Abstract
Purpose
In this review, we aimed to investigate the literature on sex-specific prevalence of meibomian gland dysfunction (MGD) and to determine whether women or men are more at risk for MGD.
Methods
A search was conducted on PubMed using the terms: (Sex OR Gender OR prevalence) AND (Meibomian gland).
Results
Twenty-four relevant studies on MGD prevalence were identified, including 10 population-based and 14 hospital-based studies. Among the population-based studies, five studies reported higher rates among men, three studies found no differences, and one study observed higher rates among women. In the hospital-based studies, 10 studies reported no difference, two found higher rates among men, and one found higher among women. In the reviewed literature, there was a considerable variation between studies in terms of quality, sample size, age ranges, diagnostic criteria.
Conclusions
While most of the population-based studies suggest a higher prevalence among men, the majority of clinic-based studies show no significant difference. Further research with larger samples and standardized criteria is needed to determine whether men are indeed more susceptible to MGD.
Keywords:
Introduction
Dry eye disease (DED) is a “multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film and accompanied by ocular symptoms, in which tear film instability and hyperosmolality, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles”.Citation1 Symptoms of DED include dryness, pain, foreign body sensation, and visual disturbances.
A large number of studies worldwide find that DED occurs more often among women.Citation2–5 Thus, female sex is considered an established risk factor for DED. However, in the research literature on which this perception is based, dry eye prevalence is often based on subjective diagnostic criteria in the form of questionnaires.
To obtain a more precise understanding of the role of sex in DED, studies looking into the different classes are needed. DED is classified into two major categories: aqueous deficiency dry eye (ADDE) and evaporative dry eye (EDE). Present literature illustrates the categories as two non-mutually exclusive entities in a continuum with a mixed type in between.Citation1
Meibomian gland dysfunction (MGD) is the most common etiology of EDE.Citation6 MGD is a chronic condition characterized by a reduction in the quality or quantity of lipids, resulting in alterations in the tear film. Accordingly, MGD is clinically associated with low meibum quality and/or expressibility, and in some cases, abnormalities of lid margins such as telangiectasia.
Several studies have investigated the prevalence of MGD, although the numbers vary depending on the population and diagnostic criteria used. A few review articles have summarized the results,Citation7,Citation8 and the global prevalence varies between 21.2% to 71%.Citation7
Two review articles have attempted to assess the influence of sex on the prevalence of MGD.Citation7,Citation8 In the broad TFOS DEWS II Epidemiology Report, the authors presented a schematic overview of diverse studies on the prevalence of MGD.Citation8 While a subset of these studies also reported sex-specific prevalence, statistical tests for differences were not presented. Further, Hassanzadeh et al. conducted meta-analyses on several factors tied to MGD prevalence globally, and found that MGD affected men more frequently than women, although there was a wide heterogeneity among the studies.Citation7 Despite these findings, many questions tied to the relationship between sex and MGD remain unanswered, and the current review article dedicated to the sex-specific differences in MGD, allows us to include, and critically review, a wider range of studies and perspectives on this specific question.
In this review article, we first summarize the global prevalences stratified by sex and provide an overview of the age of population and diagnostic criteria used among the included studies. Secondly, we summarize the clinical studies with outcomes of meibomian gland parameters stratified by sex. The aim of this review is to provide more knowledge of the currently unsettled role of sex in the widespread disease MGD.
Methods
Search strategy
A literature search was conducted on PubMed on the 25th of September 2022 using the following search term: (sex OR gender OR prevalence) AND (meibomian gland).
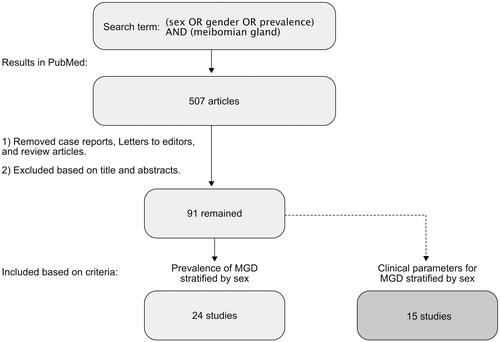
All published articles available in English were included in the initial search results. Case reports, letters to the editor, and review articles were excluded. The remaining articles were then evaluated by title and abstract to ensure relevance to the topic. The full text was then evaluated based on the following primary inclusion criteria: (1) studies that investigated the prevalence of MGD stratified by sex. Subsequently, the articles were evaluated against the secondary inclusion criteria: (2) studies that investigated clinical outcomes on MGD stratified by sex. This process is shown in .
Results
The search term “(sex OR gender OR prevalence) AND (meibomian gland)” yielded 507 results. After screening the entries based on title and abstract, and excluding review articles, letters-to-editor, and case reports, 91 entries were selected for further full-text screening. For the remaining 91 articles, the full text was reviewed for relevance according to the primary inclusion criteria. This yielded the final 24 articles. Subsequently, the 91 potentially relevant articles were then assessed again against the secondary inclusion criteria to also include studies on clinical parameters on MGD, yielding an additional 15 articles.
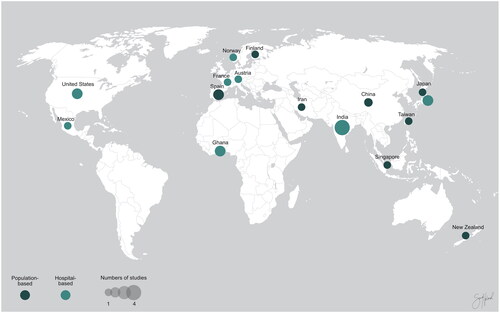
The 39 studies included in this review were published between 1990 and 2022 and conducted in 20 different countries: 6 in Japan;Citation9–14 4 in India;Citation15–18 3 in Ghana,Citation19–21 USA,Citation22–24 and Germany;Citation25–27 2 in Norway,Citation28,Citation29 Mexico,Citation30,Citation31 Iran,Citation32,Citation33 China,Citation34,Citation35 and Spain;Citation36,Citation37 1 in France,Citation38 Austria,Citation39 Finland,Citation40 New Zealand,Citation41 Singapore,Citation42 Taiwan,Citation43 South Korea,Citation44 the Netherlands,Citation45 Poland,Citation46 and Australia.Citation47
Of the 24 studies assessing the prevalence of MGD, 10 were population-based () and 14 was hospital-based (). shows the geographical locations of the included prevalence studies. Of the 10 population-based studies, three studies relied on both subjective and objective diagnostic criteria for MGD,Citation11,Citation32,Citation41 while seven studies relied on objective criteria alone.Citation33,Citation35–37,Citation40,Citation42,Citation43 All 14 hospital-based studies relied on objective diagnostic criteria for MGD, of which five studies used the diagnostic criteria suggested by the international workshop on meibomian gland dysfunction.Citation48 There were 15 studies presenting clinical parameters for MGD stratified by sex. A summary of study characteristics and key findings of each group are presented in , Citation2 and Supplemental Table 1. A quality assessment of the included studies was conducted using the NHLBI quality assessment tool for observational cohort and cross-sectional studies. The results of the quality assessment are summarized in Supplemental Tables.
Table 1. Population-based studies.
Table 2. Hospital-based studies.
Overview of population-based studies
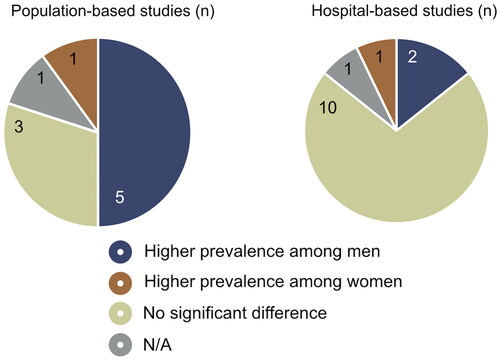
The majority of the 10 population-based studies included populations based on random samples of all inhabitants in selected regions or cities,Citation11,Citation32,Citation33,Citation36,Citation37,Citation40–43 whereas one study only relies on staff from a university.Citation35 The studies were conducted in 8 different countries between 2003 and 2022. Sample sizes ranged from 356 to 4700, with a median sample size of 2246. Across all studies, the sexes were quite equally represented, with 54% females and 46% males in the total number of subjects included. The age range of the populations varied in the included studies: 7 studies only include middle-aged and elderly participants, while 2 studies include participants of all ages. The mean age was 59.7 years. The total prevalence of MGD ranged from 7% to 71%. 5 studies found a significantly higher prevalence among men than women,Citation11,Citation32,Citation37,Citation40,Citation42 1 found a higher prevalence among women,Citation41 three found no sex difference,Citation33,Citation35,Citation43 and the remaining article did not report statistical tests indicating whether a sex difference existed.Citation36 The results are shown in
Overview of hospital-based studies
The included populations of the 14 hospital-based studies are studied in eye clinics,Citation9,Citation10,Citation15–18,Citation20,Citation22,Citation24,Citation29,Citation30,Citation38,Citation39 except for one study which was performed at a diabetes clinic.Citation19 There were variations in inclusion criteria: five studies included only patients with dry eye,Citation15,Citation18,Citation22,Citation29,Citation39 while the remaining included all patients recruited during routine vision examination or based on other scheduled appointments, such as cataract surgery.Citation9,Citation10,Citation16,Citation17,Citation19,Citation20,Citation24,Citation30,Citation38 The studies were conducted in eight different countries between 1990 and 2022. Sample sizes ranged from 60 to 1372, with a median sample size of 325. Most of the studies had a higher percentage of female participants, resulting in an overall ratio of 63% females and 37% males. As far as age range was presented, most studies included participants from all ages,Citation15–18,Citation22,Citation29,Citation30 while three only included those over middle age,Citation9,Citation10,Citation19 and one included only those under 40 years of age.Citation20 The mean age was 53 years. The total prevalence of MGD ranged from 25.5% to 93.8%. 10 studies found no significant difference between the sexes,Citation9,Citation10,Citation15–17,Citation19,Citation20,Citation24,Citation29,Citation38 two studies found a higher prevalence among men,Citation22,Citation30 one found a higher prevalence among women,Citation18 and the last article did not indicate whether a sex difference existed ().Citation39
Overview of studies with clinical measures
15 studies presented clinical parameters for MGD stratified by sex.Citation12–14,Citation21,Citation23,Citation25–28,Citation31,Citation34,Citation44–47 The studies varied in selection criteria; some only include dry eye patients,Citation21,Citation25,Citation28,Citation44,Citation45 while others include a broad sample of patients at a general eye clinic.Citation14,Citation23,Citation26,Citation34 The clinical studies examined a range of relevant clinical parameters, from glands’ functional assessment (ME and MQ) to MG dropout rate measured with meibography. The studies were conducted in 12 different countries between 2006 and 2021. Sample sizes ranged from 17 to 1662, with a median sample size of 120. All but one study had a higher share of female participants, resulting in an overall ratio of 70% females and 30% males. As far as age range was presented, most studies included participants of all ages, while only one included those middle-aged or older.Citation34 The mean age was 46 years. Across all 15 studies, nine studies found no significant difference between the sexes.Citation12,Citation21,Citation23,Citation25,Citation26,Citation31,Citation44,Citation45,Citation47 Among the three studies that measured MQ and ME, 2 found worse scores among women.Citation28,Citation46 Among the 8 studies that measured meibomian gland loss with meibography, 6 studies found no sex difference,Citation12,Citation21,Citation26,Citation31,Citation44,Citation46 1 study showed greater loss in women,Citation27 and 1 demonstrated greater loss in men.Citation13 The one study that measured meibomian gland dropout incidence showed higher incidence among men.Citation14 However, in subgroup age analysis, this higher incidence was only significant for those over 70 years of age.
Discussion
The overall prevalence of MGD in the population-based studies ranged from 7% to 71%, a slightly wider range than previous estimates of the global prevalence of 21% to 71%.Citation7,Citation8 A majority of studies found a significantly higher prevalence among men, with five studies finding higher rates among men, 1 study finding higher rates among women, and three studies finding no differences. The biggest sex difference was found by the Japanese researchers Arita et al. who reported the prevalence among men and women to be 42.1% and 27.4%, respectively. In the hospital-based studies, the overall prevalence of MGD ranged from 25.5% to 93.8%. In terms of sex-specific prevalence, most of the hospital-based studies found no sex difference. This also applied to studies of clinical measures.
The diagnostic criteria used and the risk of bias varied across the included studies. Interestingly, the only population-based study that found women to have more MGD required the participants to first meet diagnostic criteria for DED, including a SANDE score over 30, in order to be diagnosed with MGD.Citation41 This was also the study finding the lowest prevalence of MGD at only 7%. Conversely, the population-based studies finding a higher prevalence of MGD in men more often used less stringent criteria for diagnosis relying more on observable changes in meibum excretion or meibomian gland health and tended to report much higher prevalence of MGD.Citation11,Citation32,Citation37,Citation40,Citation42 Thus, it is important to bear in mind these diagnostic differences when interpreting the findings.
The current review shows a clear difference between the findings of population-based and hospital-based studies in the sex-specific prevalence of MGD. As the higher prevalence in men observed in many population-based studies does not translate to higher rates in the hospital-based studies, it is essential to distinguish the findings of these studies from each other. In the previous meta-analysis on sex differences in MGD prevalence by Hassanzadeh et al. this was not done.Citation7 The conclusions of Hassanzadeh et al. that men have a higher prevalence of MGD is based on only seven studies, without distinguishing between population-based and hospital-based studies. Our analysis encompasses the same seven studies, and due to our semi-systematic design, we have also critically reviewed a substantially greater number of studies. This allowed us to separate population-based and hospital-based studies, enhancing the granularity, and making our results more comprehensively reflect the overall landscape. Our finding that MGD is most prevalent among men in the population-based studies is, thus, in line with the findings of their meta-analyses. However, this interpretation must be approached with caution, as these ten studies exhibited considerable heterogeneity and used varying diagnostic criteria.
The findings of the population-based studies indicate a higher proportion of male patients with MGD, which may appear unexpected given that female sex is a well-established risk factor for DED in general, and MGD constitutes a significant portion of the DED population.Citation49 An essential aspect to consider in this context is the use of subjective versus objective criteria. The assertion that women are much more susceptible to dry eyes in general populations is based on studies where diagnosis includes self-reported symptoms.Citation50,Citation51 It has been noted that women have lower symptoms reporting thresholds and lower symptom-sign correlations, which might account for their higher prevalence in such studies.Citation25,Citation45,Citation52 In contrast, the MGD diagnosis relies mainly on objective criteria. Although MGD is a common cause of DED, and thus it could be argued that diagnosis require symptoms, it is a broadly used medical term that encompasses various subgroups, such as low-delivery versus high-delivery and symptomatic versus asymptomatic.Citation53 The discrepancies in the definition poses a challenge to agree on one unifying set of diagnostic criteria for MGD, as is evident from the diverse criteria seen in this study. Despite this, most of the current studies report “total MGD” which comprises both symptomatic and asymptomatic cases, with objective criteria being the only requirement for diagnosis. Only two population-based studies mandate a symptom score for diagnosis,Citation11,Citation41 with one of these finding a higher proportion of female patients, albeit with a low total prevalence. This was the only population-based study that detected more females with MGD.Citation41 Such a symptom-driven selection of female patients is also visible in another study that investigated sex-specific MGD prevalence, which discovered that asymptomatic MGD was more prevalent among males, while symptomatic MGD had a similar prevalence in both sexes.Citation37 The use of objective criteria in the population-based studies included in this review, as opposed to other studies on dry eye, may account for a higher prevalence of MGD in men.
The disparity in results between the population-based and hospital-based studies strengthens the hypothesis of a selection effect in hospital-based studies. Unlike in the population-based studies, the overall sex-specific prevalence in hospital-based studies are more similar. Although these studies do not require symptoms in diagnosis, they are mainly conducted on patients who visit eye clinics due to symptoms. Just as women tend to have lower thresholds for reporting symptoms,Citation54,Citation55 studies also show that they have lower thresholds for visiting health clinics.Citation56 This might help explain why the hospital-based studies have a higher share of female participants than the population-based studies (63% vs 54%). A similar trend is also observed in the studies with clinical measures, where as much as 70% of participants were females. The TFOS DEWS II report on Sex, Gender and Hormones highlights the issue of selection bias in clinical-based studies due to gender differences in care-seeking behavior, arguing that sex differences are best studied in population-based studies.Citation57 Hence, even though women tend to report more symptoms and are more frequently represented in clinics,Citation25,Citation56 it is possible that men are actually more prone to the development of MGD. This is consistent with the TFOS report, which suggests that asymptomatic MGD are more prevalent among men.Citation57
One factor that may influence the results is age. In fact, higher age is found to be the strongest predictor for the development of MGD.Citation24 Therefore, studies with more elderly participants are expected to report higher prevalences of MGD, which has been statistically confirmed in another review of the global prevalence of MGD.Citation7 Further, a crucial question in this context is whether sex differences are age-dependent. A challenge is that few studies control for or separate by age, which was also noted in the TFOS DEWS II Epidemiology report.Citation8 In this review, some studies included adult participants of all ages, yet many included only the elderly population. Of the five population-based studies that found higher prevalence among men, four were conducted on an elderly population (over 40 or over 60).Citation32,Citation37,Citation40,Citation42 It is unclear whether this indicates an age-dependent sex difference or just a coincidence. Only one study reported sex-specific prevalences stratified by age groups, which found a higher proportion of men across all four age groups with no significant age trend; however, the entire study population was over 40 years old.Citation42 However, the same study revealed significant increase from pre- to postmenopausal women.Citation42
Age-related sex differences were also noted in the studies with clinical measures. For instance, Den et al. investigated the incidence of meibomian gland dropout and reported no sex difference in the 21–60 y group, but an overrepresentation of men in the >70-year-old group.Citation14 Another study which investigated age effects on meibography outcomes found that MGD loss was more profound in men in the two oldest age groups (60–69 and >80 y), yet similar in the younger groups.Citation13 Their correlation analysis also revealed that the first changes appeared in men in their 20s and in women in their 30s. Taken together, it seems possible that changes in the elderly are more severe in men than in women and changes in meibomian glands develop earlier in men than in women.
Sex hormones are an essential factor to consider when exploring sex- and age-related differences in MGD. It is widely accepted that differences in sex hormones, particularly androgens, play a significant role in sex-related differences in MGD.Citation57 The meibomian gland is an androgen target organ, and androgen deficiency has been identified as a risk factor for MGD and a corresponding evaporative DED.Citation57 For instance, researchers have observed that patients undergoing anti-androgen treatment have significant alterations in their meibomian glands, such as orifice metaplasia, reduced quality of secretions and a marked shift in the neutral lipid profile of meibum.Citation58,Citation59 Furthermore, these patients have a greater frequency of lid abnormalities, corneal staining and tear film instability, as well as an increased level of symptoms.Citation58 Conversely, studies have shown that topical application of dehydroepiandrosterone (DHEA) to a human, as well as to rabbits and dogs, stimulates the elaboration and release of meibomian gland lipids and prolongs the tear film breakup time.Citation60
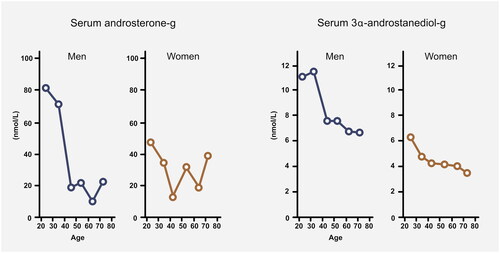
Androgen insufficiency occurs during aging in both sexes, during menopause in women, and as a result of anti-androgen medication use (e.g. for prostatic hypertrophy). A relationship between androgen levels, age and meibomian gland function is well established.Citation57 In fact, a study showed that age-dependent alterations in sebaceous glands directly coincides with the decline in androgen in both sexes.Citation61 The decline in androgen levels occurs at different rates in men and women throughout life, raising the question of whether sex-specific androgen decline could explain the possible sex-specific onset of MGD development. illustrates the decline in the serum concentration of conjugated dihydrotestosterone metabolites, androsterone-glucoronide and 3α-androstanediol-glucoronide.Citation62 These metabolites are considered the most accurate indicators of the total androgen pool, as they directly mirror the intracellular synthesis of androgens in the tissues.Citation63 Although men maintain a higher concentration throughout life, men experience a greater absolute and relative drop in androgens at an earlier stage than women. This could support the findings that men are particularly prone to the effects of increasing age for their meibomian gland health, due to a potentially greater protective effect of high androgens in young age, followed by a more rapid fall in androgen levels. Sullivan et al. discovered disparate gene responses in lacrimal and meibomian glands induced by testosterone in male and female mice.Citation64 Moreover, a recent study on hormone signaling in meibomian glands found that estrogen can counteract the effect of androgen in a “yin-yang” relationship.Citation65 This is in line with previous findings of dose-dependent anti-androgenic actions of estrogens on sebaceous glands.Citation66,Citation67 As the effects of androgens and estrogens are different in males and females, it is plausible that the relative change in androgens may be more important than the absolute serum concentration.
Figure 4. Decline of conjugated dihydrotestosterone metabolites in men and women (Redrawn figure based on results by Labrie et al.Citation62).
The question arises as to whether estrogen concentration influences sex-related disparities in MGD prevalence. If estrogen counteracts the protective effect of androgen, the postmenopausal estrogen drop may result in a relatively heightened protective effect for aging women. Although age-related reduction in meibomian gland health occurs in both sexes,Citation24 one may anticipate a milder age-related deterioration in women. While some studies suggest such a sex-dependent age effect,Citation13,Citation14 additional research is needed to investigate the factor of age in the question of sex differences in MGD is needed. The degree to which sex-specific androgen and estrogen decline contributes to sex differences in MGD remains uncertain.
When investigating sex differences in MGD, it is important to consider all potential causal factors, including those related to gender and behavior. One of the limitations of this article is that the majority of included studies did not control for additional risk factors for MGD. Behavior-related factors such as smoking, medication use, sleep, makeup use and prolonged use of visual displays have been linked to an increased risk of MGD.Citation68–70 Given the different behavioral patterns between the genders concerning these factors, they may serve as significant confounders in the observed sex differences in MGD. For instance, a hospital-based study by Martinez initially identified male sex as a significant risk factor for MGD; however, subsequent adjustments for other factors, including anti-hypertension medication, arthritis, and contact lens wear, through multiple logistic regression analysis revealed that the observed sex difference was no longer significant.Citation30 Thus, the increased use of certain medication tied to higher risk of MGD among men may amplify or confound any biological mechanisms present.Citation30,Citation42 Conversely, women use more cosmetics, a factor associated with increased risk of DED, and appear to have a negative impact on the meibomian glands.Citation71 As numerous biological and behavioral factors may influence the sex disparities observed in MGD prevalence, determining which factors are most significant remains a challenging task and more studies are needed.
A central weakness of this article is the large variation in diagnostic criteria and study populations. Although 5 of the hospital-based studies have used the same diagnostic criteria suggested by the international workshop on meibomian gland dysfunction,Citation68 the prevalence differed widely (between 25.5% and 93.8%). Further, none of these 5 studies reported any statistical sex difference. The workshop report also suggests evaluating morphological lid features in the assessment of MGD, which was included in many of the studies. However, some studies set the diagnosis based on abnormal lid features alone.Citation36,Citation37,Citation42 Siak et al. diagnosed MGD by either orifice plugging or telangiectasia in at least one eye of each participant.Citation42 Viso et al. also included telangiectasia alone as a diagnosis for MGD in two studies,Citation36,Citation37 which weakens the validity of the results. Even though the presence of MGD can cause telangiectasia, it is not an intrinsic factor. Telangiectasia may have multiple other causes, including genetics, environmental causes, alcohol intake, corticosteroid treatment, dermatomyositis, and lupus.Citation72,Citation73 Therefore, their findings that men are overrepresented may, in reality, be influenced by factors other than MGD.
Conclusion
In conclusion, this review provides a comprehensive assessment of the current literature on sex differences in prevalence of MGD. The evidence indicates that MGD is a widespread disorder among both sexes. While population-based studies lean towards men being more affected than women, most clinic-based studies find no significant difference. The disparity of results underscores the impact of selection bias on epidemiological studies on sex and gender differences. Although men being less likely to visit health care practitioners, clinicians should be aware that MGD affects men in the general population as frequently as women. Further research is needed to establish whether men are indeed more susceptible to MGD than women. There is a considerable variation between studies in terms of quality, sample size, age ranges and diagnostic criteria. Future studies should include large samples, make comparisons based on sex, control for age, and use standardized criteria for evaluating MGD.
Supplemental Material
Download Zip (489.4 KB)Disclosure
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
- Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, Liu Z, Nelson JD, Nichols JJ, Tsubota K, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15(3):276–283. doi:10.1016/j.jtos.2017.05.008.
- Kim H, An Y, Hwang WJ. Gender differences in dry eye disease symptoms associated with psychological health indicators among adults using mobile mental health apps. PLoS One. 2023;18(1):e0278921. doi:10.1371/journal.pone.0278921.
- Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118(9):1264–1268. doi:10.1001/archopht.118.9.1264.
- Ahn JM, Lee SH, Rim TH, Park RJ, Yang HS, Kim TI, Yoon KC, Seo KY, Epidemiologic Survey Committee of the Korean Ophthalmological Society. Prevalence of and risk factors associated with dry eye: the Korea National Health and Nutrition Examination Survey 2010-2011. Am J Ophthalmol. 2014;158(6):1205–1214.e7. doi:10.1016/j.ajo.2014.08.021.
- Castro JS, Selegatto IB, Castro RS, Miranda ECM, de Vasconcelos JPC, de Carvalho KM, Arieta CEL, Alves M. Prevalence and Risk Factors of self-reported dry eye in Brazil using a short symptom questionnaire. Sci Rep. 2018;8(1):2076. doi:10.1038/s41598-018-20273-9.
- Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, Knop E, Markoulli M, Ogawa Y, Perez V, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438–510. doi:10.1016/j.jtos.2017.05.011.
- Hassanzadeh S, Varmaghani M, Zarei-Ghanavati S, Heravian Shandiz J, Azimi Khorasani A. Global Prevalence of Meibomian Gland Dysfunction: a Systematic Review and Meta-Analysis. Ocul Immunol Inflamm. 2021;29(1):66–75. doi:10.1080/09273948.2020.1755441.
- Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, Na KS, Schaumberg D, Uchino M, Vehof J, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15(3):334–365. doi:10.1016/j.jtos.2017.05.003.
- Amano S, Inoue K. Clinic-Based Study on Meibomian Gland Dysfunction in Japan. Invest Ophthalmol Vis Sci. 2017;58(2):1283–1287. doi:10.1167/iovs.16-21374.
- Amano S, Inoue K. Estimation of Prevalence of Meibomian Gland Dysfunction in Japan. Cornea. 2017;36(6):684–688. doi:10.1097/ICO.0000000000001208.
- Arita R, Mizoguchi T, Kawashima M, Fukuoka S, Koh S, Shirakawa R, Suzuki T, Morishige N. Meibomian gland dysfunction and dry eye are similar but different based on a population-based study: the Hirado-Takushima Study in Japan. Am J Ophthalmol. 2019;207:410–418. doi:10.1016/j.ajo.2019.02.024.
- Ban Y, Shimazaki-Den S, Tsubota K, Shimazaki J. Morphological evaluation of meibomian glands using noncontact infrared meibography. Ocul Surf. 2013;11(1):47–53. doi:10.1016/j.jtos.2012.09.005.
- Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology. 2008;115(5):911–915. doi:10.1016/j.ophtha.2007.06.031.
- Den S, Shimizu K, Ikeda T, Tsubota K, Shimmura S, Shimazaki J. Association between meibomian gland changes and aging, sex, or tear function. Cornea. 2006;25(6):651–655. doi:10.1097/01.ico.0000227889.11500.6f.
- Singh J, Priya Y, Bhat V. Prevalence, severity, and treatment outcomes of meibomian gland dysfunction in patients with dry eye symptoms at a Tertiary Care Center in South India. Cureus. 2022;14(6):e25703. doi:10.7759/cureus.25703.
- Yadav S, Gupta N, Makwana T, Vanathi M, Tandon R. Noninvasive ocular surface analyzer as an adjunct in diagnosis and estimating prevalence of meibomian gland dysfunction: hospital-based comparative study. Indian J Ophthalmol. 2022;70(5):1539–1545. doi:10.4103/ijo.IJO_2245_21.
- Chatterjee S, Agrawal D, Sharma A. Meibomian gland dysfunction in a hospital-based population in central India. Cornea. 2020;39(5):634–639. doi:10.1097/ICO.0000000000002217.
- Rathnakumar K, Ramachandran K, Baba D, Ramesh V, Anebaracy V, Vidhya R, Vinothkumar R, Poovitha R, Geetha R. Prevalence of dry eye disease and its association with dyslipidemia. J Basic Clin Physiol Pharmacol. 2018;29(2):195–199. doi:10.1515/jbcpp-2017-0001.
- Abu EK, Ofori AO, Boadi-Kusi SB, Ocansey S, Yankah RK, Kyei S, Awuku AY. Dry eye disease and meibomian gland dysfunction among a clinical sample of type 2 diabetes patients in Ghana. Afr Health Sci. 2022;22(1):293–302. doi:10.4314/ahs.v22i1.36.
- Asiedu K, Kyei S, Dzasimatu SK, Morny EKA. Meibomian gland dysfunction in a youthful clinical sample in Ghana. Optom Vis Sci. 2018;95(4):349–353. doi:10.1097/OPX.0000000000001192.
- Osae EA, Ablorddepey RK, Horstmann J, Kumah DB, Steven P. Assessment of meibomian glands using a custom-made meibographer in dry eye patients in Ghana. BMC Ophthalmol. 2018;18(1):201. doi:10.1186/s12886-018-0869-0.
- Mussi N, Haque W, Robertson DM. The association between risk factors for metabolic syndrome and meibomian gland disease in a dry eye cohort. Clin Ophthalmol. 2021;15:3821–3832. doi:10.2147/OPTH.S322461.
- Brooks CC, Gupta PK. Meibomian gland morphology among patients presenting for refractive surgery evaluation. Clin Ophthalmol. 2021;15:315–321. doi:10.2147/OPTH.S292919.
- Hom MM, Martinson JR, Knapp LL, Paugh JR. Prevalence of Meibomian gland dysfunction. Optom Vis Sci. 1990;67(9):710–712. doi:10.1097/00006324-199009000-00010.
- Borrelli M, Frings A, Geerling G, Finis D. Gender-specific differences in signs and symptoms of dry eye disease. Curr Eye Res. 2021;46(3):294–301. doi:10.1080/02713683.2020.1801758.
- Pult H. Relationships between meibomian gland loss and age, sex, and dry eye. Eye Contact Lens. 2018;44 Suppl 2: S318–S324. doi:10.1097/ICL.0000000000000467.
- Pult H, Riede-Pult BH. Non-contact meibography: keep it simple but effective. Cont Lens Anterior Eye. 2012;35(2):77–80. doi:10.1016/j.clae.2011.08.003.
- Nøland ST, Badian RA, Utheim TP, Utheim ØA, Stojanovic A, Tashbayev B, Raeder S, Dartt DA, Chen X. Sex and age differences in symptoms and signs of dry eye disease in a Norwegian cohort of patients. Ocul Surf. 2021;19:68–73. doi:10.1016/j.jtos.2020.11.009.
- Badian RA, Utheim TP, Chen X, Utheim ØA, Ræder S, Ystenæs AE, Aakre BM, Sundling V. Meibomian gland dysfunction is highly prevalent among first-time visitors at a Norwegian dry eye specialist clinic. Sci Rep. 2021;11(1):23412. doi:10.1038/s41598-021-02738-6.
- Martinez JD, Galor A, Ramos-Betancourt N, Lisker-Cervantes A, Beltrán F, Ozorno-Zárate J, Sánchez-Huerta V, Torres-Vera MA, Hernández-Quintela E. Frequency and risk factors associated with dry eye in patients attending a tertiary care ophthalmology center in Mexico City. Clin Ophthalmol. 2016;10:1335–1342. doi:10.2147/OPTH.S106451.
- Crespo-Treviño RR, Salinas-Sánchez AK, Amparo F, Garza-Leon M. Comparative of meibomian gland morphology in patients with evaporative dry eye disease versus non-dry eye disease. Sci Rep. 2021;11(1):20729. doi:10.1038/s41598-021-00122-y.
- Hashemi H, Asharlous A, Aghamirsalim M, Yekta A, Pourmatin R, Sajjadi M, Pakbin M, Asadollahi M, Khabazkhoob M. Meibomian gland dysfunction in geriatric population: tehran geriatric eye study. Int Ophthalmol. 2021;41(7):2539–2546. doi:10.1007/s10792-021-01812-2.
- Hashemi H, Rastad H, Emamian MH, Fotouhi A. Meibomian gland dysfunction and its determinants in Iranian adults: a population-based study. Cont Lens Anterior Eye. 2017;40(4):213–216. doi:10.1016/j.clae.2017.05.003.
- Lin X, Wu Y, Chen Y, Zhao Y, Xiang L, Dai Q, Fu Y, Zhao Y, Zhao YE. Characterization of meibomian gland atrophy and the potential risk factors for middle aged to elderly patients with cataracts. Transl Vis Sci Technol. 2020;9(7):48. doi:10.1167/tvst.9.7.48.
- Gao JG, Chen J, Tang Y, Chen DN. Prevalence of meibomian gland dysfunction in staffs and faculty members of a Chinese university. Int J Ophthalmol. 2020;13(10):1667–1670. doi:10.18240/ijo.2020.10.23.
- Viso E, Gude F, Rodríguez-Ares MT. The association of meibomian gland dysfunction and other common ocular diseases with dry eye: a population-based study in Spain. Cornea. 2011;30(1):1–6. doi:10.1097/ICO.0b013e3181da5778.
- Viso E, Rodríguez-Ares MT, Abelenda D, Oubiña B, Gude F. Prevalence of asymptomatic and symptomatic meibomian gland dysfunction in the general population of Spain. Invest Ophthalmol Vis Sci. 2012;53(6):2601–2606. doi:10.1167/iovs.11-9228.
- Robin M, Liang H, Rabut G, Augstburger E, Baudouin C, Labbé A. The role of meibography in the diagnosis of meibomian gland dysfunction in ocular surface diseases. Transl Vis Sci Technol. 2019;8(6):6. doi:10.1167/tvst.8.6.6.
- Rabensteiner DF, Aminfar H, Boldin I, Schwantzer G, Horwath-Winter J. The prevalence of meibomian gland dysfunction, tear film and ocular surface parameters in an Austrian dry eye clinic population. Acta Ophthalmol. 2018;96(6):e707–e711. doi:10.1111/aos.13732.
- Aapola U, Nättinen J, Suurkuukka I, Tuomilehto J, Keinänen-Kiukaanniemi S, Saramies J, Uusitalo H. Ocular surface health of the Finnish elderly population. Acta Ophthalmol. 2022;100(8):894–902. doi:10.1111/aos.15130.
- Craig JP, Wang MTM, Ambler A, Cheyne K, Wilson GA. Characterising the ocular surface and tear film in a population-based birth cohort of 45-year old New Zealand men and women. Ocul Surf. 2020;18(4):808–813. doi:10.1016/j.jtos.2020.08.005.
- Siak JJ, Tong L, Wong WL, Cajucom-Uy H, Rosman M, Saw SM, Wong TY. Prevalence and risk factors of meibomian gland dysfunction: the Singapore Malay eye study. Cornea. 2012;31(11):1223–1228. doi:10.1097/ICO.0b013e31823f0977.
- Lin PY, Tsai SY, Cheng CY, Liu JH, Chou P, Hsu WM. Prevalence of dry eye among an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology. 2003;110(6):1096–1101. doi:10.1016/S0161-6420(03)00262-8.
- Jeon YJ, Song MY, Kim KY, Hwang KY, Kwon YA, Koh K. Relationship between the partial blink rate and ocular surface parameters. Int Ophthalmol. 2021;41(7):2601–2608. doi:10.1007/s10792-021-01819-9.
- Vehof J, Sillevis Smitt-Kamminga N, Nibourg SA, Hammond CJ. Sex differences in clinical characteristics of dry eye disease. Ocul Surf. 2018;16(2):242–248. doi:10.1016/j.jtos.2018.01.001.
- Machalińska A, Zakrzewska A, Safranow K, Wiszniewska B, Machaliński B. Risk factors and symptoms of meibomian gland loss in a healthy population. J Ophthalmol. 2016;2016:7526120. doi:10.1155/2016/7526120.
- Yeotikar NS, Zhu H, Markoulli M, Nichols KK, Naduvilath T, Papas EB. Functional and morphologic changes of meibomian glands in an asymptomatic adult population. Invest Ophthalmol Vis Sci. 2016;57(10):3996–4007. doi:10.1167/iovs.15-18467.
- Subcommittee of the International Dry Eye WorkShop. Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):108–152. doi:10.1016/s1542-0124(12)70083-6.
- Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472–478. doi:10.1097/ICO.0b013e318225415a.
- Rapoport Y, Singer JM, Ling JD, Gregory A, Kohanim S. A comprehensive review of sex disparities in symptoms, pathophysiology, and epidemiology of dry eye syndrome. Semin Ophthalmol. 2016;31(4):325–336. doi:10.3109/08820538.2016.1154168.
- Begley CG, Chalmers RL, Abetz L, Venkataraman K, Mertzanis P, Caffery BA, Snyder C, Edrington T, Nelson D, Simpson T. The relationship between habitual patient-reported symptoms and clinical signs among patients with dry eye of varying severity. Invest Ophthalmol Vis Sci. 2003;44(11):4753–4761. doi:10.1167/iovs.03-0270.
- Hashmi JA, Davis KD. Deconstructing sex differences in pain sensitivity. Pain. 2014;155(1):10–13. doi:10.1016/j.pain.2013.07.039.
- Nichols KK, Foulks GN, Bron AJ, Glasgow BJ, Dogru M, Tsubota K, Lemp MA, Sullivan DA. The International Workshop on Meibomian Gland Dysfunction: executive Summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922–1929. doi:10.1167/iovs.10-6997a.
- Ladwig KH, Marten-Mittag B, Formanek B, Dammann G. Gender differences of symptom reporting and medical health care utilization in the German population. Eur J Epidemiol. 2000;16(6):511–518. doi:10.1023/a:1007629920752.
- Almeida SA, Trone DW, Leone DM, Shaffer RA, Patheal SL, Long K. Gender differences in musculoskeletal injury rates: a function of symptom reporting? Med Sci Sports Exerc. 1999;31(12):1807–1812. doi:10.1097/00005768-199912000-00017.
- Thompson AE, Anisimowicz Y, Miedema B, Hogg W, Wodchis WP, Aubrey-Bassler K. The influence of gender and other patient characteristics on health care-seeking behaviour: a QUALICOPC study. BMC Fam Pract. 2016;17(1):38. doi:10.1186/s12875-016-0440-0.
- Sullivan DA, Rocha EM, Aragona P, Clayton JA, Ding J, Golebiowski B, Hampel U, McDermott AM, Schaumberg DA, Srinivasan S, et al. TFOS DEWS II Sex, Gender, and Hormones Report. Ocul Surf. 2017;15(3):284–333. doi:10.1016/j.jtos.2017.04.001.
- Krenzer KL, Dana MR, Ullman MD, Cermak JM, Tolls DB, Evans JE, Sullivan DA. Effect of androgen deficiency on the human meibomian gland and ocular surface. J Clin Endocrinol Metab. 2000;85(12):4874–4882. doi:10.1210/jcem.85.12.7072.
- Sullivan BD, Evans JE, Krenzer KL, Reza Dana M, Sullivan DA. Impact of antiandrogen treatment on the fatty acid profile of neutral lipids in human meibomian gland secretions. J Clin Endocrinol Metab. 2000;85(12):4866–4873. doi:10.1210/jcem.85.12.7066.
- Zeligs M, Gordon KB. Dehydroepiandrosterone therapy for the treatment of dry eye disorders. 1994. WO1994004155 A1. PCT/US1992/007096.
- Bolognia JL. Aging skin. Am J Med. 1995;98(1a):99s–103s. doi:10.1016/s0002-9343(99)80066-7.
- Labrie F, Bélanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997;82(8):2396–2402. doi:10.1210/jcem.82.8.4160.
- Labrie F, Bélanger A, Cusan L, Candas B. Physiological changes in dehydroepiandrosterone are not reflected by serum levels of active androgens and estrogens but of their metabolites: intracrinology. J Clin Endocrinol Metab. 1997;82(8):2403–2409. doi:10.1210/jcem.82.8.4161.
- Sullivan DA, Jensen RV, Suzuki T, Richards SM. Do sex steroids exert sex-specific and/or opposite effects on gene expression in lacrimal and meibomian glands? Mol Vis. 2009; Aug 1015:1553–72.
- Reneker LW, Lubahn DB, Huang AJW. The Cross-talk between Androgen and Estrogen Signaling in Meibomian Glands. IOVS. 2023;64(8):676–676.
- Pochi PE, Strauss JS. Endocrinologic control of the development and activity of the human sebaceous gland. J Invest Dermatol. 1974;62(3):191–201. doi:10.1111/1523-1747.ep12676783.
- Sweeney TM, Szarnicki RJ, Strauss JS, Pochi PE. The effect of estrogen and androgen on the sebaceous gland turnover time. J Invest Dermatol. 1969;53(1):8–10. doi:10.1038/jid.1969.100.
- Tomlinson A, Bron AJ, Korb DR, Amano S, Paugh JR, Pearce EI, Yee R, Yokoi N, Arita R, Dogru M. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):2006–2049. doi:10.1167/iovs.10-6997f.
- Yazdani M, Elgstøen KBP, Utheim TP. Eye Make-up Products and Dry Eye Disease: a Mini Review. Curr Eye Res. 2022;47(1):1–11. doi:10.1080/02713683.2021.1966476.
- Fenga C, Aragona P, Cacciola A, Spinella R, Di Nola C, Ferreri F, Rania L. Meibomian gland dysfunction and ocular discomfort in video display terminal workers. Eye (Lond)). 2008;22(1):91–95. doi:10.1038/sj.eye.6703025.
- Wang J, Liu Y, Kam WR, Li Y, Sullivan DA. Toxicity of the cosmetic preservatives parabens, phenoxyethanol and chlorphenesin on human meibomian gland epithelial cells. Exp Eye Res. 2020;196:108057. doi:10.1016/j.exer.2020.108057.
- Kritharis A, Al-Samkari H, Kuter DJ. Hereditary hemorrhagic telangiectasia: diagnosis and management from the hematologist’s perspective. Haematologica. 2018;103(9):1433–1443. doi:10.3324/haematol.2018.193003.
- Jain NP, Shao K, Stewart C, Grant-Kels JM. The effects of alcohol and illicit drug use on the skin. Clin Dermatol. 2021;39(5):772–783. doi:10.1016/j.clindermatol.2021.05.005.