ABSTRACT
Purpose
Evidence-based treatments for fatigue after brain injury are scarce and often not personalized. An approach to foster personalization is Experience Sampling Methodology (ESM), consisting of repeated daily measurements of fatigue and related factors in daily life. We investigated the feasibility and usability of a novel six-week ESM-based intervention for fatigue after brain injury.
Materials and methods
Ten individuals with acquired brain injury (six men; four women) aged between 36–70 years (M = 53.3, SD = 12.9) used a mHealth application for three days each week during six-weeks; seven completed the intervention. Momentary fatigue, activities, mood, worrying, and social context were assessed with ESM and participants received weekly personalized feedback by a therapist..
Results
56% of ESM-questionnaires (568/1008) were completed, providing detailed insights into individual fatigue patterns. No statistically significant decrease in response rate was found over the course of treatment. Qualitative feedback from participants revealed increased insight into factors underlying fatigue, and no problems with treatment duration or difficulties using the app. Five participants showed a decline in fatigue level during treatment.
Conclusions
This pilot study provides initial support for the feasibility and usability of this novel blended-care intervention, aimed at alleviating fatigue through personalized feedback and treatment strategies.
Introduction
Acquired brain injury (ABI) refers to nondegenerative damage to the brain sustained after birth. This injury can, for instance, result from stroke, mild to severe traumatic brain injury (TBI), hypoxic brain injury, inflammation of the brain, or a brain tumor (Citation1,Citation2). Moreover, it is estimated that more than 69 million individuals worldwide suffer an ABI each year (Citation3). Long-term consequences of ABI are multifaceted and range from sensory and/or motor to cognitive, emotional, and behavioral disturbances. One of the most common and persistent complaints among individuals with ABI is fatigue (Citation4). Individuals with ABI often report fatigue that differs from what is considered to be normal fatigue in that it appears more quickly, even during relatively non-demanding tasks, and requires long recovery with rest (Citation5).
It has been posited that in the first months after an ABI, fatigue is primarily a consequence of the dysfunction of brain interactions, that is, a primary brain injury-induced symptom (Citation6). After the recovery phase, fatigue may become determined by other factors such as mood, behavior, social or physical activities (Citation7–12). Research has demonstrated that the factors found to be associated with fatigue may differ significantly between individuals with ABI (Citation13,Citation14). Therefore, it is important to develop targeted personalized treatments to manage (chronic) fatigue after brain injury.
Among available non-pharmacological treatments are psychological interventions such as cognitive behavioral therapy or mindfulness-based stress reduction (MBSR) (Citation14–18). Physical exercise interventions have also been used, including aquatic physical activity, fitness-center-based exercise, Tai Chi, and aerobic training (Citation19–22). A combination of physical training and cognitive behavioral therapy has been shown to be effective in alleviating fatigue after stroke (Citation18,Citation23). However, evidence-based treatments for fatigue after ABI are still scarce and focus only on one or two factors that may influence fatigue (Citation13,Citation14). Moreover, a systematic review (Citation14) showed that the quality of evidence supporting some of these available treatment options has been assessed as (very) low according to the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system. All these interventions have investigated the manipulation of psychological or physical factors related to fatigue. Some of these interventions, especially those combining psychological and physical training elements, are highly intensive, time-consuming, and expensive, which may limit the implementation in clinical settings. Most interventions are non-personalized (i.e. standardized) and conducted at group level, where all individuals receive the same treatment protocol. Information about a person’s unique living conditions and the factors that are associated with fatigue for this person are not consistently measured and incorporated in existing treatments (Citation13,Citation16,Citation24–26), whereas we know that fatigue after ABI is a multifactorial symptom that includes physical, psychological, motivational, contextual, and activity-related components and is therefore person dependent (Citation12,Citation27). A more personalized intervention is needed to tackle the problem of heterogeneity of factors that may contribute to fatigue after ABI. A promising approach to achieve personalized treatment of fatigue is the use of the ‘Experience Sampling Method’ (ESM) as the basis for an intervention (Citation28,Citation29).
ESM is a structured diary method that allows measuring symptoms, experience, behavior and contextual information through repeated daily measurements in the flow of daily life (Citation30,Citation31). Based on these daily measurements, ESM allows detailed and personalized insight into fatigue and the factors that contribute to the maintenance of fatigue, which may differ across individuals (Citation32,Citation33). Recent ESM research (Citation34) showed that the relation between fatigue and physical activity largely differs between individuals, with some showing more fatigue after physical activity but others showing no association at all or even less fatigue. These results suggest that the potential effectiveness of a one-size-fits-all physical exercise intervention for fatigue is a priori limited because different individuals require different approaches. Moreover, ESM overcomes memory bias since it uses real-time measurements of experiences instead of retrospective questionnaires relying on memory recall (Citation35). This is important because persons with ABI often experience cognitive problems that may undermine accurate recollection from memory of information relevant to the diagnostic and treatment process (Citation36). ESM has been shown to be a feasible method in ABI populations with compliance rates ranging from 65% to 75% (Citation32,Citation34,Citation35,Citation37,Citation38). Ecological momentary intervention (EMI) combines ESM assessment with providing personalized feedback and encouraging behavioral change within the context of a person’s daily life (Citation39). For instance, in the treatment of depression, the application of ESM as part of treatment has been proven to be effective in reducing depressive symptoms (Citation40). Further, a recent review that investigated mobile health (mHealth) technology for long-term assessment in ABI individuals, revealed that giving feedback based on personalized data from individuals creates a sense of control by the individual, so-called sense of agency (Citation41). Such interventions have not been investigated for the treatment post-ABI fatigue yet.
The purpose of the current study was to investigate the feasibility and usability of an ESM-based intervention aimed at alleviating fatigue in an ABI individual population. To achieve this, a pilot study was conducted, with a newly developed six-week ESM intervention for fatigue using the mobile application PsyMateTM (www.Psymate.eu) in a small sample of individuals with ABI. The blended-care intervention combines ESM data collection by individuals with ABI with weekly, personalized feedback sessions with a therapist. The added value of this intervention lies in detailed insight into daily symptom patterns that may be unique to an individual, combined with behavioral interventions in daily life. This intervention thereby creates the possibility to promote healthy and adaptive behavioral change based on participants’ own data, displayed in graphs, and supported by feedback from healthcare professionals. Based on earlier research and results with ESM in ABI individuals (Citation32,Citation34,Citation35,Citation37,Citation38) we hypothesize that the intervention is feasible and usable. Feasibility is investigated by determining the response rates to the ESM questionnaires on a weekly basis during this six-week intervention and the responses of individuals to debriefing questionnaires. In addition, the individuals’ fatigue progression as response to the intervention is explored.
Methods
Participants
Participants were recruited at the Maastricht University Medical Center (MUMC+) and at the Adelante rehabilitation clinic in Hoensbroek, the Netherlands. Individuals who finished a rehabilitation treatment, but still experienced fatigue symptoms at the end of their rehabilitation program, were approached for participation in this study.
Inclusion criteria for the study were:
age 18 or above;
being diagnosed with ABI;
reporting fatigue symptoms;
good comprehension of Dutch;
capable of handling smartphone;
willing and able to give informed consent.
Exclusion criteria were:
1. Participation assessed as potentially too burdening based on clinical judgment. Such as patients with:
– motor limitations, due to hemiparesis or limitations with fine motor skills
– language problems, such as aphasia
2. Diagnosis of chronic fatigue syndrome or fibromyalgia or currently undergoing cancer treatment (self-reported).
The assessment of fatigue symptoms prior to study participation was based on self-reported fatigue complaints of the patient to their treating therapist. Subsequently, prior to the start of the intervention, all participants completed the Dutch Multifactorial Fatigue Scale as well. All participants gave their informed consent.
Ethics Review Committee Psychology and Neuroscience of Maastricht University approved the study.
Experience sampling method
We used a mobile Health application, PsyMateTM (http://www.psymate.eu), that was developed by the department of Psychiatry of Maastricht UMC+ and Maastricht University, the Netherlands. The PsyMate, a mHealth-application, is a structured diary technique that allows investigating fatigue and other symptoms in daily life through repeated real-time assessment in natural environments (Citation32).
The app was programmed to emit eight notifications a day, 3 days per week, at random moments between 7:30 in the morning and 22:30 in the evening, with the restriction that notifications were separated by at least 15 min and no more than 270 min. The average interval was set to 90 min. After each notification, participants were requested to fill in a 28-item questionnaire in the app, taking approximately 2–3 min to complete. If a person did not respond to a notification within 15 min, that specific questionnaire was skipped and registered as missing data. The participants have received the instruction to answer as much as they could from these eight notifications.
Questions contained the following themes, firstly, affective state was measured in eight items using Likert scales ranging from 1 to 7, with 1 signifying ‘strongly disagree’ and 7 signifying ‘strongly agree.’ Examples of questions measuring affective state are ‘I feel confident’ and ‘I am worrying’. Secondly, participants rated their currently experienced fatigue on a 7-point Likert scale, with 1 signifying ‘no fatigue’ and 7 signifying ‘extreme fatigue.’ There were three fatigue items measuring, respectively, general (‘I feel tired’), physical (‘I feel physically tired’) and mental fatigue (‘I feel mentally tired’). Thirdly, questions about physical or mental exertion since the last notification were asked. Fourthly, activities were measured by means of multiple-choice questions that list possible activities, as well as by questions concerning the appraisal of the activity, for example ‘I enjoyed this activity.’ Fifthly, location was measured by indicating where the participant was at the time of the notification signal, for example at home or in a public place. Participants also indicated if they were alone or had company, including who they were with (e.g., partner, family, friends). All questionnaires were completed in Dutch. Appendix A presents the layout of the PsyMate app questions.
Blended-care tied by tiredness intervention
Why the Tied by Tiredness intervention? Fatigue is a multifactorial phenomenon in which the brain injury itself obviously plays a causal role but has little predictive value in the chronic phase. Cognitive, emotional, and behavioral factors play an increasingly central role in what can become a vicious cycle for many patients. Tied by Tiredness aims to reduce fatigue by, on the one hand, offering the patient (and the therapist) detailed insight into the unique mix of factors associated with the individual’s fatigue and, on the other hand, providing the tools to actively and independently improve functioning. Tied by Tiredness is a program that distinguishes itself because it is specifically designed for personalized intervention in the daily life of patients. The treatment advice can take many forms such as improvement of physical condition, daily planning of rest and activity, targeted activities, sleep hygiene or targeting maladaptive thoughts about fatigue through cognitive behavioral principles – the important point here is that this is tailored to the individual through personalized data collection and feedback. As such, this intervention offers a toolbox to respond flexibly to the unique situation of each patient based on current scientific insights and recommendations on effective interventions. The intervention protocol was based on the treatment protocol used in an earlier RCT study and tailored to the specific context of fatigue after brain injury (Citation40).
Why blended care intervention? Blended care refers to the combination of traditional face-to-face conversations with the use of technology. This study involves an app that is installed on patients’ smartphones. The app assesses patients’ complaints in their daily lives for the duration of the intervention. In this way, we gain a better insight into how patients are really doing and can tailor treatment accordingly. In concrete terms, patients will record in the app several times a day during treatment how tired they are, how they feel, where they are, what they are doing and with whom (ESM) after personal feedback was given. In this way, both the healthcare professional and the patient gained detailed insight into fluctuations in fatigue symptoms (e.g., when does it get better/worse?), but also into the factors associated with this. By accurately mapping out for each patient which factors in daily life cause or maintain fatigue, therapists can adapt their treatment in a highly personalized way.
Who provides the therapy? The intervention can be performed by healthcare professionals (occupational therapists, psychologists, physiotherapists, etc.) who have knowledge and experience (they work in a setting where people with brain injury are treated) with brain injury patients with fatigue complaints.
How, when and how much? The intervention lasts a total of 6 weeks. Each of these 6 weeks has a recurring pattern consisting of two components: (a) the patient gets started with the m-ESM app; (b) and then discusses the collected data with the healthcare professional in a face-to-face conversation.
Face-to-face feedback sessions
In total, there were six feedback sessions that the therapist scheduled with the participant. During these sessions, the data entered in the participant’s app (data from the week before) served as a starting point. The feedback sessions were mainly intended to give the participants (and healthcare professionals) more insight into fatigue complaints and the factors that may be related to fatigue ().
Figure 1. Example of a feedback graph for level of fatigue over the intervention period (6 weeks, 3 days per week).
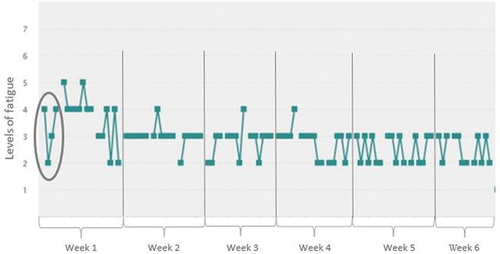
Each session lasted a maximum of 1 h, with direct contact lasting 45 min. This gave the healthcare professionals the first 15 min to view the data collected by the participant online and recognize any patterns. Each session had a specific topic and builds on the previous session(s). Based on the insights obtained using the ESM app, the healthcare professionals then provided personalized feedback and asked the participant to look at possible adjustments in daily behavior that could lead to fewer fatigue complaints. This intervention was intended as an additional aid to treatment by providing detailed and personalized insight into the complaints. The intervention protocol also contains elements from cognitive behavioral therapy (CBT), with the necessary explanation, so that every healthcare professional can use it where and when necessary. This approach was chosen because research has shown that cognitive-behavioral principles can have a positive influence on fatigue complaints after brain injury.
Where? Sessions took place in the outpatient clinics of participating rehabilitation center and departments of medical psychology in participating hospital in The Netherlands.
Tailoring. The treatment protocol consisted of six treatment modules (Citation1 and Citation2): fatigue during the day and in relation to mood (Citation3); fatigue during the day in relation to physical exertion and compared to previous weeks (Citation4); fatigue during the day in relation to mental effort and compared to previous weeks(Citation5); fatigue during the day in relation to activities and compared to previous weeks(Citation6); fatigue during the day in relation to social contacts and location and compared to previous weeks. The content of the treatment modules is described in .
Table 1. Tied by tiredness intervention treatment modules.
Figuur 1
Measurements
Dutch Multifactor Fatigue Scale (DMFS). This questionnaire was developed (Citation42) to assess the unique characteristics of fatigue and coping with fatigue in the chronic phase of ABI. The DMFS consists of five factors: Impact of fatigue, Mental fatigue, Signs and Direct consequences of fatigue, Physical fatigue and Coping with fatigue. The questionnaire consists of 38 items with a 5-point Likert scale ranging from ‘I totally disagree’ to ‘I totally agree.’ Scores range from 11 to 54, with higher scores indicating higher levels of fatigue. The subscales of the DMFS were found to have sufficient to good reliability, good convergent validity, and good divergent validity.
Usability questionnaire
A debriefing questionnaire about participants’ experiences of the intervention and the PsyMate application was administered to further assess the feasibility and usability of the intervention. Questions about feasibility ranged from readability and operation of the app to opinions about the number of notifications and length of the ESM questionnaire. Questions about the intervention ranged from opinions about how the intervention sessions and mails were experienced and if the interventions helped understanding the participant’s own fatigue. Several open-ended questions were also included to obtain more in-depth opinions about the app/interventions and to receive suggestions for improvements. The questionnaire consisted of 13 closed-ended items using a 7-point Likert scale ranging from 1 ‘not at all’ to 7 ‘very much’ and four open-ended items. The usability evaluation questions are present in .
Table 3. Weekly number of signals responded to (out of 24) and corresponding response rate in percentage per participant.
Procedure
The recruitment of participants took place through healthcare professionals at both study locations in the period from June 2019 to April 2020. Information about the study was then sent to the individuals. After giving informed consent, a researcher conducted a short telephone interview to establish whether inclusion criteria were met. Next, during a face-to-face briefing meeting with the healthcare professional, participants completed the DMFS questionnaire and received detailed instructions about the ESM procedure. During this briefing session, participants could also do a practice run of the ESM questionnaire in the PsyMate app to ensure that participants could navigate through the app and correctly understood the questions. During the intervention period, questions and/or problems could be reported. After the six-week intervention, a debriefing session with the healthcare professional was held to evaluate participants’ experiences of the ESM procedure and to assess the feasibility of the intervention. Also, at this stage, the DMFS questionnaire was post-intervention completed. DMFS-scores pre- and post-intervention are included in .
Table 2. Demographics of participants.
Analysis
To assess the feasibility of this blended-care intervention, response rates per participant per week and over the entire treatment period were calculated as a measure of adherence to the intervention. Further, a within-between subjects t-test using SPSS version 25 was run to compare adherence during the first week of treatment to the final week of treatment. We also ran these within-subject t-tests for general, physical, and mental fatigue. For fatigue however, these tests were exploratory in nature, as this pilot study was designed to assess feasibility and not efficacy. Alpha level was set at .05. Momentary levels of overall fatigue were measured at every notification signal with one questionnaire item ranked on a seven-point Likert scale. The evolution of fatigue (general, mental, and physical) was captured by calculating weekly averages of fatigue ratings per participant. Further, the results of the evaluation questionnaire were used to describe participants’ experiences with the app and during treatment.
Results
Sample characteristics
In total, 11 participants were recruited to take part in the study. However, one participant, immediately after admission, for the start of the intervention, terminated participation due to personal reasons. The final sample consisted of 10 participants, six men and four women, with an age range of 36–70 years (M = 53.3, SD = 12.9). Seven participants had suffered a stroke, one had a cerebral abscess, one had meningitis and one had a brain tumor. Descriptive statistics of the sample are shown in .
Reasons for subject withdrawal
Seven out of ten participants completed the six-week intervention. Two participants (participants 3 and 9) reported that they were experiencing technical problems with the use of the app and only received the notifications sporadically of not at all. Due to this, participants 3 and 9 were not able to complete all questionnaires and had to end the intervention prematurely after 5 and 4 weeks, respectively. Participant 7 stopped the intervention after three weeks due to personal reasons.
These 3 participants were excluded from the analyses.
Feasibility/Response rate
provides an overview of the number of PsyMate notifications that each participant responded to per week (out of a total of 24 weekly notifications). In total, 56.3% of signals (568 out of 1008 signals) were responded to. The average compliance rate differed between participants: from the highest rate at 70.8% (participant 8), to the lowest rate at 38.2% (participant 5). Within participants, compliance rates varied from week-to-week with; both increases and decreases over the course of the intervention period. The highest response rate (overall) was registered in week 4 (60.7%) and the lowest in week 2 (51.8%).
A paired samples t-test indicated that response rate was not significantly lower at the end of the intervention (week 6) relative to the beginning of the intervention (week 1), mean difference = 0, SD difference = 5.5, t (Citation6) = 0, p = 0.999.
Usability
Regarding the usability of the app (see ), none of the participants reported difficulties (except the technical one) using or operating the app. This means that participants did not experience problems with reading of filling in the questions. The number of notifications and time spent filling out an ESM questionnaire was evaluated as ‘not at all a burden’ by the majority of participants. Participants experienced no problems reading the questions in the app, although some reported difficulty in understanding one or more questions. Examples given by participants were the questions ‘I feel cheerful’ or ‘I feel gloomy.’ One participant suggested using more open questions instead of closed questions alone, and another participant suggested including a blank field that could be filled in manually for the question ‘What are you doing?’ Two participants reported technical difficulties, which reportedly led to them receiving no or fewer notifications per day, for example week 2 for participant 2 and for participant 5. In total that 4 out 10 participants had experiencing technical issues. Two out of 10 participants have experiencing serious technical problems, therefore they could not complete the intervention, and they were excluded from the analyses. In this case, the participants were receiving no or fewer notifications (set of questions) and was neither a motivation problem from the participants, nor an issue with the intervention/protocol.
Table 4. Evaluation questionnaire about usability app, 7-point likert scale with 1 ‘not at all’ and 7 ‘very’.
With respect to the intervention, most participants reported an enhanced understanding of fatigue (see ). No participants reported problems with the duration of the intervention. The participants experienced the intervention sessions equally useful as the feedback provided by e-mail.
Table 5. Evaluation questionnaire about the intervention, 7-point likert scale with 1 ‘not at all’ and 7 ‘very’.
Fatigue level
and shows the weekly averaged ratings of general fatigue per participant. Five out of seven participants (80%) showed a decline in fatigue level from the first to the last measurement week. Two participants showed a slight increase in their fatigue ratings. Decline rates range from −0.1 to 1.9 points in fatigue rating (M = 0.8, SD = 1.6). Within participants, fatigue progression is not linear; most participants display fluctuations in fatigue level from week to week. With respect to physical and mental fatigue, the decline rates ranged from −1 to 2 points in physical fatigue rating (M = 0.4, SD = 1.9) and mental fatigue ranged from −0.1 to 2 points (M = 1, SD = 1.6).
Figure 2. Weekly average of momentary fatigue rating per participant and the mean of the three fatigue measurements.
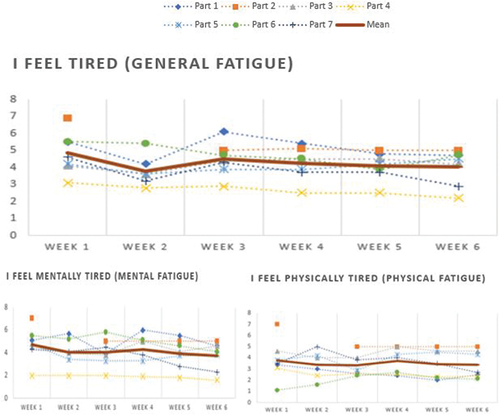
Table 6. Weekly average of momentary fatigue rating per participant on a 7-point likert scale and the average of the three types of fatigue ratings.
A paired samples t-test indicated that general fatigue, as measured by ESM, was significantly lower at the end of the intervention (M difference = 0.8, SD difference = 0.82), t (6) = 2.477, p = 0.048. Physical fatigue was not significantly lower at the end of the intervention (M difference = 0.4, SD difference = 0.99), t (6) = 1.097, p = 0.315. Mental fatigue was significantly lower at the end of the intervention (M difference = 1.02, SD difference = 0.81), t (6) = 3.344, p = 0.016.
Mean scores on the Dutch Multifactor Fatigue Scale were lower for three out of five factors from pre- vs. post-intervention: impact of fatigue (−4.6 points), mental fatigue (−2.5 points), and physical fatigue (−1.9 points). Consequences of fatigue were stable and coping with fatigue (+1 points) increased slightly. However, paired samples t-tests of pre-intervention fatigue scores versus post-intervention fatigue scores were not significant (all p’s > .190).
Discussion
The current pilot study examined the feasibility and usability of a six-week blended-care intervention aimed at alleviating fatigue after acquired brain injury. Results showed that the intervention was feasible in terms of individual burden and adherence to treatment. The overall compliance rate during the intervention period – reflecting the number of notification signals in the PsyMate app responded to – was 56.3%. This number exceeds the minimum percentage of 33% that has been posited to be the minimal required for the method to have sufficient validity (Citation43). As mentioned, there are some studies that have investigated ESM assessment in ABI individuals and concluded that is a feasible method with compliance rates ranging from 65% to 75% (Citation32,Citation34,Citation35,Citation37,Citation38). However, these studies were conducted only for assessment of symptoms without an intervention or feedback component, and therefore had a much shorter measurement period (e.g., 6 days). This study investigated a six-week ESM-based intervention with weekly personalized graphic feedback, for which we found an overall response rate of 56.3% to be acceptable. However, it should be noted that three out of ten participants were not able to finish the treatment, which was caused by either personal problems for one individual or technical issues with the application reportedly not sending all programmed notification signals for two individuals. Technical issues may be inherent to newly emerging mHealth interventions and technological innovations in the field of neuropsychology but should be addressed appropriately moving forward. Technical problems such as user error (an error made by the participant) or internet connection difficulties, which interfered with the completion of assessments, were also signaled as barriers in a systematic review on evidence on the use of ecological momentary assessments after brain injury (Citation33).
Besides the response rate, the results of the evaluation questionnaire were also considered to obtain user feedback about the intervention. Participants found the app easy to use and experienced no problems with the duration of the intervention or the number of questionnaires. Again however, two participants mentioned technical problems interfering with following and completing the treatment. Alternative applications can be considered in future studies.
Previous research into mHealth interventions in ABI individuals showed that blended care interventions that combine standardized protocols and reliable, bias-free, and detailed monitoring are most appreciated by individuals (Citation44). The blended care intervention presented in this study also meets several needs expressed by individuals with ABI. Moreover, a recent review that investigated mHealth technology for long-term assessment in ABI individuals revealed that a two-way system is also prefered by ABI individuals (Citation41). A two-way system consists of reciprocal exchange (back-and-forth) of information between individual and health professional. Thus, although it certainly lies within the possibilities of technological innovation to replace the face-to-face feedback of our intervention with automated feedback within the app environment, such modification would primarily meet cost-efficiency concerns but could go against individual preference. Further, it is (Citation41) noted that a consistent theme that was mentioned by individuals with ABI is personalization, namely that individual differences require personalized approaches and that the use of mHealth technology should allow capturing the unique needs and abilities of each person.
With respect to the current Tied by Tiredness intervention, the majority of participants reported an enhanced understanding of fatigue, which was achieved through verbal and visual feedback. In a recent systematic review, the authors (Citation33) reported that procedures that make use of momentary assessments such as ESM may pose a burden on participants due to intense data collection protocols. However, participants in the current study did not experience the duration of the intervention as burdensome. One possible explanation for these findings is that the current intervention does not involve a high cognitive load, with a limited set of recurring statements that are easy to understand (e.g., ‘I feel tired’). Further, recently it has been found (Citation45) a negative association between the length of the ESM questionnaires and rate of compliance. Longer ESM questionnaires are associated with lower compliance. However, these authors also found that high-sampling frequencies (e.g., many notification signals per day) do not seem to be associated with negative consequences regarding compliance. Hence, compliance of this and other future ESM intervention studies may benefit from reducing the number of items in the ESM questionnaire, while having the flexibility to adjust the sampling frequency to the needs of the study or the specific study population.
Further, although the intervention and the total number of notifications were not experienced as burdensome by participants, the total response rate across the sample was 56%. On the one hand, this may be due to our explicit instruction to participants that notifications could be missed due to circumstances (e.g., in traffic, going to bed early, etc.). This was done to ensure that participants went about their daily lives without adjusting their behavior to the study protocol. On the other hand, it remains possible that some participants ‘self-managed’ the burden experienced because of the study by occasionally not responding to notifications.
The third aim of the study was to explore the evolution of individual fatigue ratings over the six-week intervention period. Overall, the majority of participants showed a lower weekly average of fatigue after 6 weeks than at the beginning of the intervention. A statistically significant decrease was found for general (i.e., ‘I feel tired’) and mental fatigue (i.e., ‘I feel mentally tired’). However, scores on the DMFS questionnaire were not significantly lower after the intervention relative to baseline scores. Because evaluating feasibility was the primary goal of this study, our sample size was small. Controlled studies with larger sample sizes are needed to evaluate effectiveness. Finally, this study also shows that fatigue often varies from moment to moment and that participants can distinguish between mental and physical fatigue. These findings are important because they demonstrate that during the diagnostic process and especially treatment, specific forms of fatigue can be zoomed in on.
Limitations and future research
A first limitation of this study concerns the specific app (PsyMate) that was used. Some participants reported not receiving (all of the) notification signals throughout the ESM data collection period. The problems with the PsyMate app are both due to a programming bug within the app and due to settings of individual smartphones (e.g., some types of smartphones may automatically mute signals from apps if they come in high frequency, as is the case in this study). These technical problems do represent an extra hurdle in implementing mHealth interventions in daily life. Therefore, in a further study, other ESM app’s may be taken into consideration to be used. Further, this is a pilot study, with a very low sample size, that aimed to determine feasibility and usability of the treatment, and therefore there was no control group. In order to draw conclusions about the effectiveness of this intervention, a controlled trial is needed in which this intervention can be compared with treatment as usual. Further, in order to learn whether this intervention may be effective in reducing fatigue complaints after brain injury, a bigger sample is needed, and the technological issues need to be resolved. Finally, our sample consisted of ABI individuals who already completed a rehabilitation program. It is possible that ABI individuals who are recruited earlier in their rehabilitation experience more benefits of this blended-care intervention.
Conclusion
In summary, this pilot study provides initial support for the feasibility and usability of this Ecological Momentary Intervention with personalized feedback in an ABI population. ESM provides a promising method to bridge the gap between treatment settings and the daily lives of individuals, and it can facilitate the process of personalized diagnosis and treatment (Citation29). Together with weekly feedback from therapists, this intervention embraces technological innovation while also accommodating preferences expressed by individuals of having real, reciprocal interactions with their treating therapist.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Feigin VL, Nichols E, Alam T, Bannick MS, Beghi E, Blake N, Culpepper WJ, Dorsey ER, Elbaz A, Ellenbogen RG, et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the global burden of disease study 2016. The Lancet Neurology. 2019;18(5):459–80. doi:10.1016/S1474-4422(18)30499-X.
- Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson LM, Truelsen T, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the global burden of disease study 2010. Lancet Glob Heal. 2013;1(5):e259–81. doi:10.1016/S2214-109X(13)70089-5.
- Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M, Agrawal A, Adeleye AO, Shrime MG, Rubiano AM, et al. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2018;130(4):1080–97. doi:10.3171/2017.10.JNS17352.
- Subbarao BS, Stokke J, Martin SJ. Telerehabilitation in Acquired Brain Injury. Phys Med Rehabil Clin N Am. 2021;32(2):223–38. [cited 2022 Jan 17] https://pubmed.ncbi.nlm.nih.gov/33814054/.
- Guggisberg AG, Chauvigné L, Pignat JM. Fatigue after acquired brain injury and its impact on socio-professional reintegration. Rev Med Suisse. 2020;16(692):901–03. [cited 2022 Jul 27] https://europepmc.org/article/med/32374533.
- Schönberger M, Herrberg M, Ponsford J. Fatigue as a cause, not a consequence of depression and daytime sleepiness: a cross-lagged analysis. J Head Trauma Rehabil. 2014;29(5):427–31. doi:10.1097/HTR.0b013e31829ddd08.
- Levine J, Greenwald BD. Fatigue in parkinson disease, stroke, and traumatic brain injury. Phys Med Rehabil Clin N Am. 2009;20(2):347–61. [cited 2021 Nov 27] https://pubmed.ncbi.nlm.nih.gov/19389616/.
- De Doncker W, Dantzer R, Ormstad H, Kuppuswamy A. Mechanisms of poststroke fatigue. J Neurol Neurosurg Psychiatry. 2018;89(3):287–93. [cited 2020 Oct 3] https://pubmed.ncbi.nlm.nih.gov/28939684/.
- Aarnes R, Stubberud J, Lerdal A. A literature review of factors associated with fatigue after stroke and a proposal for a framework for clinical utility. Neuropsychol Rehabil. 2020;30(8):1449–76. doi:10.1080/09602011.2019.1589530.
- Schönberger M, Reutens D, Beare R, O’Sullivan R, Rajaratnam SMW, Ponsford J. Brain lesion correlates of fatigue in individuals with traumatic brain injury. Neuropsychol Rehabil. 2017;27(7):1056–70. doi:10.1080/09602011.2016.1154875.
- Mollayeva T, Kendzerska T, Mollayeva S, Shapiro CM, Colantonio A, Cassidy JD. A systematic review of fatigue in individuals with traumatic brain injury: the course, predictors and consequences. Neurosci Biobehav Rev. 2014;47:684–716. doi:10.1016/j.neubiorev.2014.10.024.
- Bruijel J, van Heugten CM, Murray J, Grima N, Ymer L, Walters EM, Sinclair K, Stapert SZ, Vermeeren A, Ponsford JL, et al. The bidirectional relationship between sleep and physical activity following traumatic brain injury. J Sleep Res. 2021;30(5). doi:10.1111/jsr.13334.
- Wu S, Kutlubaev MA, Chun HYY, Cowey E, Pollock A, Macleod MR, Dennis M, Keane E, Sharpe M, Mead GE. Interventions for post-stroke fatigue. Cochrane Database Syst Rev. 2015;2015(7). doi:10.1002/14651858.CD007030.pub3.
- Xu GZ, Li YF, Wang M-D, Cao DY. Complementary and alternative interventions for fatigue management after traumatic brain injury: a systematic review. Ther Adv Neurol Disord. 2017;10(5):229. doi:10.1177/1756285616682675.
- Johansson B, Bjuhr H, Rönnbäck L. Mindfulness-based stress reduction (MBSR) improves long-term mental fatigue after stroke or traumatic brain injury. Brain Inj. 2012;26(13–14):1621–28. doi:10.3109/02699052.2012.700082.
- Clarke A, Barker-Collo SL, Feigin VL. Poststroke fatigue: does group education make a difference? A randomized pilot trial. Top Stroke Rehabil. 2012;19(1):32–39. doi:10.1310/tsr1901-32.
- Johansson B, Carlsson A, Carlsson ML, Karlsson M, Nilsson MKL, Nordquist-Brandt E, Rönnbäck L. Placebo-controlled cross-over study of the monoaminergic stabiliser (−)-OSU6162 in mental fatigue following stroke or traumatic brain injury. Acta Neuropsychiatr. 2012;24(5):266–74. doi:10.1111/j.1601-5215.2012.00678.x.
- Zedlitz AMEE, Rietveld TCM, Geurts AC, Fasotti L. Cognitive and graded activity training can alleviate persistent fatigue after stroke: a randomized, controlled trial. Stroke. 2012;43(4):1046–51. doi:10.1161/STROKEAHA.111.632117.
- Hassett LM, Moseley AM, Tate RL, Harmer AR, Fairbairn TJ, Leung J. Efficacy of a fitness centre-based exercise programme compared with a home-based exercise programme in traumatic brain injury: a randomized controlled trial. J Rehabil Med. 2009;41(4):247–55. doi:10.2340/16501977-0316.
- Bateman A, Culpan FJ, Pickering AD, Powell JH, Scott OM, Greenwood RJ. The effect of aerobic training on rehabilitation outcomes after recent severe brain injury: a randomized controlled evaluation. Arch Phys Med Rehabil. 2001;82(2):174–82. https://pubmed.ncbi.nlm.nih.gov/11239307/.
- Gemmell C, Leathem JM. A study investigating the effects of Tai Chi Chuan: individuals with traumatic brain injury compared to controls. Brain Inj. 2006;20(2):151–56. doi:10.1080/02699050500442998.
- Driver S, Ede A. Impact of physical activity on mood after TBI. Brain Inj. 2009;23(3):203–12. doi:10.1080/02699050802695574.
- Amorós-Aguilar L, Rodríguez-Quiroga E, Sánchez-Santolaya S, Coll-Andreu M. Effects of combined interventions with aerobic physical exercise and cognitive training on cognitive function in stroke individuals: a systematic review. Brain Sciences. 2021;11(4):473. https://pubmed.ncbi.nlm.nih.gov/33917909/.
- Brown DL, Chervin RD, Kalbfleisch JD, Zupancic MJ, Migda EM, Svatikova A, Concannon M, Martin C, Weatherwax KJ, Morgenstern LB, et al. Sleep apnea treatment after stroke (SATS) trial: is it feasible? J Stroke Cerebrovasc Dis. 2013;22(8):1216–24. doi:10.1016/j.jstrokecerebrovasdis.2011.06.010.
- Jonasson A, Levin C, Renfors M, Strandberg S, Johansson B. Mental fatigue and impaired cognitive function after an acquired brain injury. Brain Behav. 2018;8(8):1–7. doi:10.1002/brb3.1056.
- Zedlitz AMEE, Fasotti L, Geurts ACH. Post-stroke fatigue: a treatment protocol that is being evaluated. Clin Rehabil. 2011;25(6):487–500. [cited 2021 Mar 4] https://pubmed.ncbi.nlm.nih.gov/21402652/.
- Wu S, Mead G, Macleod M, Chalder T. Model of understanding fatigue after stroke [internet]. Stroke. 2015;46(3):893–98. [cited 2021 Apr 5] https://pubmed.ncbi.nlm.nih.gov/25649798/.
- Myin-Germeys I, Oorschot M, Collip D, Lataster J, Delespaul P, Van Os J. Experience sampling research in psychopathology: opening the black box of daily life. Psychol Med. 2009;39(9):1533–47. [cited 2022 Jan 19] https://pubmed.ncbi.nlm.nih.gov/19215626/.
- van Os J, Verhagen S, Marsman A, Peeters F, Bak M, Marcelis M, Drukker M, Reininghaus U, Jacobs N, Lataster T, et al. The experience sampling method as an mHealth tool to support self-monitoring, self-insight, and personalized health care in clinical practice. Depress Anxiety. 2017;34(6):481–93. doi:10.1002/da.22647.
- Stone AA, Shiffman S. Ecological momentary assessment (EMA) in behavioral medicine. Ann Behav Med. 1994;16(3):199–202. [cited 2020 Oct 7] https://academic.oup.com/abm/article/16/3/199/4648561.
- Sohl SJ, Friedberg F. Memory for fatigue in chronic fatigue syndrome: relationships to fatigue variability, catastrophizing, and negative affect. Behav Med. 2008;34(1):29–38. [cited 2020 Oct 7] https://pubmed.ncbi.nlm.nih.gov/18400687/.
- Lenaert B, Colombi M, van Heugten C, Rasquin S, Kasanova Z, Ponds R. Exploring the feasibility and usability of the experience sampling method to examine the daily lives of individuals with acquired brain injury. Neuropsychol Rehabil. 2019;29(5):754–66. doi:10.1080/09602011.2017.1330214.
- Mitchell RJ, Goggins R, Lystad RP. Synthesis of evidence on the use of ecological momentary assessments to monitor health outcomes after traumatic injury: rapid systematic review. BMC Med Res Methodol. 2022;22(1). doi:10.1186/s12874-022-01586-w.
- Lenaert B, Neijmeijer M, van Kampen N, van Heugten C, Ponds R. Poststroke fatigue and daily activity patterns during outindividual rehabilitation: an experience sampling method study. Arch Phys Med Rehabil. 2020;101(6):1001–08. [cited 2020 Nov 25] http://www.archives-pmr.org/article/S0003999320300265/fulltext.
- Forster SD, Gauggel S, Petershofer A, Völzke V, Mainz VEcological Momentary Assessment in Individuals With an Acquired Brain Injury: A Pilot Study on Compliance and Fluctuations. Front Neurol. 202011. doi: 10.3389/fneur.2020.00115.
- Hinkle JL, Becker KJ, Kim JS, Choi-Kwon S, Saban KL, McNair N, Mead GE. Poststroke fatigue: emerging evidence and approaches to management: a scientific statement for healthcare professionals from the American heart association. Stroke. 2017;48(7):e159–70. [cited 2021 Feb 28] https://pubmed.ncbi.nlm.nih.gov/28546322/.
- Johansson B, Rönnbäck L. Assessment and treatment of mental fatigue after a traumatic brain injury. Neuropsychol Rehabil. 2017;27(7):1047–55. doi:10.1080/09602011.2017.1292921.
- Cumming TB, Yeo AB, Marquez J, Churilov L, Annoni JM, Badaru U, Ghotbi N, Harbison J, Kwakkel G, Lerdal A, et al. Investigating post-stroke fatigue: An individual participant data meta-analysis. J Psychosom Res. 2018;113(June):107–12. doi:10.1016/j.jpsychores.2018.08.006.
- Colombo D, Fernández-Álvarez J, Patané A, Semonella M, Kwiatkowska M, García-Palacios A, Cipresso P, Riva G, Botella C. Current state and future directions of technology-based ecological momentary assessment and intervention for Major depressive disorder: a systematic review. J Clin Med. 2019;8(4):465. doi:10.3390/jcm8040465.
- Kramer I, Simons CJP, Hartmann JA, Menne-Lothmann C, Viechtbauer W, Peeters F, Schruers K, van Bemmel AL, Myin-Germeys I, Delespaul P, et al. A therapeutic application of the experience sampling method in the treatment of depression: a randomized controlled trial. World Psychiatry. 2014;13(1):68–77. doi:10.1002/wps.20090.
- Juengst SB, Terhorst L, Nabasny A, Wallace T, Weaver JA, Osborne CL, Burns SP, Wright B, Wen P-S, Kew C-LN, et al. Use of mHealth technology for Individual-Reported Outcomes in Community-Dwelling Adults with acquired brain injuries: a scoping review. Int J Envir Res Pub Health. 2021;18(4):1–19. doi:10.3390/ijerph18042173.
- Visser-Keizer AC, Hogenkamp A, Westerhof-Evers HJ, Egberink IJL, Spikman JM. Dutch multifactor fatigue scale: a new scale to measure the different aspects of fatigue after acquired brain injury. Arch Phys Med Rehabil. 2015;96(6):1056–63. doi:10.1016/j.apmr.2014.12.010.
- Delespaul PAEG. Assessing schizophrenia in daily life: the experience sampling method. 1995. doi:10.26481/dis.19950504pd
- Juengst SB, Hart T, Sander AM, Nalder EJ, Pappadis MR. Mobile health interventions for traumatic brain injuries. Curr Phys Med Rehabil Reports. 2019;7(4):341–56. doi:10.1007/s40141-019-00240-9.
- Eisele G, Vachon H, Lafit G, Kuppens P, Houben M, Myin-Germeys I, Viechtbauer W. The effects of sampling frequency and questionnaire length on perceived burden, compliance, and careless responding in experience sampling data in a student population. Assessment. 2022;29(2):136–51. [cited 2022 Oct 26] https://pubmed.ncbi.nlm.nih.gov/32909448/.

