Abstract
Objective: The Asthma Salford Lung Study demonstrated the effectiveness of initiating once-daily fluticasone furoate/vilanterol (FF/VI) versus continuing usual care in asthma patients in UK primary care [Citation1]. Here, we report a secondary analysis in a subset of patients with fluticasone propionate/salmeterol (FP/Salm) as their baseline intended maintenance therapy, to evaluate the relative effectiveness of initiating FF/VI versus continuing FP/Salm. Methods: Adults with symptomatic asthma were randomised to initiate FF/VI 100[200]/25 µg or continue FP/Salm. The Asthma Control Test (ACT), Asthma Quality of Life Questionnaire (AQLQ), Work Productivity and Activity Impairment Asthma questionnaire, severe exacerbations, salbutamol inhaler prescriptions and serious adverse events (SAEs) were recorded throughout the 12-month treatment period. Results: One thousand two hundred and sixty-four patients (FF/VI 646; FP/Salm 618) were included in this subset analysis; 978 had baseline ACT score <20 and were included in the primary effectiveness analysis (PEA) population. At week 24, odds of patients being ACT responders (total score ≥20 and/or improvement from baseline ≥3) were significantly higher with FF/VI versus FP/Salm (71% vs. 56%; odds ratio 2.03 [95% CI: 1.53, 2.68]; p < 0.001 [PEA]). Significant benefit with FF/VI versus FP/Salm was also observed for AQLQ responders, activity impairment due to asthma, exacerbation rates, and salbutamol inhalers prescribed. No significant between-group differences were observed for impairment while working or work absenteeism due to asthma. Conclusions: For patients in primary care, initiating FF/VI was significantly better than continuing with FP/Salm for improving asthma control and quality of life, and reducing asthma exacerbations, with no notable difference in SAEs.
ClinicalTrials.gov: NCT01706198.
Introduction
The Salford Lung Study in asthma (SLS asthma) assessed the effectiveness and safety of initiating treatment with the once-daily inhaled combination of fluticasone furoate and vilanterol (FF/VI) compared with continuing usual care (UC, comprising an inhaled corticosteroid ± long-acting beta2-agonist [ICS ± LABA]) over 52 weeks [Citation1]. The study was conducted in a real-world population of asthma patients in a primary care setting in and around Salford, UK. Patients initiating treatment with FF/VI had twice the odds of achieving or improving asthma control (as measured using the Asthma Control Test [ACT]) compared with those continuing on UC; this benefit was consistent regardless of patients’ baseline maintenance therapy (ICS or ICS/LABA) [Citation1].
Asthma is a common condition affecting more than 300 million people worldwide [Citation2]. Asthma management guidelines recommend the addition of a LABA to ICS for patients whose asthma is not adequately controlled on low-dose ICS alone [Citation3,Citation4]. A number of ICS/LABA combination products are available for asthma and most are indicated for twice-daily use. FF/VI is the only once-daily ICS/LABA currently available. One of the most commonly used twice-daily ICS/LABAs is fluticasone propionate/salmeterol (FP/Salm). A Phase III efficacy trial comparing FF/VI with FP/Salm showed no significant difference between the treatments at week 24 for the primary endpoint of 0–24 h weighted mean forced expiratory volume in 1 s [Citation5] or in standardised measures of health-related quality of life (HR-QoL; Asthma Quality of Life Questionnaire [AQLQ] and EuroQol-5D). However, post-hoc analyses indicated clinically relevant improvements in AQLQ total score responders with FF/VI versus FP/Salm.
Although viewed as the highest form of scientific evidence, traditional randomised controlled trials (RCTs) have limited relevance to everyday clinical practice, due to their strict inclusion/exclusion criteria for patient enrolment. In one study by Herland et al., only 3.3% of patients with asthma visiting their doctor’s surgery were eligible for recruitment into a typical asthma clinical trial [Citation6]. The generalisability of data from such trials to a broader population of patients with asthma is therefore questionable. Furthermore, in a traditional Phase III double-blind, double-dummy trial, there would be little opportunity to demonstrate the impact on patient outcomes, of features of FF/VI such as once-daily use, long duration of action of the components, and delivery via an easy-to-use ELLIPTA inhaler. Such features may be better explored in real-world effectiveness studies, which more closely reflect the routine clinical practice setting.
We conducted pre-planned and post-hoc analyses in a subset of patients who participated in SLS asthma who had FP/Salm as their baseline intended maintenance therapy prior to randomisation, in order to evaluate the relative effectiveness of initiating treatment with FF/VI versus continuing FP/Salm.
Methods
Study design and patients
SLS asthma was a prospective, open-label RCT conducted at 74 primary care practices in Salford and South Manchester, UK. Details of the study design have been published previously [Citation1,Citation7]. Patients were aged ≥18 years with a general practitioner (GP) diagnosis of symptomatic asthma maintained on ICS ± LABA. Randomisation to initiate FF/VI or continue UC was stratified according to baseline intended asthma maintenance therapy (ICS or ICS/LABA) and baseline ACT total score (≥20, 16–19, ≤15). For inclusion in the subset analysis reported here, patients were required to have been included in the ICS/LABA randomisation stratum and have had branded FP/Salm (Seretide) as their intended prescription at randomisation. Patients in the subset described herein were randomly assigned to initiate treatment with FF/VI 100/25 µg or 200/25 µg (FF/VI group) or to continue on FP/Salm (Seretide; FP/Salm group).
In SLS asthma, patients were recruited and managed by the GPs who provided their usual clinical care. Data were captured remotely and continuously via patients’ electronic health records (EHRs) using a primary and secondary care-linked database system developed by NorthWest EHealth. All patients provided written informed consent. The study was conducted in accordance with International Conference on Harmonisation Good Clinical Practice and with the principles founded in the Declaration of Helsinki 2008. The trial was approved by the National Research Ethics Service Committee North West, Greater Manchester South (approval number: 12/NW/0455). The study is registered with ClinicalTrials.gov (NCT01706198; GSK study HZA115150).
Study procedures
At Visit 1, patients were given information about the trial and informed consent was obtained. At Visit 2 (randomisation visit [baseline]), GPs were required to write a prescription for patients’ intended asthma medication. Patients were then randomised 1:1 to initiate FF/VI or to continue their UC, where UC was the prescription patients received prior to randomisation. Only those patients whose intended prescription at baseline was FP/Salm were included in this subset analysis. At Visit 2, baseline assessments of asthma control using the ACT, AQLQ and the Work Productivity and Activity Impairment Asthma questionnaire (WPAI: asthma) were collected. At 52 weeks following Visit 2 or at the early withdrawal visit, patients were required to complete the same questionnaires. Additionally, patients were contacted by telephone at 12, 24 and 40 weeks; ACT was completed at each time point. Assessment time points are shown in .
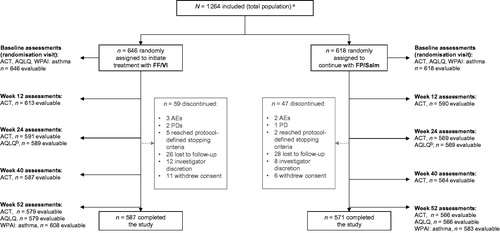
Figure 1. Patient flow through study.
Notes. ACT: Asthma Control Test; AE: adverse event; AQLQ: Asthma Quality of Life Questionnaire; FF/VI: fluticasone furoate/vilanterol; FP/Salm: fluticasone propionate/salmeterol; PD: protocol deviation; WPAI: asthma: Work Productivity and Activity Impairment: Asthma questionnaire. aThe total population includes all randomly assigned patients who received at least one prescription of study medication, were included in the ICS/LABA stratum and whose prerandomisation intended prescription was FP/Salm. bWeek 24 AQLQ data are not reported for this FP/Salm subset analysis. Other endpoints, including safety, exacerbations and prescribed/dispensed/collected medications, were captured throughout the study.
Safety was monitored throughout the study; only information regarding non-serious adverse drug reactions (ADRs; events which the investigator assesses as having a reasonable possibility of being related to treatment) and serious adverse events (SAEs) were collected. Safety data were monitored remotely via patients’ EHRs using the NorthWest EHealth linked database system. Patients were also contacted via telephone at Weeks 12, 24 and 40 by a study team member who assessed patients for non-serious ADRs and SAEs. The GP/investigator or site staff were responsible for detecting, documenting in patients’ electronic case report forms, and reporting events that met the definition for a non-serious ADR or an SAE.
To maintain the real-world nature of the trial, patient care was kept as close as possible to everyday clinical practice. Patients could modify their treatment and remain in the study; patients randomised to receive FF/VI could modify to UC but patients randomised to receive UC (i.e. FP/Salm for this subset analysis) could not receive FF/VI. Patients accessed their medication from community pharmacists in their usual way.
Study assessments/outcomes
As per the main SLS asthma study, the primary outcome of interest in this subset analysis was the percentage of patients at week 24 achieving an ACT total score ≥20 and/or improvement from baseline ≥3 (termed ‘responders’). The ACT is a validated, simple, self-administered questionnaire comprising five questions that assess asthma control during the past 4 weeks on a 5-point categorical scale. The cut-off for well-controlled asthma is ≥20 points [Citation8] and an increase of ≥3 points has been reported to be the minimal clinically important difference (MCID) [Citation9]. For this subset analysis, the percentage of ACT responders at week 24 was analysed, as well as ACT responders and change from baseline in ACT score at weeks 12, 24, 40 and 52. ACT responder analyses were conducted a priori with p-values calculated post-hoc.
Several secondary study outcomes were also evaluated in this subset analysis, including AQLQ, WPAI: asthma, severe exacerbation rates, salbutamol inhalers prescribed, adherence and safety. With the exception of exacerbation rates (a priori analysis with p-values calculated post-hoc), all secondary outcomes were analysed post-hoc.
The AQLQ is a disease-specific, self-administered questionnaire comprising 32 questions in four domains (environmental stimuli, symptoms, activity limitation, and emotional function). The total and domain scores are defined on a range of 1–7 with higher scores indicating better HR-QoL. For this subset analysis, the percentage of AQLQ responders (defined as patients who achieved an increase from baseline of ≥0.5 points, the MCID for AQLQ [Citation10]) for AQLQ total score and for each of the individual AQLQ domains was assessed at week 52.
The WPAI: asthma questionnaire is a validated, self-administered, 6-item questionnaire [Citation11,Citation12] designed to quantitatively assess patients’ overall work impairment and overall activity impairment due to asthma over the previous 7 days. Change from baseline in derived scores of the WPAI: asthma questionnaire was analysed at week 52/early withdrawal visit.
Mean annual rates of severe asthma exacerbations (defined as any worsening of symptoms treated with systemic corticosteroids or antibiotics, an inpatient hospitalisation, or emergency department visit due to asthma that required systemic corticosteroids or antibiotics) were calculated.
The number of salbutamol inhalers prescribed during the study, time-to-first treatment modification, and adherence (as measured by the proportion of days covered by study medication) were also assessed.
Safety endpoints included SAEs of pneumonia, all SAEs and non-serious ADRs. Serious adverse events of special interest (SAESI) were defined, a priori, as groups of events of interest based on the known pharmacological action of ICS or LABA therapy and were defined in one of two ways. Standardised Medical Dictionary for Regulatory Activities (MedDRA) queries (SMQs; predefined MedDRA lists of preferred terms allowing for comprehensive review of safety data not limited to a specific preferred term) were used where available for specific SAESI. Where SMQs were not available in MedDRA, a sponsor-defined list of all relevant preferred terms was used.
The pneumonia SAESI group was defined by 69 MedDRA preferred terms; any verbatim term for an SAE that mapped to one of these terms was counted as a pneumonia SAESI. A diagnostic X-ray was not required, although it was possible to identify patients who had an X-ray and review whether the imaging showed pneumonia.
Statistical analyses
All effectiveness analyses were conducted according to the intention-to treat principle, i.e. patients were analysed according to the treatment group to which they had been randomly assigned regardless of any treatment modification during the study. Safety data were analysed according to the actual treatment patients were taking at the time of the event, with the exception of pneumonia, which was analysed according to the treatment patients were receiving for the majority of the time in the 28 days preceding the event, as well as by randomised treatment group as requested by regulators.
The FP/Salm subset of the overall SLS asthma population was defined as patients who were randomised and received at least one prescription of study medication, and who were included in the ICS/LABA randomisation stratum with FP/Salm as their intended pre-randomisation prescription (referred to hereafter as the total population). ACT responder analyses were additionally conducted in patients in the FP/Salm subset total population who had an ACT total score of <20 at baseline (referred to hereafter as the primary effectiveness analysis [PEA] population).
Analyses of between-group differences for the percentage of ACT responders at week 24 and mean annual rates of severe asthma exacerbations were conducted a priori, but p values were calculated post-hoc; all other analyses were conducted post-hoc. All analyses were performed at the 5% significance level with no adjustment for multiplicity.
The primary endpoint of percentage of ACT responders (patients achieving ACT total score ≥20 and/or improvement from baseline ≥3) at week 24 was analysed using logistic regression adjusting for randomised treatment, baseline ACT total score, baseline ACT total score squared, age and gender. Odds ratios (ORs) and associated 95% confidence intervals (CIs) and p values for the difference between the treatment groups (initiating FF/VI vs. continuing FP/Salm) were calculated. ACT responder analyses were also conducted at weeks 12, 40 and 52.
Change from baseline in ACT total score at weeks 12, 24, 40 and 52 was analysed using mixed model repeated measures adjusting for randomised treatment, baseline ACT total score, randomised treatment-by-baseline ACT total score interaction, gender, age, visit and randomised treatment-by-visit interaction with an unstructured covariance matrix. Least squares (LS) mean changes from baseline were calculated for each treatment group together with LS mean differences and the associated 95% CIs and p values.
ORs, 95% CIs and p values for the treatment difference in the percentage of AQLQ responders at week 52 were calculated using logistic regression analysis adjusting for randomised treatment, ACT total score at baseline per randomisation stratum, age, gender and baseline AQLQ score.
The mean annual rate of severe asthma exacerbations was analysed using a generalised linear model assuming the negative binomial distribution, adjusting for randomised treatment, ACT total score at baseline per randomisation stratification, age, gender and number of severe asthma exacerbations in the previous year prior to randomisation categorised. The offset variable was logarithm of time on treatment and the LS mean annual rate and associated 95% CIs were calculated for each treatment group. Treatment differences were evaluated by estimating the ratio of LS mean annual rates, associated 95% CIs, and p values. The percentage reduction in the mean annual rate of exacerbations and 95% CI was subsequently calculated.
Changes from baseline in WPAI: asthma derived scores at week 52/early withdrawal visit were analysed using an analysis of covariance (ANCOVA) model adjusting for randomised treatment, ACT total score at baseline per randomisation stratification, gender, age and baseline WPAI score. LS mean change from baseline for each treatment group, LS mean differences with associated 95% CIs, and p values were calculated.
The number of salbutamol inhalers prescribed during the study was adjusted for each patient, taking time on treatment into account so that it corresponded to 12 months on treatment. One inhaler was defined as 200 actuations. An ANCOVA model adjusting for randomised treatment, ACT total score at baseline per randomisation stratification, gender, age and number of salbutamol inhalers prescribed in the year prior to randomisation, was used to calculated LS means for each treatment group and the LS mean difference with associated 95% CI and p values.
Time-to-modification of initial therapy was analysed using a Cox proportional hazards model adjusting for randomised treatment, ACT total score at baseline per randomisation stratification, gender and age. The hazard ratio (HR) for the difference between the FF/VI and FP/Salm groups was calculated with the associated 95% CI and p values.
Adherence to treatment was estimated by the proportion of days covered (PDC) by study medication, as calculated from prescription data captured in patients’ electronic case report forms. Summary statistics were descriptive only.
Results
Of the 4233 patients randomised to treatment in the SLS asthma study, 1264 were included in the ICS/LABA stratum and had FP/Salm as their intended prescription prior to randomisation; these patients comprised the total population for this subset analysis. Of these, 978 had a baseline ACT score of <20 and were included in the PEA population.
In the total population, the FF/VI (n = 646) and FP/Salm (n = 618) groups were well matched for age, gender, duration of asthma and asthma symptoms (). Around 45% had ≥1 exacerbation in the previous year and >90% reported daytime symptoms occurring more than twice a week. In total, 1158/1264 (92%) of patients in the total population completed the study (); the most frequent reason for study withdrawal was loss to follow-up.
Table 1. Patient demographics and baseline asthma characteristics (total population).
In the FF/VI group, 138 (21%) patients modified their treatment, with the most common modification being switching from FF/VI to UC (119 patients, 18%). In the FP/Salm group, 147 patients (24%) modified their treatment at least once during the study; the most common modification was switching brand of medication (98 patients, 16%). Where a reason was specified, the most common reason cited for treatment modification was patient preference in the FF/VI group (25%) and an increase in dose due to need for better control in the FP/Salm group (25%). There was no significant difference between the FF/VI and FP/Salm groups for time-to-modification of initial treatment (HR 0.94 [95% CI 0.76, 1.16], p = 0.571). Adherence, as measured by the PDC, was high in both treatment groups; FF/VI 82.4% (standard deviation [SD] 23.1), FP/Salm 76.6% (SD 25.7).
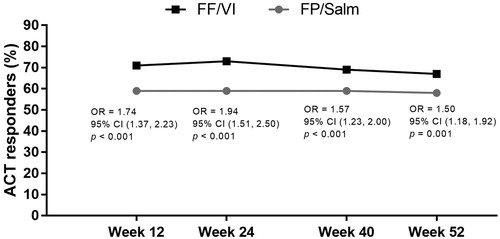
For the primary effectiveness endpoint of the percentage of ACT responders at week 24 in the PEA population, the odds of being a responder were significantly higher in the FF/VI group in than the FP/Salm group (FF/VI n = 323 [71%], FP/Salm n = 253 [56%]; OR 2.03 [95% CI 1.53, 2.68], p < 0.001). This benefit was consistent across all time points in the PEA population (). Similar effects were observed at week 24 in the total population (FF/VI n = 431 [73%], FP/Salm n = 335 [59%]; OR 1.94 [95% CI 1.51, 2.50], p < 0.001). This benefit was also consistent across all time points in the total population ().
Figure 2. ACT responders over time in the FF/VI and FP/Salm groups (total population).a.b
Notes. ACT: Asthma Control Test; CI: confidence interval; FF/VI: fluticasone furoate/vilanterol; FP/Salm: fluticasone propionate/salmeterol; OR: odds ratio. aResponders defined as patients who achieved an ACT total score of ≥20 and/or improvement from baseline of ≥3 points. bLogistic regression analysis adjusting for randomised treatment, baseline ACT total score, baseline ACT total score squared, gender, and age.
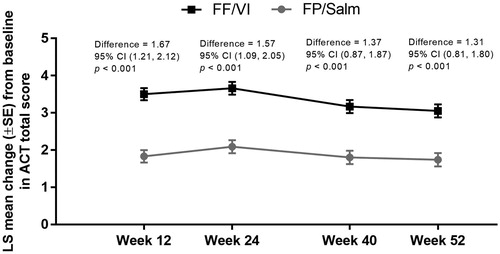
The LS mean ACT total score in the total population increased from baseline by 3.66 (standard error [SE] 0.17) in the FF/VI group and 2.09 (SE 0.18) in the FP/Salm group at week 24 (difference 1.57 [95% CI 1.09, 2.05], p < 0.001). Similar results were observed at weeks 12, 40 and 52 ().
Figure 3. LS mean change from baseline in ACT total score over time in the FF/VI and FP/Salm groups (total population).a
Notes. ACT: Asthma Control Test; CI: confidence interval; FF/VI: fluticasone furoate/vilanterol; FP/Salm: fluticasone propionate/salmeterol; LS: least squares; SE: standard error. aMixed model repeated measures analysis adjusted for randomised treatment, baseline ACT total score, randomised treatment-by-baseline ACT total score interaction, gender, age, visit and randomised treatment-by-visit interaction with an unstructured covariance matrix.
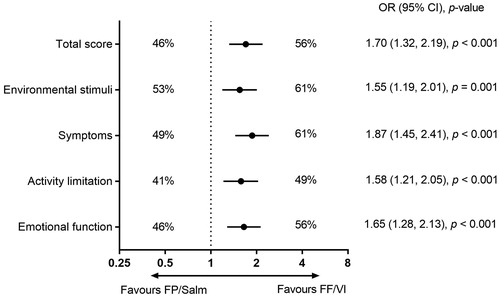
The proportion of patients classified as responders for AQLQ total score at week 52 was significantly higher in the FF/VI group than in the FP/Salm group (FF/VI n = 325 [56%], FP/Salm n = 258 [46%]; OR 1.70 [95% CI 1.32, 2.19], p < 0.001). A similar treatment effect favouring FF/VI over FP/Salm was observed across each of the four AQLQ domains at week 52 ().
Figure 4. Percentage of responders for AQLQ total score and individual AQLQ domains in the FF/VI and FP/Salm groups (total population).a,b
Notes. AQLQ: Asthma Quality of Life Questionnaire; CI: confidence interval; FF/VI: fluticasone furoate/vilanterol; FP/Salm: fluticasone propionate/salmeterol; OR: odds ratio. aResponders defined as patients with a change from baseline of ≥0.5 points in AQLQ total score or AQLQ domain score. bLogistic regression analysis adjusting for randomised treatment, ACT total score at baseline per randomisation stratum, gender, age and baseline AQLQ score.
A significant 20% reduction in the LS mean annual rate of severe exacerbations was observed in the FF/VI group compared with FP/Salm group (FF/VI 0.47, FP/Salm 0.59; rate ratio 0.80 [95% CI 0.66, 0.95], p = 0.014).
Patients initiated on FF/VI reported a greater decrease in activity impairment due to asthma on WPAI than those who continued on FP/Salm (–10.4% vs. –5.9%; difference –4.5% [95% CI –7.2%, –1.8%], p < 0.001). No significant between-group differences were observed for changes in the WPAI measures of percent work time missed due to asthma, percent impairment while working due to asthma, and percent overall work impairment due to asthma.
Patients in the FF/VI group were prescribed significantly fewer salbutamol inhalers during the study than patients in the FP/Salm group (LS mean number of salbutamol inhalers prescribed: FF/VI 8.3, FP/Salm 9.5; difference –1.2 [95% CI –1.8, –0.6] p < 0.001).
On-treatment SAEs of special interest, based on the actual treatment patients were receiving at the time of the event, are summarised in . The incidence of pneumonia was low (n = 6 in each treatment group, regardless of whether assessed by randomised treatment group or actual treatment at the time of the event). There were four fatalities, two in each treatment group (FF/VI group: deep vein thrombosis and pulmonary embolism in one patient and myocardial ischaemia with diabetes and hypertension in another patient; FP/Salm group: colorectal cancer in one patient and myocardial ischaemia in another patient); these deaths were not considered to be drug-related.
Table 2. On-treatment SAEs of special interest (total population).
Discussion
The Salford Lung Study in asthma was revolutionary in that it is the largest randomised comparative effectiveness study conducted in patients with GP-diagnosed asthma in an everyday clinical practice setting. The study showed that initiating once-daily FF/VI was superior to continuing UC (as optimised by GP at start of trial with the opportunity to modify as deemed necessary by the GP during the trial) for improving asthma control [Citation1]. The present subset analysis was conducted specifically in the group of patients whose intended pre-randomisation prescription was FP/Salm. As FP/Salm is one of the most widely used ICS/LABA combination products for asthma, we considered this subset analysis to be of particular relevance, and the analyses of the primary endpoint of the percentage of ACT responders at week 24 and severe asthma exacerbation rates in this subset were planned a priori.
In the FP/Salm subset, significant benefit was seen in patients initiating once-daily FF/VI versus continuing FP/Salm for asthma control, quality of life, severe asthma exacerbation rates, and number of salbutamol inhalers prescribed. With the exception of exacerbation rates, these findings are broadly consistent with those in the overall SLS asthma population [Citation1]. It is interesting to note the consistent benefit across a number of endpoints in this analysis when a Phase III asthma study did not show significant differences between FF/VI and FP/Salm [Citation5]. The reason for the different outcomes is mostly likely due to the differences in study design. SLS recruited a broad range of asthma patients with a GP diagnosis of asthma, with protocol-mandated study visits at the start and end of the 12-month study period only and was open-label with the opportunity to modify treatment throughout the study. In contrast, the Phase III study was a double-blind, double-dummy study with very strict entry criteria and frequent clinic visits; adherence was also closely monitored and actively encouraged. In the present subset analysis, adherence as measured by PDC (FF/VI 82%, FP/Salm 77%) was higher than might be expected of a study conducted in a real-world setting; however, PDC is a measure of prescriptions issued and does not necessarily inform whether prescriptions were fulfilled or whether patients in fact took the medication as instructed. Thus, attributes of FF/VI such as true 24 h efficacy [Citation13] and delivery via the easy-to-use ELLIPTA inhaler with a low critical error rate [Citation14,Citation15] are unlikely to have the opportunity to impact outcome in a highly controlled Phase III study.
It is of note that a significant reduction in the rate of asthma exacerbations was seen in patients initiating FF/VI versus continuing FP/Salm in the subset of patients reported here, whereas no significant difference was seen between FF/VI and UC in the overall SLS asthma study population [Citation1]. This observation may have been influenced by the fact that the subset population reported here had more severe asthma than the overall group, with 45% of patients in this subset reporting at least one exacerbation in the 12 months prior to randomisation in contrast to 36% in the overall study. A mean annual exacerbation rate in the year prior to the study of 0.9 was seen in the current analysis compared with 0.6 in the overall SLS asthma study population. Thus, the population reported here had more opportunity to achieve a reduction in exacerbation rate despite the subset being approximately one-third the size of the overall SLS study population.
As was seen in the overall study, there was a significant reduction in the number of salbutamol inhalers prescribed during the study for the FF/VI group compared with the FP/Salm group. This provides corroboration of superiority in the percentage of patients achieving or improving control as patients will use more rescue medication when symptomatic. The Global Initiative for Asthma [Citation3] guidelines state that reducing the need for reliever therapy is important as both a goal of asthma management and a measure of the success of asthma treatment.
Significant improvement with FF/VI versus FP/Salm was also seen for AQLQ total score and across each of the four individual AQLQ domains. Although the main goal of asthma treatment is to achieve asthma control and reduce the risk of future exacerbations (initiating FF/VI showed significant benefit over continuing FP/Salm for both), patients tend to focus on how their symptoms make them feel and how their symptoms impact on their everyday lives [Citation16]. Therefore, as well as improving clinical outcomes, these HR-QoL outcomes are likely to be of great importance to patients [Citation17]. Significant benefit was also seen for reduction in activity impairment due to asthma on WPAI, again corroborating the positive treatment effect with FF/VI on asthma control and quality of life.
There is evidence in the literature to suggest that changes in ACT scores are strongly associated with changes in percent predicted forced expiratory volume in 1 s (r = 0.81) and moderately associated with changes in specialist rating of asthma control (r = 0.43) [Citation18]. Additionally, changes in ACT scores were correlated to an extent with changes in asthma-related QoL scores (r = 0.62) [Citation18]. Antonova et al. (2016) reported a correlation between improvement in ACT score and improvement in productivity, as measured using the WPAI; however, statistical significance was not tested [Citation19].
The benefit of FF/VI on HR-QoL outcomes in the FP/Salm patient subset is supported by similar findings in the overall SLS asthma patient population [Citation20], where FF/VI demonstrated a consistent improvement on patient-reported outcomes across the subpopulations analysed, as measured by ACT, AQLQ and WPAI. Patient experience captured in follow-up interviews with a subset of 400 patients who completed the SLS furthermore indicated that FF/VI was more commonly associated with a perceived positive change in asthma versus UC [Doward et al., manuscript in preparation]. This suggests that initiating FF/VI versus continuing UC could result in an “everyday” positive effect on asthma control and HR-QoL.
One of the strengths of this subset analysis is the pre-planned nature of the ACT and exacerbations endpoints, with highly statistically significant results reported. Other strengths include the use of a real-world patient population with GP-diagnosed asthma, no requirement for spirometry testing for inclusion in the trial, the focus on patient-relevant outcomes, and the consistency of findings across different endpoints and populations. The present study had some weaknesses, including the open-label nature of the trial and the post-hoc nature of some of the conducted analyses. The lack of correction for multiplicity of statistical testing could also be perceived as a weakness. However, our findings were mostly highly statistically significant, with p < 0.001 in the majority of cases. If a conservative method of accounting for multiplicity (such as the Bonferroni method) had been applied across the 24 statistical tests conducted for this subset analysis, then for each test a p value of < 0.002 would have been required to conclude statistical significance. Hence, the strong evidence accrued here indicates that the benefit of initiating FF/VI over continuing FP/Salm is unlikely to be a chance finding. These results provide important information on the effectiveness of a simple once-daily treatment regimen with FF/VI in patients with symptomatic asthma seen in routine clinical practice.
Conclusions
For patients with asthma in a primary care setting, initiating treatment with FF/VI was significantly better than continuing with FP/Salm for improving asthma control, improving HR-QoL, reducing the need for prescriptions for rescue medication, and reducing asthma exacerbations, with no notable difference in SAEs.
Declarations of interest
LJ, HS, JL-F, and DAL report employment with and stock/share ownership in GSK. NDB reports employment by the organisation that provided IT support for automated data collection in SLS (NorthWest EHealth) and receipt of financial support to attend meetings in the form of non-restricting educational grants from GSK, Novartis, AstraZeneca and Boehringer Ingelheim. JPN reports grants and personal fees from GSK.
Supplemental Material
Download MS Word (113 KB)Acknowledgements
Editorial support in the form of formatting, assembling tables and figures, collating author comments, grammatical editing and referencing was provided by Emma Landers, PhD, at Gardiner-Caldwell Communications (Macclesfield, UK) and was funded by GSK.
Additional information
Funding
References
- Woodcock A, Vestbo J, Bakerly ND, New J, Gibson JM, McCorkindale S, Jones R, et al. Effectiveness of fluticasone furoate plus vilanterol on asthma control in clinical practice: an open-label, parallel group, randomised controlled trial. Lancet 2017;390:2247–2255.
- Global Asthma Network. Global Asthma Report (2014). Available from: http://www.globalasthmareport.org/resources/Global_Asthma_Report_2014.pdf [last accessed 15 February 2018].
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention 2017. Available from: http://ginasthma.org/ [last accessed 15 Feb 2018].
- British Thoracic Society (BTW). British Guideline on the Management of Asthma. A National Clinical Guideline 2016. Available from: https://www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-asthma-guideline-2016/ [last accessed 15 Feb 2018].
- Woodcock A, Bleecker ER, Lötvall J, O'Byrne PM, Bateman ED, Medley H, Ellsworth A, et al. Efficacy and safety of fluticasone furoate/vilanterol compared with fluticasone propionate/salmeterol combination in adult and adolescent patients with persistent asthma: a randomized trial. Chest 2013;144:1222–1229.
- Herland K, Akselsen JP, Skjønsberg OH, Bjermer L. How representative are clinical study patients with asthma or COPD for a larger “real life” population of patients with obstructive lung disease? Respir Med 2005;99:11–19.
- Woodcock A, Bakerly ND, New J, Gibson JM, Wu W, Vestbo J, Leather D. The Salford Lung Study protocol: a pragmatic randomised phase III real-world effectiveness trial in asthma. BMC Pulm Med 2015;15:160.
- Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol 2004;113:59–65.
- Schatz M, Kosinski M, Yarlas AS, Hanlon J, Watson ME, Jhingran P. The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol 2009;124:719–723. e1
- Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific quality of life questionnaire. J Clin Epidemiol 1994;47:81–87.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993;4:353–365.
- Andréasson E, Svensson K, Berggren F. The validity of the work productivity and activity impairment questionnaire for patients with asthma (WPAI-asthma): results from a web-based study. Value Health 2003;6:780.
- O'Byrne PM, Bleecker ER, Bateman ED, Busse WW, Woodcock A, Forth R, Toler WT, et al. Once-daily fluticasone furoate alone or combined with vilanterol in persistent asthma. Eur Respir J 2014;43:773–782.
- Grant AC, Walker R, Hamilton M, Garrill K. The ELLIPTA® Dry Powder Inhaler: design, functionality, in vitro dosing performance and critical task compliance by patients and caregivers. J Aerosol Med Pulm Drug Deliv 2015;28:474–485.
- van der Palen J, Thomas M, Chrystyn H, Sharma RK, van der Valk PD, Goosens M, Wilkinson T, et al. A randomised open-label cross-over study of inhaler errors, preference and time to achieve correct inhaler use in patients with COPD or asthma: comparison of ELLIPTA with other inhaler devices. NPJ Prim Care Respir Med 2016;26:16079.
- Svedsater H, Roberts J, Patel C, Macey J, Hilton E, Bradshaw L. Life impact and treatment preferences of individuals with asthma and chronic obstructive pulmonary disease: results from qualitative interviews and focus groups. Adv Ther 2017;34:1466–1481.
- Malik M, Khan A, Hussain A, Javaid AO. Health related quality of life in asthma: a systematic review. Arch Palliat Care 2017;2:1012.
- Kwon SH, Lee SH, Yang MS, Lee SM, Kim SG, Kim DI, Sohn SW, et al. Correlation between the Korean version of asthma control test and health-related quality of life in adult asthmatics. J Korean Med Sci 2008;23:621–627.
- Antonova E, Trzaskoma B, Omachi TA, Schatz M. Poor asthma control is associated with overall daily activity impairment: 3-year data from the EXCELS study of omalizaumab. J Allergy Clin Immunol Pract 2016;137:Suppl, Page AB14.
- Svedsater H, Jones R, Bosanquet N, Jacques L, Lay-Flurrie J, Leather DA, Vestbo J, et al. Patient-reported outcomes with initiation of fluticasone furoate/vilanterol versus continuing usual care in the asthma Salford Lung Study. Respir Med 2018;141:198–206.
