ABSTRACT
Background
Patients who suffer traumatic brain injury (TBI) often experience a constellation of physical, cognitive, and emotional/behavioral symptoms called “post-concussion symptoms” and subsequent long-term disability. This study aimed to investigate the incidence of persistent post-concussion symptoms and possible predictors of long-term disability focusing on demographic, injury, and psychological factors. It was hoped to identify groups at high risk.
Methods
A prospective cohort of 1322 individuals admitted with TBI were assessed in a specialist neurorehabilitation clinic at 10 weeks and 1-year post injury between August 2011 and July 2015. The outcome (post-concussion symptoms) was measured using the Rivermead Post-concussion Questionnaire (RPQ) at 1-year post injury.
Results
At 1 yr, 1131 individuals were identified (>90% follow-up). Over 20% exhibited moderate or severe symptom levels on RPQ. A linear regression model showed that previous psychiatric history, lower Glasgow Coma Scale (GCS), severe CT abnormalities, injury caused by assault, pre-injury unemployment, and inability to return to work at 6 weeks post-injury were associated with worse symptoms at 1 yr. The adjusted R2 of the model was 25.1%.
Conclusion
These findings confirm the high incidence of post-concussion symptoms at 1 yr and identify certain associated features that increase risk. This may allow targeting of certain groups, e.g., return to work or victims of assault.
Introduction
The incidence of Traumatic Brain Injury varies widely by age and between countries with an annual incidence of Traumatic Brain Injury ranging between 235 and 435 per 100 000 (Citation1,Citation2). It is a major cause of disability, with 1.4 million patients admitted to the Emergency Department annually and the most common deaths of individuals under 40 years of age in England and Wales (Citation3). After a mild Traumatic Brain Injury, 50% of patients will experience a constellation of self-reported physical, cognitive, and emotional/behavioral symptoms within 7–10 days that will usually dissipate within 3 months (Citation4,Citation5). These “Post-concussion symptoms” include headaches, dizziness, fatigue, irritability, reduced concentration, sleep disturbance, memory dysfunction, sensitivity to noise or light, double or blurred vision, nausea, anxiety, and depression (Citation6). While such symptoms resolve in the majority of individuals who experience these symptoms (Citation7), 8–15% termed the “miserable minority” (Citation8), persist with these symptoms at 1-year (Citation4,Citation9,Citation10). These persistent symptoms are often intrusive, unresponsive to treatment, predispose to lifetime disability (Citation8), and compromise neuropsychological function (Citation11). Quality of life is directly proportional to symptom severity (Citation4). The term post-concussion syndrome as defined by the International Classification of Diseases (ICD-10) (Citation12) and Diagnostic and Statistical Manual (DSM-V) (Citation13) has been considered controversial because of limitations in diagnostic reliability (Citation14,Citation15)
The Rivermead Post-concussion Questionnaire (RPQ) is a validated tool for assessing the severity of post-concussion symptomatology (Citation11). It was developed to identify symptoms that are worse than the pre-morbid state by subjectively assessing the ability of the patient to compare the symptom level before the injury (Citation16)
To identify predictors of severity using the RPQ, previous studies have used acute clinical signs and symptoms, serum s100b, medical history, and medications as early predictors (Citation17–19). Other studies, albeit using small sample sizes, used a combination of demographics, injury severity, and psychological factors as predictors (Citation4,Citation5,Citation20,Citation21). However, there is little agreement on risk factors in the literature.
Identifying patients on a trajectory for poor recovery post-injury may allow clinicians to direct interventions at those with higher risk factors (Citation22). We reasoned that certain demographic, injury, radiological, or psychological features might contribute to the onset and persistence of these symptoms in the long term. Therefore, in this study, while the primary aim was to document the scale of symptoms and their change over time, we also aimed to investigate the influence of other factors on the severity of post-concussion symptoms in a large prospective, consecutively recruited, mixed cohort 1-year post Traumatic Brain Injury. There was no specific risk factor to be investigated but rather a range of population or injury factors were documented at a brain injury clinic requiring minimal patient inconvenience. The size of the cohort, its representative nature for the TBI population, and the proven ability of the clinic to facilitate high face-to-face follow-up rates presented an excellent opportunity to try and identify the scale of post-concussion symptoms and the associated features. It was hoped that such a study would be of interest to any clinician treating TBI.
Materials and methods
Consecutive admissions with TBI between August 2011 and July 2015, to a large teaching hospital, were recruited as the Sheffield Brain Injury after Trauma (SHEFBIT) study. All admissions spent at least 1 day as in-patient and underwent CT imaging.
Patients were reviewed within 24 hr by the Acute Brain Injury Team (ABIT) that comprises a Brain injury specialist doctor, a Clinical nurse specialist, and a Brain injury social worker.
A follow-up clinic was arranged at 8–10 weeks after injury in a neurorehabilitation brain injury clinic. The patients were encouraged to attend these clinics via letters, phone messages, and phone calls from clinic staff. During each clinic visit, informed consent was obtained from each patient, a detailed history taken, and the Rivermead post-concussion questionnaire was filled in by the patients and used as outcome measures of TBI recovery. The inclusion criteria for the study were a confirmed diagnosis of traumatic brain injury using the Common Data Elements, registration with a local GP to ensure appropriate follow-up, age >16 yrs, and CT scanning within 24 hr. Patients who had previously received in-patient care for TBI were excluded from the study.
Another clinic was scheduled a year after injury and the process was repeated. All assessments were done by the principal investigator (RS).
The primary outcome measure was the Rivermead Post-Concussion Questionnaire (RPQ). This is a well-validated and established questionnaire used in the clinical setting with good test-retest and inter-rater reliability (Citation11). The RPQ contains 16 common head injury symptoms, and each symptom is rated on a Likert scale of 0 (never experienced the symptom), 1 meaning “not much of a problem compared to pre-injury” to 4 (severe symptom) giving a total score of 64 (Citation11). Therefore, the higher the RPQ scores, the more the burden of symptoms experienced by the patient (Citation23,Citation24). According to cutoffs used in previous studies (Potter et al.), scores of 0–12, 13–24, 25–32, and >33 were classed as minimal, mild, moderate, and severe symptoms, respectively (Citation25).
CT scans were classified using the “overall appearance classification” (Citation26). This system categorizes scans as normal, mild (lesions limited to one lobe), moderate (lesions in adjacent lobes), and severe (lesions diffusely distributed).
The Socioeconomic Status of individuals was classified using the National Statistics Socio-economic Classification (NS-SEC) (Citation27). This has nine categories ranging from “Professional” to “Never worked.” The term “past psychiatric history” in this study was defined as individuals who had a diagnosis of a psychiatric condition or who were taking psychoactive medication at the time of injury.
The Trauma Audit and Research Network (TARN) was used to classify the mechanism of Traumatic Brain Injury: this included falls, assault, road traffic accidents, sports, and “others” (which includes falls over 2 m and occupational injuries) (Citation28).
The severity of traumatic brain injury was classified by the Glasgow Coma Scale (GCS) (Citation12) in the ED and used in analysis as a continuous score from 3 to 15. For descriptive purposes, TBI severity was classed as mild (GCS13-15), moderate (GCS9-12), and severe (GCS <9).
An initial univariate analysis was applied to describe the data and its distribution and to identify associations between features. The Mann–Whitney test was used to compare two groups against the outcome, while the Kruskal–Wallis was used for three groups or more. Positive associations were confirmed with a more detailed multivariate analysis; this was performed with a linear regression with all independent variables entered simultaneously to find an association between the predictors and the outcome measure of RPQ score and how much each predictor contributed to the outcome. For independent variables with over two categories, dummy variables were created (etiology, CT scan, and socioeconomic class). All statistical assumptions were met. This study was completed with the ethical approval of both the Sheffield Hospital Trust and the University of Sheffield Ethics Committee (ref STH16208).
Results
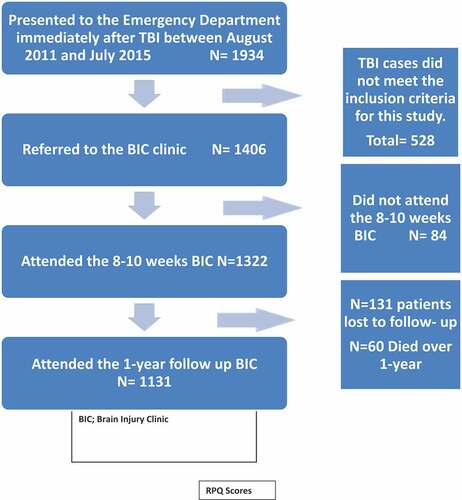
Between August 2011 and July 2015, 1934 individuals with TBI were admitted via the Emergency Department. Of these, 319 patients did not meet the criteria for the diagnosis of TBI, and 209 patients did not meet the other inclusion criteria previously stated, making a total of 528 patients excluded from the study. A total of 1406 were referred to the Brain Injury Clinics (BIC) of whom 84 patients failed to attend, leaving a total of 1322 patients who attended the 8–10 weeks BIC.
By the time of 1-year follow-up, 1191 individuals were traced of whom 60 (4.5%) had died and 1131 attended the clinic. Despite repeated attempts to contact them, 131 (10%) individuals were lost to follow-up; this represents a follow-up rate of 90%. (.)
The study cohort is described in ; out of this population, 909 (69%) were male, and 1226 (93%) of white ethnicity. The mean age at presentation was 46.9 years (SD20.1), while median age was 45.5 years. Most of the participants were employed or in full-time education (1137,86%) and were mostly engaged in semi-routine jobs (296,22.4%) and lower supervisory roles (229,17.3%). Most participants had no previous psychiatric history (1067,81%), no preexisting medical co-morbidity (953,72%), and were not intoxicated at the time of injury (997,75%).
Table 1. Demographics of study population and univariate analysis (Mann–Whitney) with RPQ at 1 yr.
Table 2. Demographics of study population and univariate analysis (Kruskal–Wallis) with RPQ at 1 yr.
The most common etiologies of injury were falls (369,33%) and road traffic collisions (323,28%) while assault constituted 214 (19%).
In terms of TBI severity on presentation to the Emergency Department, the majority had a mild TBI (571,51%), followed by moderate injury (375,33%) and severe injury (182,16%). The CT classification identified that 40% had a normal CT scan, and 19% only had one lobe involvement, a fact that reflects the relative proportions of TBI severity that are seen and admitted in ED. Individuals with diffuse lesions on CT scans were much more likely to present with severe brain injury compared to other CT lesions (p < 0.0001).
The RPQ scores at 8 weeks and 1 yr are shown in . While an almost full range of scores (0–32) were found, there was a distribution skewed slightly to the left with a tendency for lower scores to be represented. The median score dropped significantly over a year. The number of moderate and severe cases fell from 32% to 19.1%. Nevertheless, this still represents a high level of persisting symptoms in a large number of individuals.
Table 3. RPQ (Rivermead Post-concussion Questionnaire) scores at 6 weeks and 1 year.
Univariate analysis was performed on each of the demographic and injury variables to investigate the effect as predictors of worsening symptoms. Non-parametric tests (chi-square, Mann–Whitney U, Kruskal–Wallis) were used because the distribution of the Rivermead post-concussion score (RPQ) is slightly left-skewed. The Benjamin-Hochberg correction was used to control for the presence of multiple tests.
The following variables showed a significant difference between the groups: etiology (p < 0.001), failure to return to work at 6 weeks post-injury (p < 0.0001), CT lesion (p < 0.001), and socioeconomic status (p = 0.010) ().
Variables of gender (p = 0.794), ethnicity (p = 0.259), co-morbidity (0.071), and home support (p = 0.865) were not significant ().
A multivariate analysis was then performed to identify the independent predictors; all independent variables were entered into the model with RPQ as the dependent outcome ().
Table 4. Multivariate analysis of the Rivermead post-concussion questionnaire using multiple regression.
Factors that were associated with a statistically significant increment in the RPQ score were as follows: previous psychiatric history (p < 0.0001), lower GCS (p < 0.0001), inability to return to employment at 6 weeks after TBI (p < 0.0001), pre-injury unemployment (p < 0.0001), absence of medical comorbidity (p = 0.007). CT scan with moderate or diffuse abnormalities was associated with increased symptoms compared to the reference point of a normal scan. Those who sustained TBI after a fall (p = 0.005) or participation in sports (p = 0.010) had lower symptoms compared to the baseline (assault) ().
Although socioeconomic status, alcohol intoxication, and etiology were statistically significant in the univariate analysis, they were not confirmed in the multivariate model. CT lesions restricted to a single lobe, Road Traffic Collisions, and “other” forms of injuries were not significant in contributing to the model.
Gender, Age, and “home support” were also not significant in the model.
The overall model was highly significant (p < 0.0001), and the Nagelkerke R2 was 0.251, suggesting that 25.1% of the variance in the linear regression was explained by the combined variables analyzed in this study. The data met the assumptions of homogeneity and linearity and a histogram of residuals approximated to a normal distribution.
Discussion
This study aimed to investigate the level of post-concussion symptoms at 1-year post-injury and to identify any possible associations with demographic and injury features in a large prospective, consecutively recruited, mixed TBI severity cohort. This group should accurately reflect the population admitted with TBI, and the findings should be of interest to any clinician working in the field of brain injury.
We have confirmed that symptoms decline over time but there is still considerable impairment and disability caused by post-concussion symptoms at 1 year. There are many reasons why symptoms may persist in some individuals other than the severity of injury itself. Environmental stressors after a year may be different to those earlier on. For example, financial concerns, lack of home supports, pressure to return to work or other social responsibilities and legal proceedings may affect an individual’s reported symptoms.
A number of variables such as TBI severity, psychiatric history, etiology, and CT scan severity were associated with persistent symptoms. Our model was highly significant with a variance that is comparable to the TRACK-TBI pilot study with 21% and others (Citation21,Citation29) with similar study outcomes. Other models such as the IMPACT (Citation30), CRASH (Citation31), and UPFRONT (Citation33) report a variance of 20–32% and used GOSE as the long-term outcome of the overall function. While our results compare well in this regard, it is still clear that most of the outcome is still unexplained and that many other unidentified factors must influence the outcome.
Our study showed that lower GCS was associated with increased symptoms at 1-year post-injury. Other studies (Citation34,Citation35) have also found TBI severity to be associated with persistent symptoms. However, negative associations have also been demonstrated (Citation9). A healthy debate in the literature exists around whether symptoms reported by individuals are caused primarily by direct neurological damage or if they are largely psychosocial in origin. A biopsychosocial model is often used to explain the onset and persistence of post-concussion symptoms (Citation22). Although the biological components of this model, including diffuse axonal injury, neuroinflammation, and altered cerebral flow, can explain why the rates of post-concussion symptoms are higher with increasing TBI severity (Citation36,Citation37), it cannot explain why there is a high prevalence of post-concussion-like symptoms in mild injury or even in patients without brain injury (Citation38–40).
Our study also found that past psychiatric history was strongly associated with the persistence of symptoms 1-year post-injury. This is a similar finding to previous studies (Citation41,Citation42). Using the improved biopsychosocial model (Citation22) initially developed by Lishman (Citation43), the psychosocial component of this model is essential in the onset and persistence of these symptoms. This creates the “chicken and egg paradox” as it becomes difficult to establish casualty between premorbid psychiatric status and its ability to predict persistent symptoms because these symptoms might be a reaction to the persistent symptoms. Other possible factors could be resilience (Citation44), high anxiety sensitivity (Citation45), coping mechanisms (Citation46), and a low level of satisfaction with life (Citation47). It is important to note that these symptoms occur in everyday life and can be wrongly attributed to brain injury (Citation48,Citation49).
Inability to return to work at 6 weeks post-injury was associated with persistent symptoms. This is supported by similar studies (Citation46,Citation50) although other studies (Citation51,Citation52) have shown the contrary. We reason that employment provides resources for basic needs, promotes self-sufficiency, self-satisfaction, and independence. It is difficult to accurately predict if a patient will successfully return to work with the return-to-work rate fluctuating between 12% and 70% (Citation53). Reduced self-awareness leading to reduced motivation (Citation54), the severity of injury (Citation55), occupations requiring manual labor, and longer rehabilitation programs (Citation52) affect return to work rates. The role of psychosocial, economic, and cultural factors vary from region to region and is likely to contribute more to the inability to return to work than physiological reasons alone (Citation52). Patients who were initially unemployed are at risk for psychiatric comorbidities, drug use, smoking, and reduced functional outcome post-traumatic brain injury (Citation56). We reason that a complex interaction exists between the biopsychosocial, economic, and cultural factors, and the order in which each contributes differs from individual to individual and culture to culture. However, targeting the return to work may be a strategy to address concussion symptoms; this requires further investigation.
In our study, CT scan of individuals with lesions in adjacent lobes or those with a diffuse distribution of lesions was associated with increased symptoms. Those with lesions restricted to one lobe did not show any difference when compared to those with normal CT scans that was used as baseline. Post-concussion symptoms have been postulated to be partly caused by disrupted frontal lobe connections with other parts of the brain during trauma (Citation57,Citation58). In mild Traumatic Brain Injury, conventional imaging has always lacked the sensitivity to detect these nerve disruptions (Citation59). This subtle white matter axonal injury usually remains undetected with conventional imaging (Citation59). Therefore, lesions detected by conventional imaging are not consistent in accounting for cognitive deficits or recovery in those who sustain mild Traumatic Brain Injury (Citation60,Citation61). A more reliable and sensitive method for detecting these microstructural abnormalities associated with post-concussion symptoms is Diffusion Tensor Imaging (Citation62,Citation63). This may present future research opportunities. Nevertheless, it is clear that the severity of CT scan finding is associated with higher symptom level; to the best of our knowledge, this is the first study to show this relationship using the “overall classification” for CT scans in TBI.
In terms of etiology, assault contributed more to our predictor model compared to fall and road traffic crashes even though it was the 3rd most common cause of brain injury in our sample. The role of the mechanism of injury and the presence of post-concussion symptoms remains controversial, with some studies (Citation32) finding a positive association and others (Citation64) finding the contrary. Patients who experienced assault often report worse symptoms than their counterparts, likely related to the heightened emotional trauma (Citation65). Assault-related TBI is often associated with intrusive thoughts, depression, and attempts to avoid reminders of the injury (Citation66). This might be compounded if litigation is involved.
It is also important to consider the features that showed no association. Sociodemographic factors such as age, gender, ethnicity, professional status, home support, and alcohol intoxication were not associated with persistent symptoms. Although previous studies (Citation10,Citation67) have identified an association between age and gender, others report no association, and we have found no evidence of a relationship with these factors (Citation68).
This study has a number of strengths: it is the largest single-center prospective, mixed TBI severity study that we know of. The sample should represent the typical day-to-day TBI presentation to the ED and should be of relevance to all clinicians working in TBI. Many studies have very select populations, e.g., military or RTC only. While most Traumatic Brain Injury studies suffer a high attrition rate of up to 70% within 6 months (Citation69), the loss in our study was just 10%; this is impressive. To avoid inter-observer variation, a single observer was involved in the specialist clinic. RPQ is a linear measure in contrast to GOSE or dichotomized outcomes used in some studies. It is therefore more sensitive to subtle changes in symptoms of recovery that will be missed by categorical measures.
A few weaknesses should be mentioned including the lack of blinding of the investigator, absence of a control arm in this study with similar demographics, and our inability to account for confounders such as ongoing litigation and personality traits.
It is important to note that a significant number of MTBI patients underestimate the premorbid issues (past problems) and this has been described as the “the good old days” bias. This bias can affect their perception of current problems, leading to reduced quality of life, slow recovery, and return to work (Citation36).
In the future, we would like to look at possible interventions that may reduce the symptoms level or target individuals at higher risk. Individuals with psychiatric history, victims of assault, and those with severe CT changes may benefit from increased support or treatment. Efforts to expedite and support return to work may also benefit individuals from a symptom perspective quite apart from the many other benefits of work/study. It is worth noting that traditional psychotherapeutic interventions as a stand-alone approach may not be an effective intervention. In addition to traditional psychotherapy, a clinician high index of suspicion and establishing a positive therapeutic relationship between the clinician and the patient through rapport-building and patient education might be more beneficial. Future work into exploring various psychiatric disorders such as depression, schizophrenia, anxiety disorders, etc., as well as the nature, duration, and frequency of therapy will significantly contribute more to identifying those at higher risk.
Conclusion
Our findings have confirmed the high incidence of post-concussion symptoms and identified certain associated features that increase the risk of post-concussion symptoms at 1-year post TBI such as TBI severity, moderate or severe CT scan abnormality, past psychiatric history, unemployment before the injury, inability to return to work at 6 weeks post-injury, and injury caused by assault. Apart from adding to the current understanding of Traumatic Brain Injury, this may allow targeting of certain groups, e.g., return to work or victims of assault, or patients with psychiatric conditions. This study also highlights the need for further studies and multidisciplinary collaborative efforts incorporating physical, mental, and social rehabilitation to improve care.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Nguyen R, Fiest KM, McChesney J, Kwon C-S, Jette N, Frolkis AD, Atta C, Mah S, Dhaliwal H, Reid A, et al. The international incidence of traumatic brain injury: a systematic review and meta-analysis. Can J Neurol Sci J. 2016 Nov;43(6):774–85. doi:10.1017/cjn.2016.290.
- Yates PJ, Williams WH, Harris A, Round A, Jenkins R. An epidemiological study of head injuries in a UK population attending an emergency department. J Neurol Neurosurg Psychiatry. 2006 May 1;77(5):699–701. 10.1136/jnnp.2005.081901.
- Lawrence T, Helmy A, Bouamra O, Woodford M, Lecky F, Hutchinson PJ. Traumatic brain injury in England and Wales: prospective audit of epidemiology, complications and standardised mortality. BMJ Open. 2016 Nov 1;6(11):e012197. 10.1136/bmjopen-2016-012197.
- King NS, Kirwilliam S. Permanent post-concussion symptoms after mild head injury. Brain Inj. 2011 May 1;25(5):462–70. 10.3109/02699052.2011.558042.
- Broshek DK, De Marco AP, Freeman JR. A review of post-concussion syndrome and psychological factors associated with concussion. Brain Inj. 2015 Jan 28;29(2):228–37. 10.3109/02699052.2014.974674.
- King N. Mild head injury: neuropathology, sequelae, measurement and recovery. Br J Clin Psychol. 1997;36(2):161–84.doi:10.1111/j.2044-8260.1997.tb01405.x.
- Englander J, Hall K, Stimpson T, Chaffing S. Mild traumatic brain injury in an insured population: subjective complaints and return to employment. Brain Inj. 1992 Jan 1;6(2):161–66. 10.3109/02699059209029654.
- Ll Wood R. Understanding the ‘miserable minority’: a diasthesis-stress paradigm for post-concussional syndrome. Brain Inj. 2004 Nov;18(11):1135–53. doi:10.1080/02699050410001675906.
- Røe C, Sveen U, Alvsåker K, Bautz-Holter E. Post-concussion symptoms after mild traumatic brain injury: influence of demographic factors and injury severity in a 1-year cohort study. Disabil Rehabil. 2009;31(15):1235–43.doi:10.1080/09638280802532720.
- Singh R, Choudhri K, Sinha S, Mason S, Lecky F, Dawson J. Global outcome after traumatic brain injury in a prospective cohort. Clin Neurol Neurosurg. 2019 Nov;186:105526. doi:10.1016/j.clineuro.2019.105526.
- King NS, Crawford S, Wenden FJ, Moss NEG, Wade DT. The Rivermead post concussion symptoms questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995;242(9):587–92.doi:10.1007/BF00868811.
- Bellner J, Jensen SM, Lexell J, Romner B. Diagnostic criteria and the use of ICD-10 codes to define and classify minor head injury. J Neurol Neurosurg Psychiatry. 2003;74(3):351–52.doi:10.1136/jnnp.74.3.351.
- Association AP. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub; 2013.
- Boake C, McCauley SR, Levin HS, Contant CF, Song JX, Brown SA, Goodman HS, Brundage SI, Diaz-Marchan PJ, Merritt SG, et al. Limited agreement between criteria-based diagnoses of postconcussional syndrome. J Neuropsychiatry Clin Neurosci. 2004;16(4):493–99.doi:10.1176/jnp.16.4.493.
- Boake C, McCauley SR, Levin HS, Pedroza C, Contant CF, Song JX, Brown SA, Goodman H, Brundage SI, Diaz-Marchan PJ, et al. Diagnostic criteria for postconcussional syndrome after mild to moderate traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2005;17(3):350–56.doi:10.1176/jnp.17.3.350.
- Gunstad J, Suhr JA. Cognitive factors in postconcussion syndrome symptom report. Arch Clin Neuropsychol. 2004;19(3):391–405.doi:10.1016/S0887-6177(03)00073-8.
- Savola O, Hillbom M. Early predictors of post‐concussion symptoms in patients with mild head injury. Eur J Neurol. 2003;10(2):175–81.doi:10.1046/j.1468-1331.2003.00552.x.
- Dischinger PC, Ryb GE, Kufera JA, Auman KM. Early predictors of postconcussive syndrome in a population of trauma patients with mild traumatic brain injury. J Trauma Acute Care Surg. 2009;66(2):289–97.doi:10.1097/TA.0b013e3181961da2.
- Sigurdardottir S, Andelic N, Roe C, Jerstad T, Schanke A-K. Post-concussion symptoms after traumatic brain injury at 3 and 12 months post-injury: a prospective study. Brain Inj. 2009 Jan 1;23(6):489–97. 10.1080/02699050902926309.
- Morgan CD, Zuckerman SL, Lee YM, King L, Beaird S, Sills AK, Solomon GS, et al. Predictors of postconcussion syndrome after sports-related concussion in young athletes: a matched case-control study. J Neurosurg Pediatr. 2015;15(6):589–98.doi:10.3171/2014.10.PEDS14356.
- Ponsford J, Nguyen S, Downing M, Bosch M, McKenzie JE, Turner S, Chau M, Mortimer D, Gruen R, Knott J, et al. Factors associated with persistent post-concussion symptoms following mild traumatic brain injury in adults. J Rehabil Med. 2019;51(1):32–39. doi:10.2340/16501977-2492.
- Silverberg ND, Iverson GL. Etiology of the post-concussion syndrome: physiogenesis and psychogenesis revisited. NeuroRehabilitation. 2011;29(4):317–29.doi:10.3233/NRE-2011-0708.
- Asselstine J, Kristman VL, Armstrong JJ, Dewan N. The Rivermead Post-Concussion Questionnaire score is associated with disability and self-reported recovery six months after mild traumatic brain injury in older adults. Brain Inj. 2020 Jan 28;34(2):195–202. 10.1080/02699052.2019.1682670.
- Ingebrigtsen T, Waterloo K, Marup-Jensen S, Attner E, Romner B. Quantification of post-concussion symptoms 3 months after minor head injury in 100 consecutive patients. J Neurol. 1998 Aug 1;245(9):609–12. 10.1007/s004150050254.
- Potter S, Leigh E, Wade D, Fleminger S. The Rivermead post-concussion symptoms questionnaire. J Neurol. 2006 Dec;253(12):1603–14. doi:10.1007/s00415-006-0275-z.
- Wardlaw JM. Which CT features help predict outcome after head injury? J Neurol Neurosurg Psychiatry. 2002 Feb 1;72(2):188–92. 10.1136/jnnp.72.2.188.
- Rose D, Pevalin DJ. A researcher’s guide to the national statistics socio-economic classification. Sage; 2002.
- Lecky F, Woodford M, Yates D. Trends in trauma care in England and Wales 1989–97. The Lancet. 2000 May;355(9217):1771–75. doi:10.1016/S0140-6736(00)02264-9.
- Theadom A, Parag V, Dowell T, McPherson K, Starkey N, Barker-Collo S, Jones K, Ameratunga S, Feigin VL, et al. Persistent problems 1 year after mild traumatic brain injury: a longitudinal population study in New Zealand. Br J Gen Pract. 2016 Jan 1;66(642):e16–23. 10.3399/bjgp16X683161.
- Maas AIR, Marmarou A, Murray GD, Teasdale SGM, Steyerberg EW. Prognosis and Clinical Trial Design in Traumatic Brain Injury: the IMPACT Study. J Neurotrauma. 2007 Feb;24(2):232–38. doi:10.1089/neu.2006.0024.
- Collaborators MCT. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. 2008 Feb 21;336(7641):425–29.
- Kim H, Colantonio A, Dawson DR, Bayley MT. Community integration outcomes after traumatic brain injury due to physical assault. Can J Occup Ther. 2013;80(1):49–58.doi:10.1177/0008417412473262.
- van der Naalt J, Timmerman ME, de Koning Me, van der Horn Hj, Scheenen ME, Jacobs B, van der Naalt J, de Koning ME, van der Horn HJ, Hageman G, et al. Early predictors of outcome after mild traumatic brain injury (UPFRONT): an observational cohort study. Lancet Neurol. 2017 Jul 1;16(7):532–40. 10.1016/S1474-4422(17)30117-5.
- Dikmen SS, Machamer JE, Powell JM, Temkin NR. Outcome 3 to 5 years after moderate to severe traumatic brain injury. Arch Phys Med Rehabil. 2003;84(10):1449–57.doi:10.1016/S0003-9993(03)00287-9.
- Engberg AW, Liebach A, Nordenbo A. Centralized rehabilitation after severe traumatic brain injury–a population‐based study. Acta Neurol Scand. 2006;113(3):178–84.doi:10.1111/j.1600-0404.2005.00570.x.
- Iverson GL, Lange RT, Brooks BL, Lynn Ashton Rennison V. 2010. “Good old days” bias following mild traumatic brain injury. Clin Neuropsychol. 24(1):17–37. doi:10.1080/13854040903190797
- Ponsford J, Cameron P, Fitzgerald M, Grant M, Mikocka-Walus A. Long-term outcomes after uncomplicated mild traumatic brain injury: a comparison with trauma controls. J Neurotrauma. 2011;28(6):937–46.doi:10.1089/neu.2010.1516.
- Cassidy JD, Cancelliere C, Carroll LJ, Côté P, Hincapié CA, Holm LW, Hartvigsen J, Donovan J, Nygren-de Boussard C, Kristman VL, et al. Systematic review of self-reported prognosis in adults after mild traumatic brain injury: results of the international collaboration on mild traumatic brain injury prognosis. Arch Phys Med Rehabil. 2014;95(3):S132–51.doi:10.1016/j.apmr.2013.08.299.
- Marshall CM, Vernon H, Leddy JJ, Baldwin BA. The role of the cervical spine in post-concussion syndrome. Phys Sportsmed. 2015;43(3):274–84.doi:10.1080/00913847.2015.1064301.
- Iverson GL, Lange RT. Examination of” postconcussion-like” symptoms in a healthy sample. Appl Neuropsychol. 2003;10(3):137–44.doi:10.1207/S15324826AN1003_02.
- Silverberg ND, Gardner AJ, Brubacher JR, Panenka WJ, Li JJ, Iverson GL. Systematic review of multivariable prognostic models for mild traumatic brain injury. J Neurotrauma. 2015 Apr 15;32(8):517–26. 10.1089/neu.2014.3600.
- Cnossen MC, Winkler EA, Yue JK, Okonkwo DO, Valadka AB, Steyerberg EW, et al. Development of a prediction model for post-concussive symptoms following mild traumatic brain injury: a TRACK-TBI pilot study. J Neurotrauma. 2017;34(16):2396–409.doi:10.1089/neu.2016.4819.
- Lishman WA. Physiogenesis and psychogenesis in the ‘post-concussional syndrome. Br J Psychiatry. 1988 Oct;153(4):460–69. doi:10.1192/bjp.153.4.460.
- Sullivan KA, Kempe CB, Edmed SL, Bonanno GA. Resilience and other possible outcomes after mild traumatic brain injury: a systematic review. Neuropsychol Rev. 2016;26(2):173–85.doi:10.1007/s11065-016-9317-1.
- Wood RL, O’Hagan G, Williams C, McCabe M, Chadwick N. Anxiety sensitivity and alexithymia as mediators of postconcussion syndrome following mild traumatic brain injury. J Head Trauma Rehabil. 2014;29(1):E9–17.doi:10.1097/HTR.0b013e31827eabba.
- Scheenen ME, Spikman JM, de Koning ME, van der Horn HJ, Roks G, Hageman G, et al. Patients “at risk” of suffering from persistent complaints after mild traumatic brain injury: the role of coping, mood disorders, and post-traumatic stress. J Neurotrauma. 2017;34(1):31–37.doi:10.1089/neu.2015.4381.
- Stålnacke B-M. Community integration, social support and life satisfaction in relation to symptoms 3 years after mild traumatic brain injury. Brain Inj. 2007;21(9):933–42.doi:10.1080/02699050701553189.
- Evans RW. Persistent post‐traumatic headache, postconcussion syndrome, and whiplash injuries: the evidence for a non‐traumatic basis with an historical review. Headache J Head Face Pain. 2010;50(4):716–24.doi:10.1111/j.1526-4610.2010.01645.x.
- Hou R, Moss-Morris R, Peveler R, Mogg K, Bradley BP, Belli A. When a minor head injury results in enduring symptoms: a prospective investigation of risk factors for postconcussional syndrome after mild traumatic brain injury. J Neurol Neurosurg Psychiatry. 2012;83(2):217–23.doi:10.1136/jnnp-2011-300767.
- Besnard J, Richard P, Banville F, Nolin P, Aubin G, Le Gall D, et al. Virtual reality and neuropsychological assessment: the reliability of a virtual kitchen to assess daily-life activities in victims of traumatic brain injury. Appl Neuropsychol Adult. 2016;23(3):223–35.doi:10.1080/23279095.2015.1048514.
- Olver JH, Ponsford JL, Curran CA. Outcome following traumatic brain injury: a comparison between 2 and 5 years after injury. Brain Inj. 1996;10(11):841–48.doi:10.1080/026990596123945.
- Andelic N, Sigurdardottir S, Schanke A-K, Sandvik L, Sveen U, Roe C. Disability, physical health and mental health 1 year after traumatic brain injury. Disabil Rehabil. 2010;32(13):1122–31.doi:10.3109/09638280903410722.
- Shames J, Treger I, Ring H, Giaquinto S. Return to work following traumatic brain injury: trends and challenges. Disabil Rehabil. 2007;29(17):1387–95.doi:10.1080/09638280701315011.
- Ben-Yishay Y, Lakin P. Structured group treatment for brain-injury survivors. In: Neuropsychological treatment after brain injury, Springer, 1989:271–95.
- Cifu DX, Keyser-Marcus L, Lopez E, Wehman P, Kreutzer JS, Englander J, et al. Acute predictors of successful return to work 1 year after traumatic brain injury: a multicenter analysis. Arch Phys Med Rehabil. 1997;78(2):125–31.doi:10.1016/S0003-9993(97)90252-5.
- Yue JK, Rick JW, Morrissey MR, Taylor SR, Deng H, Suen CG, Vassar MJ, Cnossen MC, Lingsma HF, Yuh EL, et al. Preinjury employment status as a risk factor for symptomatology and disability in mild traumatic brain injury: a TRACK-TBI analysis. NeuroRehabilitation. 2018 Jan 1;43(2):169–82. 10.3233/NRE-172375.
- Levin H, Kraus MF. The frontal lobes and traumatic brain injury. J Neuropsychiatry Clin Neurosci. 1994;6(4):443–54.doi:10.1176/jnp.6.4.443.
- Schnider A, Gutbrod K. Traumatic brain injury. Hum Front Lobes Funct Disord. 1999;487–508.
- Hartikainen KM, Wäljas M, Isoviita T, Dastidar P, Liimatainen S, Solbakk A-K, Ogawa KH, Soimakallio S, Ylinen A, Öhman J, et al. Persistent symptoms in mild to moderate traumatic brain injury associated with executive dysfunction. J Clin Exp Neuropsychol. 2010;32(7):767–74.doi:10.1080/13803390903521000.
- Iverson GL. Complicated vs uncomplicated mild traumatic brain injury: acute neuropsychological outcome. Brain Inj. 2006;20(13–14):1335–44.doi:10.1080/02699050601082156.
- Lange RT, Iverson GL, Franzen MD. Neuropsychological functioning following complicated vs. uncomplicated mild traumatic brain injury. Brain Inj. 2009;23(2):83–91.doi:10.1080/02699050802635281.
- Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. Am J Neuroradiol. 2002;23(5):794–802.
- Wilde EA, McCauley SR, Hunter JV, Bigler ED, Chu Z, Wang ZJ, Hanten GR, Troyanskaya M, Yallampalli R, Li X, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70(12):948–55.doi:10.1212/01.wnl.0000305961.68029.54.
- Machamer JE, Temkin NR, Dikmen SS. Neurobehavioral outcome in persons with violent or nonviolent traumatic brain injury. J Head Trauma Rehabil. 2003;18(5):387–97.doi:10.1097/00001199-200309000-00001.
- Bown D, Belli A, Qureshi K, Davies D, Toman E, Upthegrove R, Seedat S. Post-traumatic stress disorder and self-reported outcomes after traumatic brain injury in victims of assault. PLoS ONE. Internet]. 2019 Feb 7 [cited 2020 Jul 23];14(2). Available from (2019);e0211684. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6366871/
- Mathias JL, Harman-Smith Y, Bowden SC, Rosenfeld JV, Bigler ED. Contribution of Psychological Trauma to Outcomes after Traumatic Brain Injury: assaults versus Sporting Injuries. J Neurotrauma. 2014 Apr;31(7):658–69. doi:10.1089/neu.2013.3160.
- Booker J, Sinha S, Choudhari K, Dawson J, Singh R. Description of the predictors of persistent post-concussion symptoms and disability after mild traumatic brain injury: the SHEFBIT cohort. Br J Neurosurg. 2019 Jul 4;33(4):367–75. 10.1080/02688697.2019.1598542.
- Zeldovich M, Wu Y-J, Gorbunova A, Mikolic A, Polinder S, Plass AM, Covic A, Asendorf T, Andelic N, Voormolen D, et al. Influence of sociodemographic, premorbid, and injury-related factors on post-concussion symptoms after traumatic brain injury. J Clin Med. 2020;9(6):1931.doi:10.3390/jcm9061931.
- Corrigan JD, Harrison-Felix C, Bogner J, Dijkers M, Terrill MS, Whiteneck G. Systematic bias in traumatic brain injury outcome studies because of loss to follow-up. Arch Phys Med Rehabil. 2003 Feb 1;84(2):153–60. 10.1053/apmr.2003.50093.