patent Application No. PCT/US2014/022468, with rights assigned to the Uniformed Services University of the Health Sciences. Dr. Ettenhofer is listed as an inventor on these patent applications. The views and opinions presented in this manuscript are those of the authors and do not necessarily represent the positions of USUHS, the Department of Defense, the Department of the Navy, or the US government.
Acknowledgments
We are greatly appreciative of the assistance that Evelyn Cordero, Ashley Safford, Jessica Kegel and other members of our staff provided in conducting the research on which this manuscript was based. We also wish to thank the many research participants who volunteered for this study, without whom this work would not have been possible.
Additional information
Funding
References
- McMahon PJ, Hricik A, Yue JK, Puccio AM, Inoue T, Lingsma HF, Beers SR, Gordon WA, Valadka AB, Manley GT. Symptomatology and functional outcome in mild traumatic brain injury: results from the prospective TRACK-TBI study. J Neurotrauma. 2014;31(1):26–33. doi:10.1089/neu.2013.2984.
- Vanderploeg RD, Belanger HG, Curtiss G. Mild traumatic brain injury and posttraumatic stress disorder and their associations with health symptoms. Arch Phys Med Rehabil. 2009;90(7):1084–93. doi:10.1016/j.apmr.2009.01.023.
- Alexander MP. Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology. 1995;45(7):1253–60.
- Binder LM, Rohling ML, Larrabee GJ. A review of mild head trauma. Part I: meta-analytic review of neuropsychological studies. J Clin Exp Neuropsychol. 1997;19(3):421–31. doi:10.1080/01688639708403870.
- Wong JL, Regennitter RP, Barrios F. Base rate and simulated symptoms of mild head injury among normals. Arch Clinical Neuropsychol. 1994;9(5):411–25. doi:10.1093/arclin/9.5.411.
- Boyle E, Cancelliere C, Hartvigsen J, Carroll LJ, Holm LW, Cassidy JD. Systematic review of prognosis after mild traumatic brain injury in the military: results of the International Collaboration on mild traumatic brain injury prognosis. Arch Phys Med Rehabil. 2014;95(3):S230–S7. doi:10.1016/j.apmr.2013.08.297.
- Belanger HG, Spiegel E, Vanderploeg RD. Neuropsychological performance following a history of multiple self-reported concussions: a meta-analysis. J Int Neuropsychol Soc. 2010;16(2):262–67. doi:10.1017/S1355617709991287.
- Rohling ML, Binder LM, Demakis GJ, Larrabee GJ, Ploetz DM, Langhinrichsen-Rohling J. A meta-analysis of neuropsychological outcome after mild traumatic brain injury: re-analyses and reconsiderations of Binder et al. (1997), Frencham et al. (2005), and Pertab et al. (2009). Clin Neuropsychol. 2011;25(4):608–23. doi:10.1080/13854046.2011.565076.
- Schretlen DJ, Shapiro AM. A quantitative review of the effects of traumatic brain injury on cognitive functioning. Int Rev Psychiatry. 2003;15(4):341–49. doi:10.1080/09540260310001606728.
- Bigler ED, Maxwell WL. Neuropathology of mild traumatic brain injury: relationship to neuroimaging findings. Brain Imaging Behav. 2012;6(2):108–36. doi:10.1007/s11682-011-9145-0.
- Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol. 2013;246:35–43. doi:10.1016/j.expneurol.2012.01.013.
- Kwok FY, Lee TM, Leung CH, Poon WS. Changes of cognitive functioning following mild traumatic brain injury over a 3-month period. Brain Inj. 2008;22(10):740–51. doi:10.1080/02699050802336989.
- Binder LM. Persisting symptoms after mild head injury: a review of the postconcussive syndrome. J Clin Exp Neuropsychol. 1986;8(4):323–46. doi:10.1080/01688638608401325.
- Kinsella G, Murtagh D, Landry A, Homfray K, Hammond M, O’beirne L, Dwyer L, Lamont M, Ponsford J. Everyday memory following traumatic brain injury. Brain Inj. 1996;10(7):499–508.
- Vanderploeg RD, Crowell TA, Curtiss G. Verbal learning and memory deficits in traumatic brain injury: encoding, consolidation, and retrieval. J Clin Exp Neuropsychol. 2001;23(2):185–95. doi:10.1076/jcen.23.2.185.1210.
- Vanderploeg RD, Curtiss G, Belanger HG. Long-term neuropsychological outcomes following mild traumatic brain injury. J Int Neuropsychol Soc. 2005;11(3):228–36. doi:10.1017/S1355617705050289.
- Deary IJ, Der G, Ford G. Reaction times and intelligence differences: A population-based cohort study. Intell. 2001;29(5):389–99. doi:10.1016/S0160-2896(01)00062-9.
- Sheppard LD, Vernon PA. Intelligence and speed of information-processing: A review of 50 years of research. Pers Individ Dif. 2008;44(3):535–51. doi:10.1016/j.paid.2007.09.015.
- Ettenhofer ML, Hershaw JN, Barry DM. Multimodal assessment of visual attention using the Bethesda Eye & Attention Measure (BEAM). J Clin Exp Neuropsychol. 2016;38(1):96–110. doi:10.1080/13803395.2015.1089978.
- Binder LM, Iverson GL, Brooks BL. To err is human:“abnormal” neuropsychological scores and variability are common in healthy adults. Arch Clinl Neuropsychol. 2009;24(1):31–46. doi:10.1093/arclin/acn001.
- Schretlen DJ, Testa SM, Winicki JM, Pearlson GD, Gordon B. Frequency and bases of abnormal performance by healthy adults on neuropsychological testing. J Int Neuropsychol Soc. 2008;14(3):436–45. doi:10.1017/S1355617708080387.
- Heitger MH, Jones RD, Macleod AD, Snell DL, Frampton CM, Anderson TJ. Impaired eye movements in post-concussion syndrome indicate suboptimal brain function beyond the influence of depression, malingering or intellectual ability. Brain. 2009;132(Pt 10):2850–70. doi:10.1093/brain/awp181.
- Klein C, Rauh R, Biscaldi M. Cognitive correlates of anti-saccade task performance. Exp Brain Res. 2010;203(4):759–64. doi:10.1007/s00221-010-2276-5.
- Heitger MH, Anderson TJ, Jones RD, Dalrymple-Alford JC, Frampton CM, Ardagh MW. Eye movement and visuomotor arm movement deficits following mild closed head injury. Brain. 2004;127(Pt 3):575–90. doi:10.1093/brain/awh066.
- Heitger MH, Jones RD, Anderson TJ. A new approach to predicting postconcussion syndrome after mild traumatic brain injury based upon eye movement function. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:3570–73. doi:10.1109/IEMBS.2008.4649977.
- Heitger MH, Jones RD, Dalrymple-Alford JC, Frampton CM, Ardagh MW, Anderson TJ. Motor deficits and recovery during the first year following mild closed head injury. Brain Inj. 2006;20(8):807–24. doi:10.1080/02699050600676354.
- Heitger MH, Jones RD, Dalrymple-Alford JC, Frampton CM, Ardagh MW, Anderson TJ. Mild head injury–a close relationship between motor function at 1 week post-injury and overall recovery at 3 and 6 months. J Neurol Sci. 2007;253(1–2):34–47. doi:10.1016/j.jns.2006.11.007.
- Heitger MH, Macaskill MR, Jones RD, Anderson TJ. The impact of mild closed head injury on involuntary saccadic adaptation: evidence for the preservation of implicit motor learning. Brain Inj. 2005;19(2):109–17.
- Suh M, Basu S, Kolster R, Sarkar R, McCandliss B, Ghajar J. Increased oculomotor deficits during target blanking as an indicator of mild traumatic brain injury. Neurosci Lett. 2006;410(3):203–07. doi:10.1016/j.neulet.2006.10.001.
- Suh M, Kolster R, Sarkar R, McCandliss B, Ghajar J. Deficits in predictive smooth pursuit after mild traumatic brain injury. Neurosci Lett. 2006;401(1–2):108–13. doi:10.1016/j.neulet.2006.02.074.
- Contreras R, Kolster R, Voss HU, Ghajar J, Suh M, Bahar S. Eye-Target Synchronization in mild traumatic brain-injured patients. J Biol Phys. 2008;34(3–4):381–92. doi:10.1007/s10867-008-9092-1.
- Rizzo JR, Hudson TE, Dai W, Birkemeier J, Pasculli RM, Selesnick I, Balcer LJ, Galetta SL, Rucker JC. Rapid number naming in chronic concussion: eye movements in the King–devick test. Ann Clin Transl Neurol. 2016;3(10):801–11. doi:10.1002/acn3.345.
- Caplan B, Bogner J, Brenner L, Cifu DX, Wares JR, Hoke KW, Wetzel PA, Gitchel G, Carne W. Differential eye movements in mild traumatic brain injury versus normal controls. J Head Trauma Rehabil. 2015;30(1):21–28. doi:10.1097/HTR.0000000000000036.
- Samadani U, Ritlop R, Reyes M, Nehrbass E, Li M, Lamm E, Schneider J, Shimunov D, Sava M, Kolecki R. Eye tracking detects disconjugate eye movements associated with structural traumatic brain injury and concussion. J Neurotrauma. 2015;32(8):548–56. doi:10.1089/neu.2014.3687.
- Ettenhofer ML, Barry DM. Saccadic impairment associated with remote history of mild traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2016;28(3):223–31. doi:10.1176/appi.neuropsych.15100243.
- Kraus MF, Little DM, Wojtowicz SM, Sweeney JA. Procedural learning impairments identified via predictive saccades in chronic traumatic brain injury. Cogn Behav Neurol. 2010;23(4):210–17. doi:10.1097/WNN.0b013e3181cefe2e.
- Maruta J, Suh M, Niogi SN, Mukherjee P, Ghajar J. Visual tracking synchronization as a metric for concussion screening. J Head Trauma Rehabil. 2010;25(4):293–305. doi:10.1097/HTR.0b013e3181e67936.
- Kraus MF, Little DM, Donnell AJ, Reilly JL, Simonian N, Sweeney JA. Oculomotor function in chronic traumatic brain injury. Cogn Behav Neurol. 2007;20(3):170–78. doi:10.1097/WNN.0b013e318142badb.
- Barker F, Cuiuffreda K, Jacobs J, Kardon R, Scheiman M, Schuchard R, Walsh D Traumatic brain injury detection using oculomotor and eye movement tracking: A Technical working group critical review. U.S. Army Medical Research & Materiel Command; 2013.
- De Haan B, Morgan PS, Rorden C. Covert orienting of attention and overt eye movements activate identical brain regions. Brain Res. 2008;1204:102–11. doi:10.1016/j.brainres.2008.01.105.
- Rizzolatti G, Riggio L, Dascola I, Umiltá C. Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia. 1987;25(1):31–40.
- Kahneman D. Attention and Effort. 1973 ed. Englewood Cliffs, New Jersey: Prentice-Hall Inc.; 1973. p. 253.
- Wickens CD. Processing resources in attention. In: Parasuraman R, Davies DR, editors. Varieties of Attention. New York, NY: Academic Press; 1984. p. 63–102.
- McDowell S, Whyte J., D’Esposito M. Working memory impairments in traumatic brain injury: evidence from a dual-task paradigm. Neuropsychologia. 1997;35(10):1341–53. doi: 10.1016/S0028-3932(97)00082-1.
- Cicerone KD. Attention deficits and dual task demands after mild traumatic brain injury. Brain Inj. 1996;10(2):79–90. doi:10.1080/026990596124566.
- Leclercq M, Couillet J, Azouvi P, Marlier N, Martin Y, Strypstein E, Rousseaux M. Dual task performance after severe diffuse traumatic brain injury or vascular prefrontal damage. J Clin Exp Neuropsychol. 2000;22(3):339–50. doi:10.1076/1380-3395(200006)22:3;1-V;FT339.
- Dockree PM, Bellgrove MA, O’Keeffe FM, Moloney P, Aimola L, Carton S, Robertson IH. Sustained attention in traumatic brain injury (TBI) and healthy controls: enhanced sensitivity with dual-task load. Exp Brain Res. 2006;168(1–2):218–29. doi:10.1007/s00221-005-0079-x.
- Azouvi P, Couillet J, Leclercq M, Martin Y, Asloun S, Rousseaux M. Divided attention and mental effort after severe traumatic brain injury. Neuropsychologia. 2004;42(9):1260–68. doi:10.1016/j.neuropsychologia.2004.01.001.
- Bogner J, Corrigan JD. Reliability and predictive validity of the Ohio State University TBI identification method with prisoners. J Head Trauma Rehabil. 2009;24(4):279–91. doi:10.1097/HTR.0b013e3181a66356.
- Corrigan JD, Bogner J. Initial reliability and validity of the Ohio State University TBI Identification Method. J Head Trauma Rehabil. 2007;22(6):318–29. doi:10.1097/01.HTR.0000300227.67748.77.
- Holdnack JA. Wechsler Test of Adult Reading: manual. San Antonio, TX: Pearson, Inc.; 2001.
- Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol. 1955;19(5):393–94. doi:10.1037/h0044509.
- Benedict RH, Schretlen D, Groninger L, Dobraski M, Shpritz B. Revision of the brief visuospatial memory test: studies of normal performance, reliability, and validity. Psychol Assess. 1996;8(2):145–53. doi:10.1037/1040-3590.8.2.145.
- Wechsler D. Wechsler adult intelligence scale - Fourth Edition: administration and scoring manual. San Antonio, TX: Pearson, Inc.; 2008.
- Wechsler D. WAIS-IV: technical and interpretive manual. San Antonio, TX: Pearson, Inc.; 2008.
- Larrabee GJ. Detection of malingering using atypical performance patterns on standard neuropsychological tests. Clin Neuropsychol: 2003; Aug 1;17(3):410–25.
- Schutte C, Axelrod BN. Use of embedded cognitive symptom validity measures in mild traumatic brain injury cases. In: Carone DA, Bush SS, editors. Mild traumatic brain injury: symptom validity assessment and malingering. New York, NY: Springer Publishing Company; 2013. p. 159–81.
- Wilson JL, Pettigrew LE, Teasdale GM. Structured interviews for the glasgow outcome scale and the extended glasgow outcome scale: guidelines for their use. J Neurotrauma. 1998;15(8):573–85. doi:10.1089/neu.1998.15.573.
- Cicerone KD, Kalmar K. Persistent postconcussion syndrome: the structure of subjective complaints after mild traumatic brain injury. J Head Trauma Rehabil. 1995;10(3):1–17. doi:10.1097/00001199-199510030-00002.
- Safford A, Kegel J, Hershaw J, Girard D, Ettenhofer M, editors. Eye-Tracking technology for estimation of cognitive load after traumatic brain injury. International Conference on Augmented Cognition; 2015: Springer.
- Kirchner WK. Age differences in short-term retention of rapidly changing information. J Exp Psychol. 1958;55(4):352. doi:10.1037/h0043688.
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14(3):340–47. doi:10.1162/089892902317361886.
- Diwakar M, Harrington DL, Maruta J, Ghajar J, El-Gabalawy F, Muzzatti L, Corbetta M, Huang M-X, Lee RR. Filling in the gaps: anticipatory control of eye movements in chronic mild traumatic brain injury. Neuroimage Clin. 2015;8:210–23. doi:10.1016/j.nicl.2015.04.011.
- Contreras R, Ghajar J, Bahar S, Suh M. Effect of cognitive load on eye-target synchronization during smooth pursuit eye movement. Brain Res. 2011;1398:55–63. doi:10.1016/j.brainres.2011.05.004.
- Mitchell JP, Macrae CN, Gilchrist ID. Working memory and the suppression of reflexive saccades. J Cogn Neurosci. 2002;14(1):95–103. doi:10.1162/089892902317205357.
- Stuyven E, van der Goten K, Vandierendonck A, Claeys K, Crevits L. The effect of cognitive load on saccadic eye movements. Acta Psychol. 2000;104(1):69–85. doi:10.1016/S0001-6918(99)00054-2.
- C, Milea DMuri RM. Eye movement control by the cerebral cortex. Curr Opin Neurol. 2004;17(1):17–25. doi: 10.1097/01.wco.0000113942.12823.e0.
- Pierrot-Deseilligny C, Muri RM, Nyffeler T, Milea D. The role of the human dorsolateral prefrontal cortex in ocular motor behavior. Ann N Y Acad Sci. 2005;1039:239–51. doi:10.1196/annals.1325.023.
- Osaka M, Osaka N. Neural bases of focusing attention in working memory: an fMRI study based on indiviudal differences. In: Osaka N, Logie RH, D’Esposito M, editors. Cognitive neuroscience of working memory. New York, NY: Oxford University Press; 2007. p. 99–117.
- Unsworth N, Schrock JC, Engle RW. Working memory capacity and the antisaccade task: individual differences in voluntary saccade control. J Exp Psychol Learn Mem Cogn. 2004;30(6):1302–21. doi:10.1037/0278-7393.30.6.1302.
- Rizzo J-R, Hudson TE, Abdou A, Lui YW, Rucker JC, Raghavan P, Landy MS. Disrupted saccade control in chronic cerebral injury: upper motor neuron-like disinhibition in the ocular motor system. Front Neurol. 2017;8:12. doi:10.3389/fneur.2017.00012.
- Ettenhofer ML, Abeles N. The significance of mild traumatic brain injury to cognition and self-reported symptoms in long-term recovery from injury. J Clin Exp Neuropsychol. 2009;31(3):363–72. doi:10.1080/13803390802175270.
- Dikmen SS, Corrigan JD, Levin HS, Machamer J, Stiers W, Weisskopf MG. Cognitive outcome following traumatic brain injury. J Head Trauma Rehabil. 2009;24(6):430–38. doi:10.1097/HTR.0b013e3181c133e9.