ABSTRACT
Purpose: To summarize the Integrated Stress Response (ISR) in the context of ophthalmology, with special interest on the cornea and anterior segment. Results: The ISR is a powerful and conserved signaling pathway that allows for cells to respond to a diverse array of both intracellular and extracellular stressors. The pathway is classically responsible for coordination of the cellular response to amino acid starvation, ultraviolet light, heme dysregulation, viral infection, and unfolded protein. Under normal circumstances, it is considered pro-survival and a necessary mechanism through which protein translation is controlled. However, in cases of severe or prolonged stress the pathway can promote apoptosis, and loss of normal cellular phenotype. The activation of this pathway culminates in the global inhibition of cap-dependent protein translation and the canonical expression of the activating transcription factor 4 (ATF4). Conclusion:The eye is uniquely exposed to ISR responsive stressors due to its environmental exposure and relative isolation from the circulatory system which are necessary for its function. We will discuss how this pathway is critical for the proper function of the tissue, its role in development, as well as how targeting of the pathway could alleviate key aspects of diverse ophthalmic diseases.
KEYWORDS:
Introduction
The Integrated Stress Response (ISR) is a critical pathway that is required for cellular response to external and internal cellular stressors.Citation1 Principally, it provides a mechanism by which a cell can alter its protein production dynamics and shut down global cap-dependent protein synthesis.Citation2 This functions as a brake on protein synthesis allowing the cell time to adapt to the new environment by slowing the rate of protein synthesis and therefore decreasing demand for energyCitation3 and amino acids.Citation4 Simultaneously, the cell can increase the production of proteins involved with cell survival,Citation5 amino acid synthesis,Citation6 and autophagyCitation7 through the key transcription factor of the ISR, activating transcription factor-4 (ATF4). The activation of the ISR is canonically carried out in response to signaling from four kinases that are principally responsive to; amino acid and glucose deprivation (general control non-depressible protein 2, GCN2),Citation4,Citation5 heme deficiency (heme-regulated inhibitor, HRI),Citation8 viral infection (double-stranded RNA-dependent protein kinase, PKR),Citation9 hypoxiaCitation10 and the accumulation of unfolded proteins in the endoplasmic reticulum (PKR like endoplasmic reticulum kinase, PERK).Citation11 Activation of the ISR results in a reduction of global protein synthesis and an induction of specific genes that aid in cellular recovery.1Citation2 A short-lived ISR is a pro-survival response aimed at relieving stresses and regaining protein homeostasis, while a sustained ISR can result in cell death.Citation12 Therefore, it is commonly believed that the ISR is a double-edged sword whose actions have been correlated with a diverse field of human diseases including neurodegeneration,Citation13 endocrine disorders,Citation14 cancer, Citation15,Citation16 autoimmune disorders,Citation17 and infections.Citation18
Unlike other tissues, the eye is uniquely exposed to all the potential stressors that can activate the ISR being both exposed to the environment, and being relatively isolated form the circulatory system.Citation19,Citation20 The response to these stressors in the eye is also limited, neovascularization in the eye has been associated with deleterious diseases and loss of vision.Citation21,Citation22 Viral infection of the eye is also pernicious with the eye being a privileged site that does not, and indeed cannot, utilize the full extent of the immune system to clear infection.Citation23,Citation24 The activation and consequences of an active ISR have often been observed, or eluded to, in diverse ocular morbidities affecting all tissues of the eye. Reports of ISR involvement have been published in, macular degeneration,Citation21 neurodegeneration,Citation25 diabetic retinopathy,Citation26 cataracts,Citation27 dry eye disease,Citation28 and keratoconus.Citation29 In animal models, the ablation of ATF4 and other components of the pathway leads to profound ocular phenotypes in development which leads us to suggest that ISR and ATF4 are key modulators of ophthalmic diseases.Citation30–32 Critically, the ISR is considered a druggable target, with several compounds that target the pathway under investigation for diverse pathologies.Citation33–35 In this review, we update the pathophysiologic roles of the ISR in ocular diseases, with special interests in disorders of the anterior segment. We further explore the potential for small molecules in modulating key components of the ISR to modulate their activity, and finally discuss the available animal models of ISR disruption, their ocular phenotypes, and their usefulness in studying the role of the ISR in the eye.
Integrated Stress Response (ISR)
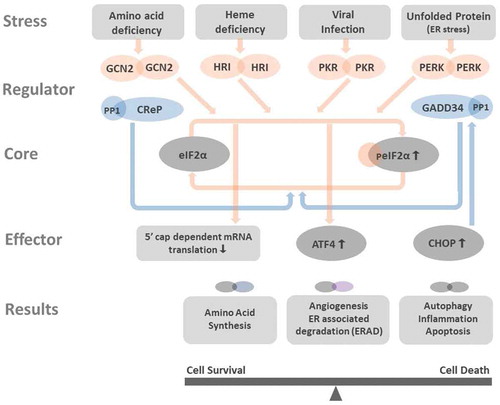
The ISR is the central node by which the cell coordinates the dynamics of protein production in response to environmental and intracellular stressors.Citation1,Citation2 The ability to decrease the rate of protein production and alter phenotype in response to acute and chronic stress situations is critical for survival of the cell, and the whole organism.Citation36 In humans, the ISR sensors comprise four eIF2α kinasesCitation37: GCN2,Citation38 HRI,Citation8 PKR,Citation39,Citation40 and PERK.Citation11,Citation41 These kinases respond to diverse stresses: GCN2 to amino acid and glucose deprivation,Citation42 HRI to iron deficiency,Citation43 PKR to viral infection,Citation9 and PERK to protein misfolding in the ER, the so-called ER stress.Citation44 Upon activation of its respective stimulus each kinase dimerizes, auto-phosphorylates, then phosphorylates Ser51 of eIF2α as demonstratedCitation37in .
Figure 1. Integrated stress response affects cell functioning Amino acid deficiency, heme deficiency, viral infection, and unfolded protein stress in the endoplasmic reticulum (ER stress) activate GCN2, HRI, PKR, and PERK. All these kinases phosphorylate eIF2α, the core member of ISR. Phosphorylated eIF2α (peIF2α) impedes the 5ʹcap-dependent mRNA translation ( in details) and results in global reduction of protein synthesis. However, few genes as ATF4 and CHOP have alternative translation machinery and are less influenced by eIF2 dysfunction. The preferentially translated ATF4 is the key effector in ISR. ATF4 couples with another ATF4 or its interacting partners to form homo- and heterodimers that bind to DNA targets and control the expression of genes that participated in amino acid synthesis, angiogenesis, ERAD, autophagy, inflammation, and apoptosis. Depends on the disease context and the duration of ISR, the downstream processes of ISR can aid in cell survival or bring cell death. CHOP is one key factor that can be promoted by ATF4. CHOP not only activates autophagy, inflammation, and apoptosis, but also induces the expression of GADD34, a phosphatase coupled with PP1 to dephosphorylate peIF2α. The dephosphorylation of peIF2α terminates ISR and resumes protein synthesis. At non-stressed cell, the CreP-PP1 complex phosphatase constantly operates for maintaining low level of peIF2α and protein homeostasis
The core mediator of the ISR response is the eIF2α subunit, being a key element in the eukaryotic initiation factor 2 complex (eIF2, consisting of α, β, and γ subunits),Citation1,Citation37 this is a central subunit required for the initiation of mRNA translation. Under normal conditions, the eIF2 complex binds a GTP, and a met-tRNA molecule and presents these to the 40S ribosomal subunit.Citation45 This assembly is a key rate-limiting step in the initiation of mRNA translation,Citation46,Citation47 therefore the function of the ISR is to regulate the availability of eIF2α by decreasing the pool of the subunit available to initiate ribosome assemblyCitation1,Citation2 ().
Figure 2. Illustration of the roles of eIF2 and peIF2 in the initiation of mRNA translation Before binding to met-tRNA, the GDP bound to the γ subunit of eIF2 must be switched to GTP. This GDP-GTP exchange process is mediated by eIF2B. The met-tRNA-GTP-eIF2 ternary complex then delivers the met-tRNA to 40S ribosome, a key step for the initiation of mRNA translation. At the stressed condition, once the ISR is activated and the GDP-eIF2 is phosphorylated in its α subunit, the structural change makes GDP-peIF2 no longer a suitable substrate of eIF2B but transforms to an inhibitor of eIF2B. The inhibition of eIF2B by GDP-peIF2 further reduces the pool of functional GTP-eIF2 to bind met-tRNA, and lowers the global protein synthesis via 5ʹcap-dependent mRNA translation. ISRIB rescues protein translation by stabilization and increasing eIF2B abundance that counteracts low level of GDP-peIF2α in treated cells
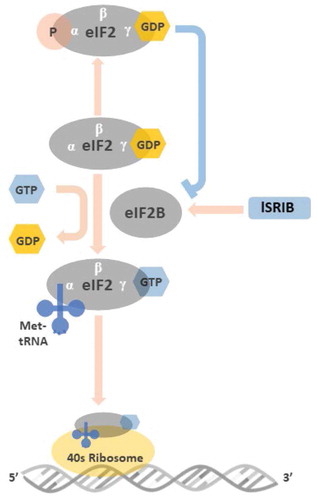
Under cellular stress, any of the above-mentioned kinases can phosphorylate Ser51 of eIF2α (peIF2α). This event causes the subunit to increase its affinity for the guanine nucleotide exchange factor, eIF2B.Citation46 Once bound peIF2α inhibits the ability of the eIF2B to exchange GDP for GTP resulting in a decrease in the available pool of free GTP-eIF2 and decreasing the rate of translational initiation.Citation33 The amount of eIF2B in the cell is significantly less than eIF2α so only a small increase in peIF2α can sequestrate all the available eIF2B and effectively shut down cap-dependent mRNA translation.Citation48,Citation49 This reduction of global protein synthesis via the 5ʹ cap-dependent mRNA translation serves a number of cytoprotective roles.Citation37 In conditions of amino acid shortageCitation50 or iron deficiency,Citation51 it reduces the rate at which these nutrients are consumed, during viral infection it slows viral replication by impeding viral protein synthesis.Citation52,Citation53 While in ER stress, its activation decreases the rate of proteins entering the ER, thereby relieving the overburdened organelle.Citation54
However, certain mRNAs that encode for proteins participating in cell responses to cellular stress are not affected or are indeed enhanced in the presence of eIF2α phosphorylation. Examples of these mRNAs include the ATF4,Citation55 C/EBP homologous protein (CHOP),Citation56 and growth arrest and DNA-damage 34 (GADD34).Citation57 This subset of mRNAs do not require 5ʹ cap recognition during translation, instead utilizing a re-initiation mechanism based on the direct recruitment of ribosomes to internal ribosome entry sites.Citation55–57 As demonstrates, ATF4 being the best-characterized effector of the ISR, is a transcription factor which has several dimerization partners that collaborate in the regulation of gene transcription and direct cellular outcomesCitation36 in amino acid transportation,Citation58 oxidative stress,Citation50 glucose metabolism,Citation59 autophagy,Citation60 angiogenesisCitation61 and protein homeostasis.Citation62
Timely termination of the ISR allows protein synthesis to recover once the ISR stimuli is relieved. As shows, at low-stressed conditions, the constitutive repressor of eIF2α -protein phosphatase 1 (CReP-PP1) complexCitation63 sustains a low level of peIF2α by removing phosphate group and restoring translational homeostasis. However, during chronic stress, ATF4 mediates activation of CHOP, which cooperates with ATF4 to induce growth arrest and GADD34 expression. GADD34 then couples with PP1 to terminate the ISR by dephosphorylation of peIF2α.Citation64 This feedback mechanism allows for protein synthesis to continue and contributes to increased ER stress and induction of cell apoptosis.Citation65 Therefore, the downstream execution of ATF4 can be cell apoptosis,Citation66 cell-cycle arrest,Citation67 and senescence.Citation68
In brief, acute activation of the ISR leads to temporary shutdown of the universal protein synthesis, whilst simultaneously activating pro-survival genes through ATF4 activation. However, in conditions such as chronic stress, prolonged activation of the ISR may activate CHOP or other pro-apoptotic genes and lead to cell death.Citation2
Pathophysiological association of ISR and ocular diseases
As summarizes, ISR activation by different stresses converge in the phosphorylation of eIF2α and the increase of ATF4. Therefore, alongside with the expression of ISR regulators (kinases), peIF2α and ATF4 are the common indicators of ISR activity in ocular disorders.Citation2 In addition, CHOP expression frequently suggests the pro-apoptotic state or cell death as the result of ISR activation in disease models.Citation65
Cornea and conjunctiva
Fuchs endothelial corneal dystrophy, a disorder of the corneal endothelium that is characterized by loss of endothelial cells and abnormalities of Descemet’s membrane, may result in progressive corneal edema.Citation69,Citation70 Enlargement of rough ER of endothelial cells has been demonstrated in Fuchs dystrophy specimens. Meanwhile, significantly higher peIF2α and CHOP expression were quantified in Fuchs dystrophy corneal endothelium as compare to the non-Fuchs dystrophy controls.Citation71 Okumura et al also reported elevated PERK activation and CHOP expression in cultivated human Fuchs endothelial cells as compared to its normal controls.Citation72 Both studies have shown that accumulation of unfolded proteins may induce corneal endothelial cell apoptosis.
Keratoconus, a multifactorial disease that is characterized by progressive thinning and weakening of the cornea, could lead to severe visual impairment in young adults.Citation73 Mass spectrometric analysis of keratoconic corneal proteins has suggested increased ER stress, oxidative stress, and apoptosis in the keratoconic stromal proteome.Citation74 We have reported elevated peIF2α and ATF4 in cultivated stromal keratocytes and corneal buttons from keratoconus patients as compared to those from normal controls.Citation29 Our findings indicate abnormal ISR activity may participate in keratoconus pathogenesis.
Another corneal disease known to be related to abnormal ISR is granular corneal dystrophy type 2 (GCD2), which is caused by the mutation in the transforming growth factor β-induced (TGFBI) gene. Diseased GCD2 corneal fibroblasts have been shown to accumulate mutant TGFBI-encoded protein (TGFBIp).Citation75 Choi et al has also demonstrated a higher level of PERK activities in GCD2 corneal fibroblasts, and GCD2 fibroblasts were more susceptible to ER-stress induced cell death than were the normal controls.Citation76
erpes simplex virus type 1 (HSV-1) is an alphaherpesvirus that is recognized as the most common cause of corneal blindness in developed countries.Citation77 Ocular involvement can present as primary infection or recurrence from latent disease.Citation78 HSV-1 is known to be able to disarm the ISR of host cells in their early stage of infection.Citation79 Us11, a HSV-1 protein, can bind dsRNA and block PKR activation.Citation80 HSV-1 also expresses an ortholog of GADD34 called γ34.5, that results in eIF2α dephosphorylation to ensure protein synthesis and offset the activation of PKR and PERK during viral infection.Citation81 By maintaining a pool of unphosphorylated eIF2α, HSV-1 avoids antiviral cellular translational arrest, meanwhile, it prevents potentially hurtful downstream ISR transcription from ATF4 and CHOP, and the induction of autophagy.Citation24 The ability of countering antiviral response of infected cells promotes HSV-1 neuroinvasion and periocular replication following corneal infection.Citation82
ER stress was also known to involve in the pathogenesis of chronic inflammatory and autoimmune diseases, including dry eye. Pflugfelder’s group has demonstrated interferon-γ (IFN-γ) could induce unfolded protein response and the expression of ATF4, and thus resulted decreased mucin synthesis and reduced goblet cell proliferation in the cultivated conjunctival goblet cell harvested from C57BL mice. Interestingly, the conjunctival goblet cells cultivated from IFN-γ knockout or IFN-γ receptor knockout mice had increased proliferation as compared to that from the wild type. In other words, inflammatory-related ER stress participates in the mucin-deficiency dry eye via modulating the mucin production and goblet cell proliferation.
Lens
Lens epithelial cells coordinate the development of the entire ocular lens. During cellular maturation, most lens fiber cells lose their nuclei and mitochondria.Citation83 However, the anterior epithelial cells remain mitotically active as a stem cell niche producing secondary fiber cells.Citation84 The ISR is highly associated with lens development and degeneration as the absence of secondary lens fibers and severe microphthalmiaCitation30 have been shown in ATF4 deficient mice. In addition, interruption of lens autophagy could lead to the loss of lens differentiation and the failure of lens resistance to stresses, resulting in cataract formation.Citation85,Citation86 Cataract, which has long been the leading cause of blindness globally,Citation87 can be induced by senility and physical and chemical stresses.Citation88 A large variety of ER stressors were found to induce the production of reactive oxygen species in cultivated lens epithelium with or without the induction of cell apoptosis.Citation89 Increased level of ATF4 and CHOP were documented in human-aged lenses.Citation89 The activation of ER stress pathways were also reported in high-myopia-related cataractCitation90 and congenital cataract.Citation27 Accordingly, ATF4 related pathways is key for the evolution of normal lenses. The ER stress and its consequences may be one of the initiation factors of many types of cataracts.Citation27,Citation91
Retina and glaucoma
Accumulation of unfolded protein activates the PERK pathway and its related pro-apoptotic circuits, which are implicated in the pathogenesis of many diseases involving different layers of the retina and optic nerve.Citation20 Diabetic retinopathy (DR), a leading cause of visual loss in the working-age population, has long been recognized as a microvascular disease.Citation92Citation93 The pathologies in DR include pericyte loss and vascular endothelial cell apoptosis in responses to hyperglycemia.Citation94 Zhang et al have proved intermittent high glucose can induce ER stress in human retinal pericytes, and is related to the inflammatory responses in DR mouse models.Citation26,Citation95 Elevated ATF4 was further demonstrated in the aqueous humor and vitreous of proliferative diabetic retinopathy patients by Wang et al. In contrast to DR, age-related macular degeneration (AMD) is the top cause of blindness among the aged group in the developed world.Citation96 AMD is characterized by dysfunction of retinal pigment epithelium (RPE) cells and their over expression of vascular endothelial growth factor (VEGF).Citation21,Citation97 Roybal et al reported oxidative stress activates the secretion of VEGF in human RPE cells by an ATF4-dependent mechanism. The ATF4 complex binds to the VEGF gene and transactivates its expression.Citation98 Therefore, activation of the ISR under oxidative stress may be one of the contributing factors to neovascularization in retinal diseases.Citation99 Alongside DR and AMD, another key contributor to global visual impairment is glaucoma, a group of optic neuropathies characterized by progressive loss of retinal ganglion cells.Citation100 ER stress was documented to play a major role in the pathogenesis of myocilin mutation associated glaucoma and glucocorticoid-induced ocular hypertension in mouse models.Citation25,Citation101 Trabecular meshwork tissue lysate form glaucoma patients also demonstrated increased ER stress markers including ATF4 and CHOP as compared to those age-matched normal controls.Citation102
In summary, the ISR aids in cellular adaptation and defense in the eye. Inactivation of the ISR by viral proteins allows viral replication and tissue destruction. Moreover, the eye is consistently under oxidative and metabolic stresses, chronic stimulation of the ISR may lead to cell death, and ISR stimulation is observed in both anterior and posterior segment ocular disorders.
Modulators of ISR and their possible therapeutic applications
Many toxins or drugs induce extensive ISR,Citation103 including arsenic,Citation104 tunicamycin,Citation105 thapsigargin,Citation106 and mitomycin C.Citation107 Environmental arsenic contamination in drinking water has been a global health issue that was associated with many cancers.Citation108 Arsenic generates oxidative stress and mediates ER and mitochondrial cross-talks that results in apoptosis via ATF4 regulated pathways.Citation104 Arsenic-induced-VEGF production in retinal-pigmented epithelium via eIF2α-ATF4 branch has suggested non-hypoxic stresses also contributed to VEGF expression.Citation98 Tunicamycin, an antibiotic mixture produced by Streptomyces lysosuperificus, has antimicrobial activity against bacteria, fungi, and viruses. Tunicamycin not only impairs protein glycosylation, but also depletes the calcium in ER which further aggravates unfolded proteins stress.Citation105 Tunicamycin has not been used as human medicineCitation103 due to its toxicity, but has been broadly applied as an ER stressor in studying various pathological and physiological processes of diabetes and asthma.Citation109,Citation110 Thapsigargin is a highly potent drug isolated from the plant Thapsia garganica L (Linnaeus),Citation106 its cytotoxicity is derived from its ability to inhibit calcium transport leading to calcium depletion in ER. Therefore, in addition to ER stress-related cell death, thapsigargin induces concomitant increase in free cytosolic calcium is also a potent pro-apoptotic signal in cells.Citation106 Mitomycin C, is a reactive oxygen species (ROS)-generating anticancer drug. Mitomycin C can induce human fibroblast apoptosis via PERK pathway,Citation107 and its anti-fibrotic effect has been applied in many ophthalmic surgeries such as pterygium excisionCitation111 and glaucoma filtering surgery.Citation112
Other than these general ISR inducers, there are several approaches for therapeutic activation or inhibition of the ISR by modulation of specific targets: (1) counteracting the effect of peIF2α; (2) activation or inhibition of the eIF2α kinases; (3) inhibition of the eIF2α phosphatase; (4) post-translational modification of ATF4; (5) altering the ATF4 downstream pathways. Here we elaborate the most studied small molecules targeting ISR pathways, especially the highly potent ISR inhibitor, ISRIB. We also summarize the molecules that have been applied in the ophthalmic field (). Detailed review of small molecules modulating the ISR network are listed., Citation2,Citation33–35,Citation113
Figure 3. Targeting the druggable ISR pathways Modulation of ISR can be achieved by: (1) Targeting the eIF2α kinases: the GCN2 activators include Indirubin-3ʹ monoxime, Sp600125, Sky inhibitor; the GCN2 inhibitors consist of halofuginone, histidinol, asparaginase, arginine deiminase. The HRI activator BtdCPU and the inhibitor, amino-pyrazolindine. The PKR activators are BEPP and Poly (I:C). The PKR inhibitors are C16 and 2-aminopurine. The selective PERK activator is CCT020312 and the inhibitors are GSK2606414 and its more selective derivative GSK2656157. (2) Targeting the peIF2α phosphatase: Salubrinal and SAL003 inhibit both the inducible phosphatase GADD34 and the constitutive expressed CReP. In addition, Sephin 1 and Guanabenz inactivate GADD34, while Nelfinavir inactivates CReP. (3) Antagonist to the peIF2α: as shows, peIF2α inhibits the eIF2B. ISRIB is hypothesized to stabilize eIF2B and offsets the peIF2α effects while the peIF2α concentration is low. (4) Inhibition of ATF4 post-translational phosphorylation: RSK2 and PKA are two kinases that phosphorylate ATF4 and increase its activity. The RSK2 inhibitors, SL0101, BI-D1870, fmk and the PKA inhibitors, H-89, KT 5720, Rp-8-Br-cAMPS are potential compounds that inhibit ATF4 phosphorylation. (5) Targeting pathways downstream of ATF4: among the cellular functions regulated by ATF4, angiogenesis, autophagy and ERAD played important roles in ocular homeostasis and pathogenesis such as dry eye, diabetic retinopathy, age-related macular degeneration. Bortezomib, a proteasome inhibitor impedes ERAD has been demonstrated with neuro-protective and anti-inflammatory effects in vivo.
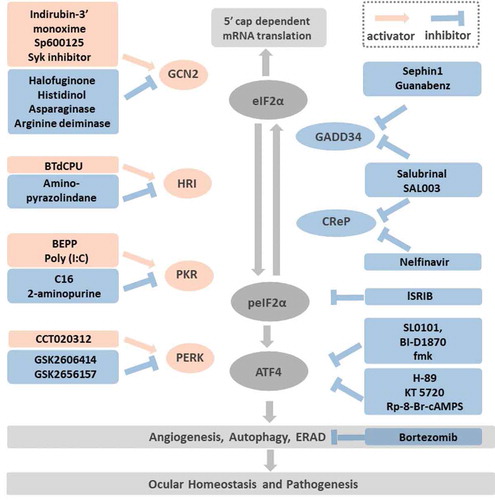
Counteract the effect of peIF2α: eIF2B activator
As illustrated, eIF2B catalyzes the GDP-GTP exchange on eIF2. While the eIF2α is phosphorylated, it transforms from a substrate into potent inhibitor of eIF2B and reduces its catalyzing ability.Citation1,Citation46 A small-molecule ISR inhibitor, ISRIB, discovered by Walter’s group, binds in the pocket formed at the junction of eIF2B β- and δ-subunits, driving the subunits to fully assemble the eIF2B decamer complex with GDP exchange ability.Citation114–116 ISRIB, therefore, rescues protein translation by stabilizing and increasing eIF2B complex abundance and counteracting low levels of peIF2α in treated cells.Citation116,Citation117 However, when the concentration of peIF2 exceeds a certain threshold-that is, when the ISR is strongly activated, more eIF2B are trapped by peIF2, and ISRIB can no longer restore the drained pool of eIF2B complex. Thus, ISRIB does not abolish the ISR’s cytoprotective effects in cells in which the ISR is strongly activated.Citation1 ISRIB has good potency (nM) and brain penetrance, and optimization of ISRIB has led to the discovery of analogs with picomolar activity.Citation117,Citation118 In vitro, ISRIB treatment partially restores the cellular protein translation rates in cultivated neuronal model of amyotrophic lateral sclerosis.Citation119 In vivo, ISRIB partially recovers the cellular protein translation rates in prion-infected mice.Citation120 ISRIB also reverses some aspects of cognitive deficit in traumatic brain injury miceCitation121 and Down syndrome mice.Citation122 In the ophthalmic field, our group was the first to report that activation of the ISR can recapitulate the keratoconus phenotype in normal corneal keratocytes in vitro. Conversely, by blocking the ISR with ISRIB, the production of matrix metalloprotease (MMP)-9 is reduced and the synthesis of collagen in keratoconic fibroblasts increases in vitro, relieving many of the hallmarks of keratoconus.Citation123 Therefore, targeting of the ISR through small molecules may be a promising therapeutic path for ophthalmic disease.
Target the eIF2α kinases
Activators of eIF2α kinases
Pharmacological activation of ISR signaling can be achieved by activating eIF2α kinases. Halofuginone,Citation124 histidinol,Citation116 asparaginase,Citation125 and arginine deiminaseCitation126 are known to be GCN2 activators; BTdCPU activates HRICitation127; BEPP and poly (I:C) are PKR activatorsCitation128,Citation129; and CCT020312Citation130,Citation131 is a selective PERK activator. These potential drugs were mostly studied for cancer treatment, and the application in ophthalmology fields is just emerging.Citation33,Citation35 In vivo, oral administrated or intraperitoneal injected halofuginone has shown potent inhibitory effects on angiogenesis progression in a mouse corneal neovascularization model.Citation132 Arginine deiminase catalyzes the deimination of proteins, a nonreversible post-translational conversion of protein-bound arginines to protein-bound citrullines. Elevated levels of protein deimination are reported in human neurological diseases,Citation133 the retina tissue of AMD patients,Citation134 and the optic nerve of open-angle glaucoma eyes.Citation135 These findings suggest GCN2 may be one of the treatment targets of AMD and glaucoma. Poly (I:C), polyinosinic:poycytidylic acid, has similar structure to the double-stranded RNA which leads to PKR activation.Citation129 In vivo, topical and systemic administrated Poly (I:C) has been known for decades to induce tear interferon against HSV in rabbit and human eyes.Citation136,Citation137
Inhibitors of eIF2α kinases
Pharmacological inhibition of the ISR can be achieved by inhibition of eIF2α kinases. Three structurally similar compounds indirubin-3ʹ-monoxamie, SP600125 and a SyK inhibitor inactivate GCN2Citation138; amino-pyrazolindine inhibits HRICitation139; C16 and 2-aminopurine are frequently used for inhibition of PKRCitation140,Citation141; and GSK260614 and its analogue GSK2656157 inactivate PERK.Citation142,Citation143 However, the application of eIF2α kinase inhibitors in ophthalmic researches is scarce. In vitro, GCN2 inhibitors, Indirubin-3ʹ-monoxamie, SP600125, and SyK inhibitor decrease the phosphorylation of eIF2α in mouse embryonic fibroblast cells after UV irradiation.Citation138 Yet, these compounds are poorly specific for GCN2, and additional studies on structure-activity relationship are essential to increase their specificity and potency in treating eye diseases. In vitro, Jiang et al has reported GSK2606414, the highly selective PERK inhibitor, suppressed RPE cell proliferation in a dose-dependent manner. Meanwhile, GSK2606414 treatment reduced the level of peIF2α, CHOP, and VEGF mRNA expression in RPE cells under ER stress.Citation144
Target eIF2α phosphatase
Dephosphorylation of eIF2α is the key step of ISR termination and restoration of protein synthesis for normal cellular functions. As shown in , in mammals, two phosphatases are responsible for the dephosphorylation of peIF2α, the CRePCitation63 and GADD34.Citation145 As the name suggested, CReP normally operates in unstressed cells for maintaining low level of peIF2α. While GADD34 expression is induced by ATF4 and CHOP, and acts as a negative feedback loop to resume protein synthesis once the stress is relieved.Citation64 Salubrinal and its analogue SAL003, are inhibitors of both GADD34 and CReP.Citation123,Citation146 Guanabenz and its derivative, Sephin 1 are known to inhibit GADD34.Citation146 Nelfinavir, a HIV protease inhibitor downregulates CReP and increases peIF2α.Citation147 Among these peIF2α phosphatase inhibitors, salubrinal has been shown to result in high levels of peIF2α. It has also been demonstrated that salubrinal blocked the replication of HSV in cell culture models.Citation148,Citation149 In vitro, Salubrinal treatment protects trabecular meshwork cells against ER stressCitation150 and RPE cells against toxins.Citation151,Citation152 However, we found SAL003 stimulates the ISR and leads to apoptosis of normal fibroblasts cultivated from donor corneas.Citation123
Post-translational modification of ATF4
There are no reported molecules that can directly bind and inhibit ATF4. Transcription factors are largely poor drug targets due to the inefficiency of small molecules to halt protein-DNA and protein-protein binding interfaces.Citation153 However, ATF4 function is dependent on extensive post-translational modifications, particularly phosphorylation, that modulates its transcriptional activity and degradation. Ribosomal S6 kinase (RSK2) and protein kinase A (PKA) are two kinases that phosphorylate ATF4 and enhance its transcriptional activity.Citation154,Citation155 Reversible RSK2 inhibitors SL0101 and BI-D1870, and the irreversible inhibitor fmk have been developed.Citation156–158 PKA inhibitors H-89 and KT 5720 are also available yet with low selectivity.Citation159 Recently, another PKA antagonist Rp-8-Br-cAMPS with better selectivity has been developed.Citation160 However, RSK2 and PKA both work on multiple protein responses for different cellular functions,Citation161,Citation162 and the use of these compounds as ATF4 inhibitors will need careful validation to support that the observed effects can be contributed to ATF4 inhibition. As yet, the application of ATF4 inhibitor in treating eye disease has not been reported.
Target pathways downstream of ATF4
As demonstrate, ATF4 transcriptionally regulates genes involved in different cell functioning.Citation33 Disruption of the pathways downstream of ATF4 is also a valid approach to interfere the effects of ISR. Insufficient vascular supply results in hypoxia, nutrient deprivation, lower ATP production and protein misfolding. These factors lead to ATF4 expression in ischemic tissue,Citation163 and drive the expression of VEGFCitation22 and pro-angiogenesis cytokine, interleukin (IL)-8.Citation164 Targeting VEGF or pro-angiogenesis interleukins has long been the promising treatment for ocular vascular neogenesis disorders.Citation165 Another potential therapeutic target is autophagy, the catabolic process responsible for lysosome-dependent degradation and recycling.Citation166 The expression of ATF4 and CHOP upregulate autophagy genes,Citation167 and dysregulated autophagy disturbs cellular homeostasis and has been linked to many ocular disorders, including dry eye, corneal dystrophy, keratoconus, cataract, glaucoma, and AMD.Citation85,Citation168 The other key mechanism for maintaining cellular homeostasis is the ER-associated protein degradation (ERAD), which through the accumulation of unfolded proteins activates the PERK-ATF4 pathway.Citation169 Bortezomib is a specific inhibitor of the proteasome that impedes ERADCitation170 which has shown neuro-protective effects in retinal ischemia-reperfusion injury in ratCitation171 and anti-inflammatory effects in experimental autoimmune uveitis (EAU) mice after intraperitoneal injection.Citation171,Citation172
Animal models in ISR research
Investigations of the ISR is an emerging field based on its established role in assorted human pathologiesCitation2 and because the pathway is considered a “druggable target”. Nonetheless, most mechanistic studies have been implemented in cell culture systems exposed to the stressors. In , we summarize relevant studies in animal models that have revealed novel and specialized functions of the ISR in distinct organisms in vivo.Citation30,Citation51,Citation59,Citation145,Citation172–189 The most profound of which is the ATF4 knockout mouse which presented with lens anomaly and microphthalmia.Citation30
Table 1. Consequence of the genetic manipulation of ISR components in mice
ATF4 knock out mice
ATF4 is a member of the cAMP-responsive element-binding protein (CREB) family and has multiple roles in cell differentiation and organ morphogenesis.Citation30,Citation173,Citation190 ATF4 heterozygous mice are viable, yet only 30% of ATF4 knockout (CREB−/-) mice survive to maturity.Citation174 ATF4 deficiency leads to mutations in eye-lens development and hair-growth, accompanied by pancreatic hypoplasia skeletal defects, and growth retardation.Citation30,Citation59,Citation173–175 Tanaka et al generated ATF4−/- mice and noted severe microphthalmia and lens degeneration due to cell apoptosis without the formation of lens secondary fiber cells. Rescue of the ATF4 mutant phenotype by lens-specific gene expression not only recovered its lens secondary fibers but also induced hyperplasia of these fibers.Citation30 Hettmann et al have also demonstrated microphthalmia due to absence of lens on ATF4−/- mice.Citation174 They noted the defect appeared to be specific for the lens, as no gross anomalies in the retina or cornea could be detected. Interestingly, the ATF4−/- and p53−/- double-homozygous mutant mice resulted in a marked suppression of microphthalmia, bringing together the DNA damage response, and the ISR in development. The lens developed in the double mutant model; however, it contained a lower number of fibers as compare to that of the wild type. Therefore, the authors proposed that there may be both p53-dependent and p53-independent effect of ATF4 deficiency participating in the differentiation and survival of lens fiber cells.Citation174 Overall, these animal studies support the functions of ATF4 in modulating cell cycle, apoptosis, and metabolism in diverse tissues.
EIF2α kinase gene knockout mice
In the 1990s, the physiologic functions of eIF2α kinases were elaborated. Kinase gene knockout mice were bred for development and phenotype observationCitation51 as listed in . Interestingly, Gcn2−/- mice have no observable phenotype mutation in a non-stressed status but present with developmental delay while being fed with leucine deficit diet.Citation176 HRI−/- mice are viable, fertile, and without gross abnormalities. Mild macrocytosis and hyperchromia were observed in the HRI−/- mice in the absence of stress that indicates the physiological adaptation of red blood cells to iron deficiency.Citation51 Pkr−/- mice have normal development and similar response to vaccine and influenza infection, which indicate the interruption of PKR is not sufficient to eliminate eIF2α phosphorylation and other eIF2α kinase family members may compensate for loss of PKR functions.Citation177 Perk−/- mice are viable, and the phenotypes involve strong changes of pancreatic β-cell secretory function and its survival. Therefore, Perk knockout animals have impaired glucose metabolism and develop early diabetes and growth retardation.Citation178,Citation179 Perk−/- mice also exhibit severe osteopenia because PERK signaling is also important for the differentiation of osteoblasts.Citation179–181 PERK signaling has divergent roles in neural tissues and cognitive function. Conditional disruption of PERK in the adult mouse forebrain was associated with altered behaviors.Citation183
EIF2α phosphatase gene knockout mice
Two somatic genes, Ppp1r15a and Ppp1r15b, encode the proteins GADD34Citation64 and CReP,Citation191 that recruit PP1 to form complex phosphatase to dephosphorylate peIF2α. The Ppp1r15a−/- mice have a normal phenotype; however, the ER stress response in their embryonic fibroblasts are different as compare to that of the wild type.Citation184 In contrast, the Ppp1r15b−/- newborns exhibit severe growth retardation, impaired erythropoiesis, and none survives the first day of postnatal life.Citation184
eIF2α gene knock in mice
In all eukaryotic cells, eIF2α is part of a major assemblage in the initiation of protein translation. The phosphorylation at serine 51 interrupts normal function of eIF2α and causes global reduction of protein synthesis except ATF4 and CHOP.Citation192 A knock in mouse model that has been developed by substitution serine 51 for alanine, creating a non-phosphorylatable eIF2α. Homozygous eIF2αS51A mutant mice die within 24 hours of birth due to impaired gluconeogenesis and hypoglycemia.Citation185 Heterogenous eIF2αS51A mutant mice develop normally into adulthood. Nevertheless, theses mice become obese and glucose-intolerant when fed a high-fat diet.Citation186 Conditional destruction of eIF2α in pancreatic β-cell led to severe diabetic mice.Citation187
CHOP knock out mice
CHOP is a well-characterized downstream target of the eIF2α-ATF4 signaling branch. Deletion of Chop in mice does not alter animal survival and development. The literature using CHOP-deficient animals for disease models are broad, and here we feature a few studies. CHOP knockout mice have reduced cell apoptosis in lung and kidney induced by ER stressors.Citation193 In the nervous system, exceptionally, CHOP knockout mice showed increased apoptosis in the hippocampal cells and decreased performance in memory-related behaviors.Citation188 However, CHOP deficiency provides significant neuroprotection in the context of brain ischemia.Citation194 Taken together, these selected studies suggest, at least in neural models, CHOP may play a protective role or enhance apoptosis depending on the disease context.
Conclusion and perspectives
The ISR widely regulates cell survival and death via peIF2-ATF4 signaling and its downstream execution. The ISR contributes to many ocular diseases such as keratoconus, corneal dystrophy, HSV keratitis, cataract, DR, AMD, and glaucoma. The use of small molecules to counteract the peIF2α effects or target the eIF2α kinases/phosphatase is promising for treating ISR-related ocular disorders. However, drug selectivity and specificity need to be cautiously inspected. In vivo models such as gene knockout or knock-in mice are invaluable for studying ISR consequences in embryogenesis, development, and phenotypes. Together with ATF4 knockout mice that present with retarded lens development, future expansion of ocular ISR-related disease models are mandatory for exploring potential therapeutic agents.
Declaration of interest
The authors declare there is no conflict of interest.
Supplemental Material
Download PDF (159.6 KB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Costa-Mattioli M, Walter P. The integrated stress response: from mechanism to disease. Science. 2020;368:6489. doi:10.1126/science.aat5314.
- Pakos-Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, Gorman AM. The integrated stress response. EMBO Rep. 2016;17(10):1374–95. doi:10.15252/embr.201642195.
- Seo J, Fortuno ES 3rd, Suh JM, Stenesen D, Tang W, Parks EJ, Adams CM, Townes T, Graff JM. Atf4 regulates obesity, glucose homeostasis, and energy expenditure. Diabetes. 2009;58(11):2565–73. doi:10.2337/db09-0335.
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6(5):1099–108. doi:10.1016/s1097-2765(00)00108-8.
- Ye J, Kumanova M, Hart LS, Sloane K, Zhang H, De Panis DN, Bobrovnikova-Marjon E, Diehl JA, Ron D, Koumenis C. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. Embo J. 2010;29(12):2082–96. doi:10.1038/emboj.2010.81.
- Kilberg MS, Shan J, Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol Metab. 2009;20(9):436–43. doi:10.1016/j.tem.2009.05.008.
- B’Chir W, Maurin AC, Carraro V, Averous J, Jousse C, Muranishi Y, Parry L, Stepien G, Fafournoux P, Bruhat A. The eIF2alpha/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013;41(16):7683–99. doi:10.1093/nar/gkt563.
- Chen JJ, Throop MS, Gehrke L, Kuo I, Pal JK, Brodsky M, London IM. Cloning of the cDNA of the heme-regulated eukaryotic initiation factor 2 alpha (eIF-2 alpha) kinase of rabbit reticulocytes: homology to yeast GCN2 protein kinase and human double-stranded-RNA-dependent eIF-2 alpha kinase. Proc Natl Acad Sci U S A. 1991;88(17):7729–33. doi:10.1073/pnas.88.17.7729.
- Clemens MJ, Elia A. The double-stranded RNA-dependent protein kinase PKR: structure and function. J Interferon Cytokine Res. 1997;17(9):503–24. doi:10.1089/jir.1997.17.503.
- Feldman DE, Chauhan V, Koong AC. The unfolded protein response: a novel component of the hypoxic stress response in tumors. Mol Cancer Res. 2005;3(11):597–605. doi:10.1158/1541-7786.Mcr-05-0221.
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397(6716):271–74. doi:10.1038/16729.
- Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D, Kaufman RJ. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4(11):e374. doi:10.1371/journal.pbio.0040374.
- Bond S, Lopez-Lloreda C, Gannon PJ, Akay-Espinoza C, Jordan-Sciutto KL. The integrated stress response and phosphorylated eukaryotic initiation factor 2α in neurodegeneration. J Neuropathol Exp Neurol. 2020;79:123–43. doi:10.1093/jnen/nlz129.
- Ariyasu D, Yoshida H, Hasegawa Y. Endoplasmic reticulum (ER) stress and endocrine disorders. Int J Mol Sci. 2017;18(2):382. doi:10.3390/ijms18020382.
- McConkey DJ. The integrated stress response and proteotoxicity in cancer therapy. Biochem Biophys Res Commun. 2017;482(3):450–53. doi:10.1016/j.bbrc.2016.11.047.
- Oakes SA. Endoplasmic reticulum proteostasis: a key checkpoint in cancer. Am J Physiol Cell Physiol. 2017;312(2):C93–c102. doi:10.1152/ajpcell.00266.2016.
- So JS. Roles of endoplasmic reticulum stress in immune responses. Mol Cells. 2018;41(8):705–16. doi:10.14348/molcells.2018.0241.
- Rodrigues L, Graca RSF, Carneiro LAM. Integrated stress responses to bacterial pathogenesis patterns. Front Immunol. 2018;9:1306. doi:10.3389/fimmu.2018.01306.
- Bashir H, Seykora JT, Lee V. Invisible Shield: review of the corneal epithelium as a barrier to uv radiation, pathogens, and other environmental stimuli. J Ophthalmic Vis Res. 2017;12(3):305–11. doi:10.4103/jovr.jovr_114_17.
- Kroeger H, Chiang W-C, Felden J, Nguyen A, Lin JH. ER stress and unfolded protein response in ocular health and disease. Febs J. 2019;286(2):399–412. doi:10.1111/febs.14522.
- Salminen A, Kauppinen A, Hyttinen JM, Toropainen E, Kaarniranta K. Endoplasmic reticulum stress in age-related macular degeneration: trigger for neovascularization. Mol Med. 2010;16(11–12):535–42. doi:10.2119/molmed.2010.00070.
- Ghosh R, Lipson KL, Sargent KE, Mercurio AM, Hunt JS, Ron D, Urano F, Blagosklonny MV. Transcriptional regulation of VEGF-A by the unfolded protein response pathway. PLoS ONE. 2010;5(3):e9575. doi:10.1371/journal.pone.0009575.
- Forrester J, Xu H. Good news–bad news: the Yin and Yang of immune privilege in the eye. Front Immunol. 2012;3(338). doi:10.3389/fimmu.2012.00338.
- Johnston BP, McCormick C. Herpesviruses and the unfolded protein response. Viruses. 2019;12(1):17. doi:10.3390/v12010017.
- Zode GS, Sharma AB, Lin X, Searby CC, Bugge K, Kim GH, Clark AF, Sheffield VC. Ocular-specific ER stress reduction rescues glaucoma in murine glucocorticoid-induced glaucoma. J Clin Invest. 2014;124(5):1956–65. doi:10.1172/JCI69774.
- Li J, Wang JJ, Yu Q, Wang M, Zhang SX. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett. 2009;583(9):1521–27. doi:10.1016/j.febslet.2009.04.007.
- Yang J, Zhou S, Gu J, Wang Y, Guo M, Liu Y, Pan C-W. Differences in unfolded protein response pathway activation in the lenses of three types of cataracts. PLoS One. 2015;10(6):e0130705. doi:10.1371/journal.pone.0130705.
- Coursey TG, Tukler Henriksson J, Barbosa FL, De Paiva CS, Pflugfelder SC. Interferon-γ–induced unfolded protein response in conjunctival goblet cells as a cause of mucin deficiency in sjögren syndrome. Am J Pathol. 2016;186(6):1547–58. doi:10.1016/j.ajpath.2016.02.004.
- Foster JW, Shinde V, Soiberman US, Sathe G, Liu S, Wan J, Qian J, Dauoud Y, Pandey A, Jun AS, et al. Integrated stress response and decreased ECM in cultured stromal cells from keratoconus corneas. Invest Ophthalmol Vis Sci. 2018;59(7):2977–86. doi:10.1167/iovs.18-24367.
- Tanaka T, Tsujimura T, Takeda K, Sugihara A, Maekawa A, Terada N, Yoshida N, Akira S. Targeted disruption of ATF4 discloses its essential role in the formation of eye lens fibres. Genes Cells. 1998;3(12):801–10. doi:10.1046/j.1365-2443.1998.00230.x.
- Starr CR, Gorbatyuk MS. Delineating the role of eIF2α in retinal degeneration. Cell Death Dis. 2019;10(6):409. doi:10.1038/s41419-019-1641-y.
- Bhootada Y, Kotla P, Zolotukhin S, Gorbatyuk O, Bebok Z, Athar M, Gorbatyuk M, Boulton ME. Limited ATF4 expression in degenerating retinas with ongoing ER stress promotes photoreceptor survival in a mouse model of autosomal dominant retinitis pigmentosa. PLoS One. 2016;11(5):e0154779. doi:10.1371/journal.pone.0154779.
- Singleton DC, Harris AL. Targeting the ATF4 pathway in cancer therapy. Expert Opin Ther Targets. 2012;16(12):1189–202. doi:10.1517/14728222.2012.728207.
- Hetz C, Chevet E, Harding HP. Targeting the unfolded protein response in disease. Nat Rev Drug Discov. 2013;12(9):703–19. doi:10.1038/nrd3976.
- Joshi M, Kulkarni A, Pal JK. Small molecule modulators of eukaryotic initiation factor 2α kinases, the key regulators of protein synthesis. Biochimie. 2013;95(11):1980–90. doi:10.1016/j.biochi.2013.07.030.
- Ameri K, Harris AL. Activating transcription factor 4. Int J Biochem Cell Biol. 2008;40(1):14–21. doi:10.1016/j.biocel.2007.01.020.
- Donnelly N, Gorman AM, Gupta S, Samali A. The eIF2alpha kinases: their structures and functions. Cell Mol Life Sci. 2013;70(19):3493–511. doi:10.1007/s00018-012-1252-6.
- Dever TE, Feng L, Wek RC, Cigan AM, Donahue TF, Hinnebusch AG. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell. 1992;68(3):585–96. doi:10.1016/0092-8674(92)90193-g.
- Kostura M, Mathews MB. Purification and activation of the double-stranded RNA-dependent eIF-2 kinase DAI. Mol Cell Biol. 1989;9(4):1576–86. doi:10.1128/mcb.9.4.1576.
- Meurs E, Chong K, Galabru J, Thomas NS, Kerr IM, Williams BR, Hovanessian AG. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990;62(2):379–90. doi:10.1016/0092-8674(90)90374-n.
- Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol. 1998;18(12):7499–509. doi:10.1128/mcb.18.12.7499.
- Masson GR. Towards a model of GCN2 activation. Biochem Soc Trans. 2019;47(5):1481–88. doi:10.1042/bst20190331.
- Chen -J-J. Translational control by heme-regulated eIF2α kinase during erythropoiesis. Curr Opin Hematol. 2014;21(3):172–78. doi:10.1097/moh.0000000000000030.
- Korennykh A, Walter P. Structural basis of the unfolded protein response. Annu Rev Cell Dev Biol. 2012;28:251–77. doi:10.1146/annurev-cellbio-101011-155826.
- Hinnebusch AG. Molecular mechanism of scanning and start codon selection in eukaryotes. Microbiol Mol Biol Rev. 2011;75(3):434–67. first page of table of contents. doi:10.1128/mmbr.00008-11.
- Kimball SR. Eukaryotic initiation factor eIF2. Int J Biochem Cell Biol. 1999;31(1):25–29. doi:10.1016/s1357-2725(98)00128-9.
- Proud CG. eIF2 and the control of cell physiology. Semin Cell Dev Biol. 2005;16(1):3–12. doi:10.1016/j.semcdb.2004.11.004.
- Rowlands AG, Panniers R, Henshaw EC. The catalytic mechanism of guanine nucleotide exchange factor action and competitive inhibition by phosphorylated eukaryotic initiation factor 2. J Biol Chem. 1988;263:5526–33.
- Rabouw HH, Visser LJ, Passchier TC, Langereis MA, Liu F, Giansanti P, van Vliet ALW, Dekker JG, van der Grein SG, Saucedo JG, et al. Inhibition of the integrated stress response by viral proteins that block p-eIF2–eIF2B association. Nat Microbiol. 2020;5(11):1361–73. doi:10.1038/s41564-020-0759-0.
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11(3):619–33. doi:10.1016/s1097-2765(03)00105-9.
- Han AP. Heme-regulated eIF2alpha kinase (HRI) is required for translational regulation and survival of erythroid precursors in iron deficiency. Embo J. 2001;20(23):6909–18. doi:10.1093/emboj/20.23.6909.
- Balachandran S, Roberts PC, Brown LE, Truong H, Pattnaik AK, Archer DR, Barber GN. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity. 2000;13(1):129–41. doi:10.1016/s1074-7613(00)00014-5.
- Garcia MA, Meurs EF, Esteban M. The dsRNA protein kinase PKR: virus and cell control. Biochimie. 2007;89(6–7):799–811. doi:10.1016/j.biochi.2007.03.001.
- Ron D. Translational control in the endoplasmic reticulum stress response. J Clin Invest. 2002;110(10):1383–88. doi:10.1172/JCI16784.
- Chan CP, Kok KH, Tang HM, Wong CM, Jin DY. Internal ribosome entry site-mediated translational regulation of ATF4 splice variant in mammalian unfolded protein response. Biochim Biophys Acta. 2013;1833(10):2165–75. doi:10.1016/j.bbamcr.2013.05.002.
- Palam LR, Baird TD, Wek RC. Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J Biol Chem. 2011;286(13):10939–49. doi:10.1074/jbc.M110.216093.
- Lee YY, Cevallos RC, Jan E. An upstream open reading frame regulates translation of GADD34 during cellular stresses that induce eIF2alpha phosphorylation. J Biol Chem. 2009;284(11):6661–73. doi:10.1074/jbc.M806735200.
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11(3):619–33. doi:10.1016/s1097-2765(03)00105-9.
- Yoshizawa T, Hinoi E, Jung DY, Kajimura D, Ferron M, Seo J, Graff JM, Kim JK, Karsenty G. The transcription factor ATF4 regulates glucose metabolism in mice through its expression in osteoblasts. J Clin Invest. 2009;119(9):2807–17. doi:10.1172/jci39366.
- Bretin A, Carriere J, Dalmasso G, Bergougnoux A, B’Chir W, Maurin AC, Muller S, Seibold F, Barnich N, Bruhat A, et al. Activation of the EIF2AK4-EIF2A/eIF2alpha-ATF4 pathway triggers autophagy response to Crohn disease-associated adherent-invasive Escherichia coli infection. Autophagy. 2016;12(5):770–83. doi:10.1080/15548627.2016.1156823.
- Wang Y, Ning Y, Alam GN, Jankowski BM, Dong Z, Nor JE, Polverini PJ. Amino acid deprivation promotes tumor angiogenesis through the GCN2/ATF4 pathway. Neoplasia. 2013;15(8):989–97. doi:10.1593/neo.13262.
- Wortel IMN, Van Der Meer LT, Kilberg MS, Van Leeuwen FN. Surviving stress: modulation of ATF4-mediated stress responses in normal and malignant cells. Trends Endocrinol Metab. 2017;28(11):794–806. doi:10.1016/j.tem.2017.07.003.
- Jousse C, Oyadomari S, Novoa I, Lu P, Zhang Y, Harding HP, Ron D. Inhibition of a constitutive translation initiation factor 2alpha phosphatase, CReP, promotes survival of stressed cells. J Cell Biol. 2003;163(4):767–75. doi:10.1083/jcb.200308075.
- Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153(5):1011–22. doi:10.1083/jcb.153.5.1011.
- Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18(24):3066–77. doi:10.1101/gad.1250704.
- Han J, Back SH, Hur J, Lin Y-H, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15(5):481–90. doi:10.1038/ncb2738.
- Frank CL, Ge X, Xie Z, Zhou Y, Tsai LH. Control of activating transcription factor 4 (ATF4) persistence by multisite phosphorylation impacts cell cycle progression and neurogenesis. J Biol Chem. 2010;285(43):33324–37. doi:10.1074/jbc.m110.140699.
- Lin H-K, Chen Z, Wang G, Nardella C, Lee S-W, Chan C-H, Yang W-L, Wang J, Egia A, Nakayama KI, et al. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature. 2010;464(7287):374–79. doi:10.1038/nature08815.
- Jun AS. One hundred years of Fuchs’ dystrophy. Ophthalmology. 2010;117(5):859–860 e814. doi:10.1016/j.ophtha.2010.03.001.
- Eghrari AO, Riazuddin SA, Gottsch JD. Fuchs corneal dystrophy. Prog Mol Biol Transl Sci. 2015;134:79–97. doi:10.1016/bs.pmbts.2015.04.005.
- Engler C, Kelliher C, Spitze AR, Speck CL, Eberhart CG, Jun AS. Unfolded protein response in fuchs endothelial corneal dystrophy: a unifying pathogenic pathway? Am J Ophthalmol. 2010;149(2):194–202.e192. doi:10.1016/j.ajo.2009.09.009.
- Okumura N, Kitahara M, Okuda H, Hashimoto K, Ueda E, Nakahara M, Kinoshita S, Young RD, Quantock AJ, Tourtas T, et al. Sustained activation of the unfolded protein response induces cell death in fuchs’ endothelial corneal dystrophy. Invest Ophthalmol Vis Sci. 2017;58(9):3697–707. doi:10.1167/iovs.16-21023.
- Ferrari G, Rama P. The keratoconus enigma: a pathogenesis review. Ocul Surf. 2020 Jul;18(3):363–73. doi:10.1016/j.jtos.2020.03.006.
- Chaerkady R, Shao H, Scott SG, Pandey A, Jun AS, Chakravarti S. The keratoconus corneal proteome: loss of epithelial integrity and stromal degeneration. J Proteomics. 2013;87:122–31. doi:10.1016/j.jprot.2013.05.023.
- Klintworth GK. Advances in the molecular genetics of corneal dystrophies. Am J Ophthalmol. 1999;128(6):747–54. doi:10.1016/s0002-9394(99)00358-x.
- Choi SI, Lee E, Jeong JB, Akuzum B, Maeng YS, Kim TI, Kim EK. 4-Phenylbutyric acid reduces mutant-TGFBIp levels and ER stress through activation of ERAD pathway in corneal fibroblasts of granular corneal dystrophy type 2. Biochem Biophys Res Commun. 2016;477(4):841–46. doi:10.1016/j.bbrc.2016.06.146.
- Shukla D, Farooq S. The role of herpesviruses in ocular infections. Virus Adapt Treat. 2010;2:115–23. doi:10.2147/vaat.s9500.
- Valerio GS, Lin CC. Ocular manifestations of herpes simplex virus. Curr Opin Ophthalmol. 2019;30(6):525–31. doi:10.1097/icu.0000000000000618.
- Burnett HF, Audas TE, Liang G, Lu RR. Herpes simplex virus-1 disarms the unfolded protein response in the early stages of infection. Cell Stress Chaperones. 2012;17(4):473–83. doi:10.1007/s12192-012-0324-8.
- Poppers J, Mulvey M, Khoo D, Mohr I. Inhibition of PKR activation by the proline-rich RNA binding domain of the herpes simplex virus type 1 Us11 protein. J Virol. 2000;74(23):11215–21. doi:10.1128/jvi.74.23.11215-11221.2000.
- He B, Gross M, Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci U S A. 1997;94(3):843–48. doi:10.1073/pnas.94.3.843.
- Charron AJ, Ward SL, North BJ, Ceron S, Leib DA, Longnecker RM. The US11 gene of herpes simplex virus 1 promotes neuroinvasion and periocular replication following corneal infection. J Virol. 2019;93(9):e02246. doi:10.1128/JVI.02246-18.
- Hejtmancik JF, Riazuddin SA, McGreal R, Liu W, Cvekl A, Shiels A. Lens biology and biochemistry. Prog Mol Biol Transl Sci. 2015;134:(169–201. doi:10.1016/bs.pmbts.2015.04.007.
- Graw J. Eye development. Curr Top Dev Biol. 2010;90:343–86. doi:10.1016/s0070-2153(10)90010-0.
- Brennan LA, Kantorow WL, Chauss D, McGreal R, He S, Mattucci L, Wei J, Riazuddin SA, Cvekl A, Hejtmancik JF, et al. Spatial expression patterns of autophagy genes in the eye lens and induction of autophagy in lens cells. Mol Vis. 2012;18:1773–86.
- Khan SY, Ali M, Kabir F, Renuse S, Na CH, Talbot CC Jr., Hackett SF, Riazuddin SA. Proteome profiling of developing murine lens through mass spectrometry. Invest Ophthalmol Vis Sci. 2018;59(1):100–07. doi:10.1167/iovs.17-21601.
- Khairallah M, Kahloun R, Bourne R, Limburg H, Flaxman SR, Jonas JB, Keeffe J, Leasher J, Naidoo K, Pesudovs K, et al. Number of people blind or visually impaired by cataract worldwide and in world regions, 1990 to 2010. Invest Ophthalmol Vis Sci. 2015;56(11):6762–69. doi:10.1167/iovs.15-17201.
- Bron AJ, Vrensen GF, Koretz J, Maraini G, Harding JJ. The ageing lens. Ophthalmologica. 2000;214(1):86–104. doi:10.1159/000027475.
- Tang HZ, Yang LM. Activation of the unfolded protein response in aged human lenses. Mol Med Rep. 2015;12(1):389–93. doi:10.3892/mmr.2015.3417.
- Yang J, Zhou S, Gu J, Guo M, Xia H, Liu Y, Nagaraj R. UPR activation and the down-regulation of alpha-crystallin in human high myopia-related cataract lens epithelium. PLoS One. 2015;10(9):e0137582. doi:10.1371/journal.pone.0137582.
- Ikesugi K, Yamamoto R, Mulhern ML, Shinohara T. Role of the unfolded protein response (UPR) in cataract formation. Exp Eye Res. 2006;83(3):508–16. doi:10.1016/j.exer.2006.01.033.
- Kroeger H, Chiang WC, Felden J, Nguyen A, Lin JH. ER stress and unfolded protein response in ocular health and disease. Febs J. 2019;286(2):399–412. doi:10.1111/febs.14522.
- Kobrin Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14(4):179–83. doi:10.1080/09286580701396720.
- Ejaz S. Importance of pericytes and mechanisms of pericyte loss during diabetes retinopathy. Diabetes, Obes Metab. 2008;10(1):53–63. doi:10.1111/j.1463-1326.2007.00795.x.
- Zhong Y, Wang JJ, Zhang SX. Intermittent but not constant high glucose induces ER stress and inflammation in human retinal pericytes. Adv Exp Med Biol. 2012;723:285–92. doi:10.1007/978-1-4614-0631-0_37.
- Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet. 2018;392(10153):1147–59. doi:10.1016/s0140-6736(18)31550-2.
- Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration—emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38(7):450–71. doi:10.1080/07853890600946724.
- Roybal CN, Hunsaker LA, Barbash O, Vander Jagt DL, Abcouwer SF. The oxidative stressor arsenite activates vascular endothelial growth factor mRNA transcription by an ATF4-dependent mechanism. J Biol Chem. 2005;280(21):20331–39. doi:10.1074/jbc.M411275200.
- Pollreisz A, Afonyushkin T, Oskolkova OV, Gruber F, Bochkov VN, Schmidt-Erfurth U. Retinal pigment epithelium cells produce VEGF in response to oxidized phospholipids through mechanisms involving ATF4 and protein kinase CK2. Exp Eye Res. 2013;116:177–84. doi:10.1016/j.exer.2013.08.021.
- Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma. JAMA. 2014;311(18):1901. doi:10.1001/jama.2014.3192.
- Zode GS, Kuehn MH, Nishimura DY, Searby CC, Mohan K, Grozdanic SD, Bugge K, Anderson MG, Clark AF, Stone EM, et al. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J Clin Invest. 2011;121(9):3542–53. doi:10.1172/JCI58183.
- Peters JC, Bhattacharya S, Clark AF, Zode GS. Increased endoplasmic reticulum stress in human glaucomatous trabecular meshwork cells and tissues. Invest Ophthalmol Vis Sci. 2015;56(6):3860–68. doi:10.1167/iovs.14-16220.
- Foufelle F, Fromenty B. Role of endoplasmic reticulum stress in drug-induced toxicity. Pharmacol Res Perspect. 2016;4(1):e00211. doi:10.1002/prp2.211.
- Srivastava RK, Li C, Ahmad A, Abrams O, Gorbatyuk MS, Harrod KS, Wek RC, Afaq F, Athar M. ATF4 regulates arsenic trioxide-mediated NADPH oxidase, ER-mitochondrial crosstalk and apoptosis. Arch Biochem Biophys. 2016;609:39–50. doi:10.1016/j.abb.2016.09.003.
- Carlberg M, Dricu A, Blegen H, Kass GEN, Orrenius S, Larsson O. Short exposures to tunicamycin induce apoptosis in SV40-transformed but not in normal human fibroblasts. Carcinogenesis. 1996;17(12):2589–96. doi:10.1093/carcin/17.12.2589.
- Treiman M, Caspersen C, Christensen SB. A tool coming of age: thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca(2+)-ATPases. Trends Pharmacol Sci. 1998;19(4):131–35. doi:10.1016/s0165-6147(98)01184-5.
- Shi K, Wang D, Cao X, Ge Y, Takehara T. Endoplasmic reticulum stress signaling is involved in mitomycin C(MMC)-induced apoptosis in human fibroblasts via PERK pathway. PLoS ONE. 2013;8(3):e59330. doi:10.1371/journal.pone.0059330.
- Martinez VD, Vucic EA, Becker-Santos DD, Gil L, Lam WL. Arsenic exposure and the induction of human cancers. J Toxicol. 2011;2011:431287. doi:10.1155/2011/431287.
- Guo Q, Li H, Liu J, Xu L, Yang L, Sun Z, Zhou B. Tunicamycin aggravates endoplasmic reticulum stress and airway inflammation via PERK-ATF4-CHOP signaling in a murine model of neutrophilic asthma. J Asthma. 2017;54(2):125–33. doi:10.1080/02770903.2016.1205085.
- Shaabani N, Honke N, Lang PA, Gorg B, Proksch P, Gailus N, Gotoh T, Haussinger D, Lang KS. Tunicamycin inhibits diabetes. Cell Physiol Biochem. 2012;29(3–4):595–602. doi:10.1159/000338513.
- Shehadeh-Mashor R, Srinivasan S, Boimer C, Lee K, Tomkins O, Slomovic AR. Management of recurrent pterygium with intraoperative mitomycin C and conjunctival autograft with fibrin glue. Am J Ophthalmol. 2011;152(5):730–32. doi:10.1016/j.ajo.2011.04.034.
- Katz GJ, Higginbotham EJ, Lichter PR, Skuta GL, Musch DC, Bergstrom TJ, Johnson AT. Mitomycin C versus 5-fluorouracil in high-risk glaucoma filtering surgery. Extended follow-up. Ophthalmology. 1995;102(9):1263–69. doi:10.1016/s0161-6420(95)30875-5.
- Doultsinos D, Avril T, Lhomond S, Dejeans N, Guedat P, Chevet E. Control of the unfolded protein response in health and disease. SLAS Discov. 2017;22(7):787–800. doi:10.1177/2472555217701685.
- Hetz C, Axten JM, Patterson JB. Pharmacological targeting of the unfolded protein response for disease intervention. Nat Chem Biol. 2019;15(8):764–75. doi:10.1038/s41589-019-0326-2.
- Rabouw HH, Langereis MA, Anand AA, Visser LJ, De Groot RJ, Walter P, Van Kuppeveld FJM. Small molecule ISRIB suppresses the integrated stress response within a defined window of activation. Proc Nat Acad Sci. 2019;116(6):2097–102. doi:10.1073/pnas.1815767116.
- Sidrauski C, Acosta-Alvear D, Khoutorsky A, Vedantham P, Hearn BR, Li H, Gamache K, Gallagher CM, Ang KK, Wilson C, et al. Pharmacological brake-release of mRNA translation enhances cognitive memory. Elife. 2013;2:e00498. doi:10.7554/eLife.00498.
- Sidrauski C, Tsai JC, Kampmann M, Hearn BR, Vedantham P, Jaishankar P, Sokabe M, Mendez AS, Newton BW, Tang EL, et al. Pharmacological dimerization and activation of the exchange factor eIF2B antagonizes the integrated stress response. Elife. 2015;4:e07314. doi:10.7554/eLife.07314.
- Hearn BR, Jaishankar P, Sidrauski C, Tsai JC, Vedantham P, Fontaine SD, Walter P, Renslo AR. Structure-activity studies of bis-O-arylglycolamides: inhibitors of the integrated stress response. ChemMedChem. 2016;11(8):870–80. doi:10.1002/cmdc.201500483.
- Bugallo R, Marlin E, Baltanas A, Toledo E, Ferrero R, Vinueza-Gavilanes R, Larrea L, Arrasate M, Aragon T. Fine tuning of the unfolded protein response by ISRIB improves neuronal survival in a model of amyotrophic lateral sclerosis. Cell Death Dis. 2020;11(5):397. doi:10.1038/s41419-020-2601-2.
- Halliday M, Radford H, Sekine Y, Moreno J, Verity N, Le Quesne J, Ortori CA, Barrett DA, Fromont C, Fischer PM, et al. Partial restoration of protein synthesis rates by the small molecule ISRIB prevents neurodegeneration without pancreatic toxicity. Cell Death Dis. 2015;6(3):e1672–e1672. doi:10.1038/cddis.2015.49.
- Chou A, Krukowski K, Jopson T, Zhu PJ, Costa-Mattioli M, Walter P, Rosi S. Inhibition of the integrated stress response reverses cognitive deficits after traumatic brain injury. Proc Natl Acad Sci U S A. 2017;114(31):E6420–E6426. doi:10.1073/pnas.1707661114.
- Zhu PJ, Khatiwada S, Cui Y, Reineke LC, Dooling SW, Kim JJ, Li W, Walter P, Costa-Mattioli M. Activation of the ISR mediates the behavioral and neurophysiological abnormalities in Down syndrome. Science. 2019;366(6467):843–49. doi:10.1126/science.aaw5185.
- Soiberman US, Shehata AEM, Lu MX, Young T, Daoud YJ, Chakravarti S, Jun AS, Foster JW. Small molecule modulation of the integrated stress response governs the keratoconic phenotype in vitro. Invest Ophthalmol Vis Sci. 2019;60(10):3422–31. doi:10.1167/iovs.19-27151.
- Sundrud MS, Koralov SB, Feuerer M, Calado DP, Kozhaya AE, Rhule-Smith A, Lefebvre RE, Unutmaz D, Mazitschek R, Waldner H, et al. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science. 2009;324(5932):1334–38. doi:10.1126/science.1172638.
- Bunpo P, Dudley A, Cundiff JK, Cavener DR, Wek RC, Anthony TG. GCN2 protein kinase is required to activate amino acid deprivation responses in mice treated with the anti-cancer agent L-asparaginase. J Biol Chem. 2009;284(47):32742–49. doi:10.1074/jbc.M109.047910.
- Long Y, Tsai WB, Wangpaichitr M, Tsukamoto T, Savaraj N, Feun LG, Kuo MT. Arginine deiminase resistance in melanoma cells is associated with metabolic reprogramming, glucose dependence, and glutamine addiction. Mol Cancer Ther. 2013;12(11):2581–90. doi:10.1158/1535-7163.MCT-13-0302.
- Chen T, Ozel D, Qiao Y, Harbinski F, Chen L, Denoyelle S, He X, Zvereva N, Supko JG, Chorev M, et al. Chemical genetics identify eIF2alpha kinase heme-regulated inhibitor as an anticancer target. Nat Chem Biol. 2011;7(9):610–16. doi:10.1038/nchembio.613.
- Hu W, Hofstetter W, Wei X, Guo W, Zhou Y, Pataer A, Li H, Fang B, Swisher SG. Double-stranded RNA-dependent protein kinase-dependent apoptosis induction by a novel small compound. J Pharmacol Exp Ther. 2009;328(3):866–72. doi:10.1124/jpet.108.141754.
- Ma C-H, Wu C-H, Jou IM, Tu Y-K, Hung C-H, Chou W-C, Chang Y-C, Hsieh P-L, Tsai K-L. PKR promotes oxidative stress and apoptosis of human articular chondrocytes by causing mitochondrial dysfunction through p38 MAPK activation—PKR activation causes apoptosis in human chondrocytes. Antioxidants. 2019;8(9):370. doi:10.3390/antiox8090370.
- Stockwell SR, Platt G, Barrie SE, Zoumpoulidou G, Te Poele RH, Aherne GW, Wilson SC, Sheldrake P, McDonald E, Venet M, et al. Mechanism-based screen for G1/S checkpoint activators identifies a selective activator of EIF2AK3/PERK signalling. PLoS One. 2012;7(1):e28568. doi:10.1371/journal.pone.0028568.
- Atkins C, Liu Q, Minthorn E, Zhang SY, Figueroa DJ, Moss K, Stanley TB, Sanders B, Goetz A, Gaul N, et al. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2013;73(6):1993–2002. doi:10.1158/0008-5472.Can-12-3109.
- Elkin M, Miao HQ, Nagler A, Aingorn E, Reich R, Hemo I, Dou HL, Pines M, Vlodavsky I. Halofuginone: a potent inhibitor of critical steps in angiogenesis progression. FASEB J. 2000;14(15):2477–85. doi:10.1096/fj.00-0292com.
- Yang L, Tan D, Piao H. Myelin basic protein citrullination in multiple sclerosis: a potential therapeutic target for the pathology. Neurochem Res. 2016;41(8):1845–56. doi:10.1007/s11064-016-1920-2.
- Bonilha VL, Shadrach KG, Rayborn ME, Li Y, Pauer GJT, Hagstrom SA, Bhattacharya SK, Hollyfield JG. Retinal deimination and PAD2 levels in retinas from donors with age-related macular degeneration (AMD). Exp Eye Res. 2013;111:71–78. doi:10.1016/j.exer.2013.03.017.
- Bhattacharya SK, Crabb JS, Bonilha VL, Gu X, Takahara H, Crabb JW. Proteomics implicates peptidyl arginine deiminase 2 and optic nerve citrullination in glaucoma pathogenesis. Invest Ophthalmol Vis Sci. 2006;47(6):2508–14. doi:10.1167/iovs.05-1499.
- Park JH, Baron S. Herpetic keratoconjunctivitis: therapy with synthetic double-stranded RNA. Science. 1968;162(3855):811–13. doi:10.1126/science.162.3855.811.
- Kaufman HE, Ellison ED, Waltman SR. Double-stranded RNA, an interferon inducer, in herpes simplex keratitis. Am J Ophthalmol. 1969;68(3):486–91. doi:10.1016/0002-9394(69)90720-x.
- Robert F, Williams C, Yan Y, Donohue E, Cencic R, Burley SK, Pelletier J. Blocking UV-induced eIF2alpha phosphorylation with small molecule inhibitors of GCN2. Chem Biol Drug Des. 2009;74(1):57–67. doi:10.1111/j.1747-0285.2009.00827.x.
- Rosen MD, Woods CR, Goldberg SD, Hack MD, Bounds AD, Yang Y, Wagaman PC, Phuong VK, Ameriks AP, Barrett TD, et al. Discovery of the first known small-molecule inhibitors of heme-regulated eukaryotic initiation factor 2alpha (HRI) kinase. Bioorg Med Chem Lett. 2009;19(23):6548–51. doi:10.1016/j.bmcl.2009.10.033.
- Jammi NV, Whitby LR, Beal PA. Small molecule inhibitors of the RNA-dependent protein kinase. Biochem Biophys Res Commun. 2003;308(1):50–57. doi:10.1016/s0006-291x(03)01318-4.
- Hu Y, Conway TW. 2-Aminopurine inhibits the double-stranded RNA-dependent protein kinase both in vitro and in vivo. J Interferon Res. 1993;13(5):323–28. doi:10.1089/jir.1993.13.323.
- Axten JM, Medina JR, Feng Y, Shu A, Romeril SP, Grant SW, Li WH, Heerding DA, Minthorn E, Mencken T, et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-p yrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK). J Med Chem. 2012;55(16):7193–207. doi:10.1021/jm300713s.
- Axten JM, Romeril SP, Shu A, Ralph J, Medina JR, Feng Y, Li WH, Grant SW, Heerding DA, Minthorn E, et al. Discovery of GSK2656157: an optimized PERK inhibitor selected for preclinical development. ACS Med Chem Lett. 2013;4(10):964–68. doi:10.1021/ml400228e.
- Jiang X, Wei Y, Zhang T, Zhang Z, Qiu S, Zhou X, Zhang S. Effects of GSK2606414 on cell proliferation and endoplasmic reticulum stressassociated gene expression in retinal pigment epithelial cells. Mol Med Rep. 2017;15(5):3105–10. doi:10.3892/mmr.2017.6418.
- Kojima E, Takeuchi A, Haneda M, Yagi F, Hasegawa T, Yamaki K-I, Takeda K, Akira S, Shimokata K, Isobe K-I. The function of GADD34 is a recovery from a shutoff of protein synthesis induced by ER stress—elucidation by GADD34‐deficient mice. Faseb J. 2003;17(11):1–18. doi:10.1096/fj.02-1184fje.
- Tsaytler P, Bertolotti A. Exploiting the selectivity of protein phosphatase 1 for pharmacological intervention. Febs J. 2013;280(2):766–70. doi:10.1111/j.1742-4658.2012.08535.x.
- De Gassart A, Bujisic B, Zaffalon L, Decosterd LA, Di Micco A, Frera G, Tallant R, Martinon F. An inhibitor of HIV-1 protease modulates constitutive eIF2alpha dephosphorylation to trigger a specific integrated stress response. Proc Natl Acad Sci U S A. 2016;113(2):E117–126. doi:10.1073/pnas.1514076113.
- Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307(5711):935–39. doi:10.1126/science.1101902.
- Bryant KF, Macari ER, Malik N, Boyce M, Yuan J, Coen DM. ICP34.5-dependent and -independent activities of salubrinal in herpes simplex virus-1 infected cells. Virology. 2008;379(2):197–204. doi:10.1016/j.virol.2008.06.028.
- Wang Y, Osakue D, Yang E, Zhou Y, Gong H, Xia X, Du Y. Endoplasmic Reticulum Stress Response of Trabecular Meshwork Stem Cells and Trabecular Meshwork Cells and Protective Effects of Activated PERK Pathway. Invest Ophthalmol Vis Sci. 2019;60(1):265–73. doi:10.1167/iovs.18-25477.
- Hirai S, Kurashima H, Nakamura D, Komatsu T, Yasuda Y, Habashita-Obata S, Ichikawa S, Katsuta O, Iwawaki T, Kohno K. 2-Phenyl-APB-144-induced retinal pigment epithelium degeneration and its underlying mechanisms. J Ocul Pharmacol Ther. 2015;31(9):570–84. doi:10.1089/jop.2014.0076.
- Zhang L, Xia Q, Zhou Y, Li J. Endoplasmic reticulum stress and autophagy contribute to cadmium-induced cytotoxicity in retinal pigment epithelial cells. Toxicol Lett. 2019;311:105–13. doi:10.1016/j.toxlet.2019.05.001.
- Imming P, Sinning C, Meyer A. Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov. 2006;5(10):821–34. doi:10.1038/nrd2132.
- Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434(7032):514–20. doi:10.1038/nature03398.
- Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, Li L, Brancorsini S, Sassone-Corsi P, Townes TM, et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell. 2004;117(3):387–98. doi:10.1016/s0092-8674(04)00344-7.
- Cohen MS, Zhang C, Shokat KM, Taunton J. Structural bioinformatics-based design of selective, irreversible kinase inhibitors. Science. 2005;308(5726):1318–21. doi:10.1126/science1108367.
- Sapkota GP, Cummings L, Newell FS, Armstrong C, Bain J, Frodin M, Grauert M, Hoffmann M, Schnapp G, Steegmaier M, et al. BI-D1870 is a specific inhibitor of the p90 RSK (ribosomal S6 kinase) isoforms in vitro and in vivo. Biochem J. 2007;401(1):29–38. doi:10.1042/bj20061088.
- Smith JA, Poteet-Smith CE, Xu Y, Errington TM, Hecht SM, Lannigan DA. Identification of the first specific inhibitor of p90 ribosomal S6 kinase (RSK) reveals an unexpected role for RSK in cancer cell proliferation. Cancer Res. 2005;65:1027–34.
- Murray AJ. Pharmacological PKA inhibition: all may not be what it seems. Sci Signal. 2008;1(22):re4. doi:10.1126/scisignal.122re4.
- Brudvik KW, Paulsen JE, Aandahl EM, Roald B, Tasken K. Protein kinase A antagonist inhibits beta-catenin nuclear translocation, c-Myc and COX-2 expression and tumor promotion in Apc(Min/+) mice. Mol Cancer. 2011;10:149. doi:10.1186/1476-4598-10-149.
- Fenton TR, Gout IT. Functions and regulation of the 70kDa ribosomal S6 kinases. Int J Biochem Cell Biol. 2011;43(1):47–59. doi:10.1016/j.biocel.2010.09.018.
- Kotani T. Protein kinase A activity and Hedgehog signaling pathway. Vitam Horm. 2012;88:273–91. doi:10.1016/B978-0-12-394622-5.00012-2.
- Wang Y, Alam GN, Ning Y, Visioli F, Dong Z, Nor JE, Polverini PJ. The unfolded protein response induces the angiogenic switch in human tumor cells through the PERK/ATF4 pathway. Cancer Res. 2012;72(20):5396–406. doi:10.1158/0008-5472.can-12-0474.
- Gargalovic PS, Imura M, Zhang B, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A, Truong A, Patel S, et al. Identification of inflammatory gene modules based on variations of human endothelial cell responses to oxidized lipids. Proc Natl Acad Sci U S A. 2006;103(34):12741–46. doi:10.1073/pnas.0605457103.
- Fallah A, Sadeghinia A, Kahroba H, Samadi A, Heidari HR, Bradaran B, Zeinali S, Molavi O. Therapeutic targeting of angiogenesis molecular pathways in angiogenesis-dependent diseases. Biomed Pharmacother. 2019;110:775–85. doi:10.1016/j.biopha.2018.12.022.
- Galluzzi L, Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cecconi F, Choi AM, Chu CT, Codogno P, Colombo MI, et al. Molecular definitions of autophagy and related processes. Embo J. 2017;36(13):1811–36. doi:10.15252/embj.201796697.
- Rouschop KM, van den Beucken T, Dubois L, Niessen H, Bussink J, Savelkouls K, Keulers T, Mujcic H, Landuyt W, Voncken JW, et al. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest. 2010;120(1):127–41. doi:10.1172/jci40027.
- Yang X, Pan X, Zhao X, Luo J, Xu M, Bai D, Hu Y, Liu X, Yu Q, Gao D. Autophagy and age-related eye diseases. Biomed Res Int. 2019;2019:1–12. doi:10.1155/2019/5763658.
- Claessen JH, Kundrat L, Ploegh HL. Protein quality control in the ER: balancing the ubiquitin checkbook. Trends Cell Biol. 2012;22(1):22–32. doi:10.1016/j.tcb.2011.09.010.
- Obeng EA, Carlson LM, Gutman DM, Harrington WJ Jr., Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107(12):4907–16. doi:10.1182/blood-2005-08-3531.
- Chen F-T, Yang C-M, Yang C-H, Mohanraj R. The protective effects of the proteasome inhibitor bortezomib (velcade) on ischemia-reperfusion injury in the rat retina. PLoS ONE. 2013;8(5):e64262. doi:10.1371/journal.pone.0064262.
- Hsu S-M, Yang C-H, Shen F-H, Chen S-H, Lin C-J, Shieh C-C. Proteasome inhibitor bortezomib suppresses nuclear factor-kappa B activation and ameliorates eye inflammation in experimental autoimmune uveitis. Mediators Inflamm. 2015;2015:1–10. doi:10.1155/2015/847373.
- Wang W, Lian N, Li L, Moss HE, Wang W, Perrien DS, Elefteriou F, Yang X. Atf4 regulates chondrocyte proliferation and differentiation during endochondral ossification by activating Ihh transcription. Development. 2009;136(24):4143–53. doi:10.1242/dev.043281.
- Hettmann T, Barton K, Leiden JM. Microphthalmia due to p53-mediated apoptosis of anterior lens epithelial cells in mice lacking the CREB-2 transcription factor. Dev Biol. 2000;222(1):110–23. doi:10.1006/dbio.2000.9699.
- Iida K, Li Y, McGrath BC, Frank A, Cavener DR. PERK eIF2 alpha kinase is required to regulate the viability of the exocrine pancreas in mice. BMC Cell Biol. 2007;8:38. doi:10.1186/1471-2121-8-38.
- Zhang P, McGrath BC, Reinert J, Olsen DS, Lei L, Gill S, Wek SA, Vattem KM, Wek RC, Kimball SR, et al. The GCN2 eIF2α kinase is required for adaptation to amino acid deprivation in mice. Mol Cell Bio. 2002;22(19):6681–88. doi:10.1128/mcb.22.19.6681-6688.2002.
- Abraham N, Stojdl DF, Duncan PI, Méthot N, Ishii T, Dubé M, Vanderhyden BC, Atkins HL, Gray DA, McBurney MW, et al. Characterization of transgenic mice with targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J Biol Chem. 1999;274(9):5953–62. doi:10.1074/jbc.274.9.5953.
- Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7(6):1153–63. doi:10.1016/s1097-2765(01)00264-7.
- Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, Gannon M, Ma K, McNaughton K, Cavener DR. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol. 2002;22(11):3864–74. doi:10.1128/mcb.22.11.3864-3874.2002.
- Wei J, Sheng X, Feng D, McGrath B, Cavener DR. PERK is essential for neonatal skeletal development to regulate osteoblast proliferation and differentiation. J Cell Physiol. 2008;217(3):693–707. doi:10.1002/jcp.21543.
- Saito A, Ochiai K, Kondo S, Tsumagari K, Murakami T, Cavener DR, Imaizumi K. Endoplasmic reticulum stress response mediated by the PERK-eIF2(alpha)-ATF4 pathway is involved in osteoblast differentiation induced by BMP2. J Biol Chem. 2011;286(6):4809–18. doi:10.1074/jbc.M110.152900.
- Gao Y, Sartori DJ, Li C, Yu QC, Kushner JA, Simon MC, Diehl JA. PERK is required in the adult pancreas and is essential for maintenance of glucose homeostasis. Mol Cell Biol. 2012;32(24):5129–39. doi:10.1128/mcb.01009-12.
- Trinh MA, Kaphzan H, Wek RC, Pierre P, Cavener DR, Klann E. Brain-specific disruption of the eIF2alpha kinase PERK decreases ATF4 expression and impairs behavioral flexibility. Cell Rep. 2012;1(6):676–88. doi:10.1016/j.celrep.2012.04.010.
- Harding HP, Zhang Y, Scheuner D, Chen -J-J, Kaufman RJ, Ron D. Ppp1r15 gene knockout reveals an essential role for translation initiation factor 2 alpha (eIF2α) dephosphorylation in mammalian development. Proc Natl Acad Sci U S A. 2009;106(6):1832–37. doi:10.1073/pnas.0809632106.
- Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7(6):1165–76. doi:10.1016/s1097-2765(01)00265-9.
- Scheuner D, Vander Mierde D, Song B, Flamez D, Creemers JW, Tsukamoto K, Ribick M, Schuit FC, Kaufman RJ. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med. 2005;11(7):757–64. doi:10.1038/nm1259.
- Back SH, Scheuner D, Han J, Song B, Ribick M, Wang J, Gildersleeve RD, Pennathur S, Kaufman RJ. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 2009;10(1):13–26. doi:10.1016/j.cmet.2009.06.002.
- Chen CM, Wu CT, Chiang CK, Liao BW, Liu SH. C/EBP homologous protein (CHOP) deficiency aggravates hippocampal cell apoptosis and impairs memory performance. PLoS One. 2012;7(7):e40801. doi:10.1371/journal.pone.0040801.
- Cornejo VH, Pihan P, Vidal RL, Hetz C. Role of the unfolded protein response in organ physiology: lessons from mouse models. IUBMB Life. 2013;65(12):962–75. doi:10.1002/iub.1224.
- Valenzuela V, Collyer E, Armentano D, Parsons GB, Court FA, Hetz C. Activation of the unfolded protein response enhances motor recovery after spinal cord injury. Cell Death Dis. 2012;3:e272. doi:10.1038/cddis.2012.8.
- Jousse C, Oyadomari S, Novoa I, Lu P, Zhang Y, Harding HP, Ron D. Inhibition of a constitutive translation initiation factor 2α phosphatase, CReP, promotes survival of stressed cells. J Cell Biol. 2003;163(4):767–75. doi:10.1083/jcb.200308075.
- Dever TE. Gene-specific regulation by general translation factors. Cell. 2002;108(4):545–56. doi:10.1016/s0092-8674(02)00642-6.
- Endo M, Oyadomari S, Suga M, Mori M, Gotoh T. The ER stress pathway involving CHOP is activated in the lungs of LPS-treated mice. J Biochem. 2005;138(4):501–07. doi:10.1093/jb/mvi143.
- Tajiri S, Oyadomari S, Yano S, Morioka M, Gotoh T, Hamada JI, Ushio Y, Mori M. Ischemia-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOP. Cell Death Differ. 2004;11(4):403–15. doi:10.1038/sj.cdd.4401365.

