Abstract
Objective: Anecdotal evidence suggests that some patients with asthma intentionally use their twice-daily (BID) inhaled controller therapy once daily (QD), thus not achieving optimal dosing levels. This study identified the prevalence of and factors associated with intentional QD use of BID-indicated controllers among adult patients with asthma. Methods: This was a cross-sectional survey study of adults using inhaled controllers intended for BID dosing for treatment of asthma and/or COPD. Survey responses were linked to administrative claims data for the prior 12 months (baseline). Results of patients indicating both an asthma diagnosis and current intentional QD or BID use of controllers are presented. Results: Of 1401 patients with asthma, 30.9% reported intentional QD use of their controller and 69.1% reported BID use. Intentional QD use was mostly a function of patients’ lack of perceived need for BID treatment (44.1%) or physician orders to take their controller QD (34.0%). Patients reporting intentional QD use tended to be healthier (higher health status scores, and lower Charlson comorbidity scores, ambulatory and ER visits, and healthcare costs) with better asthma control (lower asthma-related ER and ambulatory visits and rescue medication use, and higher Asthma Control Test scores) compared with patients reporting BID use. Conclusions: Perceptions regarding health and the necessity of controller use to control or treat asthma were the main drivers of medication-taking behavior. Patients with less severe asthma were more likely to report once daily use of their inhaled controller, but still maintained asthma control.
Introduction
The goal of asthma treatment is to preserve pulmonary function, decrease symptoms, and prevent exacerbations, allowing patients to maintain regular activity levels [Citation1]. To meet these goals, patients are often prescribed both long-term inhaled controller therapy to control persistent asthma and short-acting therapy to treat breakthrough symptoms. The effectiveness of inhaled controllers depends upon adherence to the prescribed regimen.
Adherence to inhaled controllers can be challenging due to the chronic nature of the disease and its treatment, multiple medications, and the need to consistently take medication, even during asymptomatic periods [Citation2]. The lengthier and more complicated the treatment regimen, the greater the likelihood of nonadherence. Less than 50% of patients comply with asthma treatment recommendations [Citation2]. Poor adherence contributes to increased asthma symptoms and exacerbations, leading to decreased quality of life, increased healthcare resource utilization, and asthma-related mortality [Citation3]. In addition, poorly controlled asthma is associated with one-third of direct and three-fourths of total asthma costs [Citation4].
Nonadherence to treatment can be unintentional or intentional [Citation5]. Unintentional nonadherence occurs when patients intend to take their medication as prescribed, but are unable to due to factors beyond their control (e.g. forgetfulness, poor comprehension, physical inability to manage the medication). Intentional, or deliberate, nonadherence occurs when patients choose not to take medication or to take it in a way that differs from recommendations (e.g. reduction in dosing frequency or number of medications, premature treatment discontinuation). In a survey study concerning inhaled corticosteroids (ICS), patients with asthma expressing the greatest doubt about treatment need and the most concerns regarding adverse events, had the highest nonadherence rates [Citation6]. Additionally, patients who did not perceive their asthma as chronic with serious consequences (e.g. considered themselves well when asymptomatic) questioned a need to continue with inhaled controllers.
While the most commonly prescribed inhaled controllers to treat asthma are intended to be used twice-daily (BID), anecdotal evidence suggests that a proportion of patients use their inhaled controller only once daily (QD), thus, potentially not taking a therapeutic dose. Understanding patient factors, such as beliefs about taking medications and perceived health status are important to gain insight into the reasons why patients use their BID controller medications QD. The purpose of this study was to identify the prevalence of and factors associated with intentional QD use of BID-indicated inhaled controllers among adult patients with asthma.
Methods
Design and data source
This was a cross-sectional survey study of adult commercial enrollees in large US managed care plans with COPD and/or asthma who were treated with ICS/long-acting β-agonists (LABAs) indicated for BID use. The study sample population included patients with both COPD and asthma; however, this article presents only results from the asthma cohort.
Survey candidates were identified using medical and pharmacy claims and enrollment information from a proprietary research claims database, the Optum Research Database, which includes approximately 14 million enrollees in commercial plans and 500,000 enrollees in Medicare Advantage with Part D (MAPD) plans annually. Medical claims included International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes, Current Procedural Terminology codes, and Healthcare Common Procedure Coding System codes. Pharmacy claims contained outpatient prescription pharmacy services, including drug name, dosage form, and fill date. The study was approved by the New England Institutional Review Board (Newton, MA).
Patient identification
The patient sampling frame included commercial members ≥18 years of age with ≥1 diagnosis of asthma (ICD-9-CM 493.xx) in any position on a medical claim or ≥40 years of age with ≥1 diagnosis of COPD (ICD-9-CM 491.xx, 492.xx, 496.xx) in any position on a medical claim during the 12-month identification period (August 2013–August 2014). Additional criteria included ≥2 fills for any ICS/LABA indicated for BID dosing per the FDA-approved label (aclidinium, beclomethasone, budesonide, budesonide/formoterol, ciclesonide, flunisolide, fluticasone, fluticasone/salmeterol, formoterol, mometasone/formoterol, salmeterol, and/or triamcinolone) during the last 6 months of the identification period and continuous enrollment in the health plan during the identification period.
Survey eligibility and issuance
From among the sampling frame of 15,846 members who met the study criteria, a random sample of 9996 patients with asthma and COPD were contacted to participate in the cross-sectional survey. The initial survey was mailed on October 24, 2014 and included a pre-paid $10 incentive; a postcard reminder to complete the survey was sent 2 weeks later. Four weeks after the initial mailing, non-respondents received a second survey. The survey field period ended 8 weeks following the initial mailing. Survey responses were linked to medical and pharmacy claims from the baseline period, defined as the 12-month period beginning in December 2013 and ending at the completion of the survey field period (December 2014).
Cohorts
Patients were assigned to one of three primary disease cohorts (asthma, COPD, and coexistent asthma and COPD) based on their survey responses. Only results from the asthma cohort are reported. The criteria for assignment to the asthma cohort were self-reported confirmation of an asthma diagnosis without a concurrent COPD diagnosis. Only patients that completed all asthma-specific survey measures were included in the analysis. Patients were further divided into two cohorts based on their self-reported use of an inhaled controller. Patients currently prescribed any inhaled controller indicated for BID use, but self-reported intentional QD use were assigned to the intentional QD cohort, whereas patients who reported BID use were assigned to the BID cohort. Patients who reported inhaled controller use other than intentional QD or BID were excluded from the analyses.
Patient reported measures
Patients were asked to rate their general health using a 5-point Likert scale ranging from excellent (1) to poor (5) that was transformed on a 0–100 scale, with a higher score indicating greater health status. Asthma control was assessed using the 5-item Asthma Control Test (ACT) [Citation7]. ACT scores ranged from 5 (poor asthma control) to 25 (complete control) and were divided into categories (5–15 poorly controlled; 16–19 not well controlled; 20–25 well controlled). Patients were also asked to report inhaled controller use including name of their current controller, use patterns per day (e.g. QD, BID), main reason for missed doses if QD use of BID therapy was reported (selected from a list), medication breaks of a week or longer, reasons for nonuse if patient reported not taking their inhaled controller BID, and dosing instructions per the pharmacy prescription label on the inhaler. The survey also collected information on demographic (age, gender, race, ethnicity), socioeconomic (education level, employment status, household income, marital status), and clinical (smoking history, weight, height, respiratory condition, rescue medication use) characteristics.
To further quantify nonadherence and barriers to adherence, several questionnaires were used. The 3-item Adherence Estimator was used to measure beliefs related to nonadherence (medication necessity, concerns, and affordability) [Citation8]. Total scores ranged from 0 to 36 indicating risk of nonadherence (low [0–6], medium [2–7], and high [8–36]). Subscale scores for each of the three medication belief domains were calculated and linearly transformed on a 0–100 scale, with 100 representing the most favorable belief (e.g. highest perceived medication need and affordability, fewest perceived concerns). A modified version of the Adult Asthma Adherence Questionnaire (AAAQ) was used to measure adherence to the medication plan and barriers to adherence [Citation9]. Patient satisfaction of their inhaled controller was measured using the 9-item version of the Treatment Satisfaction Questionnaire for Medication (TSQM-9), with scores ranging from 0 to 100 [Citation10].
Administrative claims measures
Select patient demographic and clinical characteristics (U.S. Census region, Charlson comorbidity score [Citation11,Citation12], dispensed medications, and total and asthma-related healthcare resource utilization and costs) were determined from the baseline administrative claims data. Costs were computed from the sum of health plan- and patient-paid amounts and represented claims for all medical (ambulatory, emergency room [ER], inpatient, and other) and pharmacy services. Costs were adjusted to 2014 USD using the annual medical care component of the Consumer Price Index to account for inflation [Citation13].
Statistical analyses
Sample size was calculated based on the power to detect a mean score difference of five points for the medication necessity and concerns domains of the Adherence Estimator between QD and BID cohorts within each condition. Assuming unequal cohort sizes with 80% power at the 5% significance level, the estimated minimum sample size was 812 surveys each for the COPD, coexistent asthma and COPD, and asthma cohorts (203 in asthma QD cohort, 609 asthma in BID cohort), which required a total mail out sample size of 10,000 patients based on the predicted response rate (30%) and the expected minimum proportion of patients reporting QD use (15%). Variables were analyzed descriptively and results were stratified by cohort. Comparisons between means of continuous measures were made using the two-sample t-test for independent groups and comparisons of binary and categorical measures were made using Fisher's exact test.
Results
Patient selection and survey response
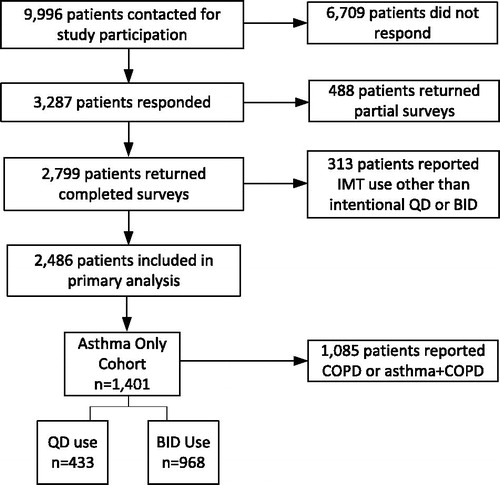
Of the 9996 surveys mailed, 3287 were returned, with 2799 complete (). The overall survey response rate was 33.1% and the survey completion rate was 28.2% based on the American Association for Public Opinion Research’s Response Rate (RR) and Standard Definitions RR4 and RR3, respectively [Citation14]. Compared with nonrespondents (n = 6709), respondents (n = 3287) were more likely to be older (mean age 55.6 years vs. 52.3 years, p < 0.001), female (57.3% vs. 52.4%, p < 0.001), and live in the Midwest (31.4% vs. 27.5%, p < 0.001), but were less likely to live in the South (42.4% vs. 47.2%, p < 0.001). A total of 1401 patients reported a diagnosis of asthma without concurrent COPD and current intentional QD or BID inhaled controller use and were included in this analysis.
Baseline characteristics
Out of 1401 patients, 433 (30.9%) reported intentional QD use of their inhaled controller, while 968 (69.1%) reported BID use. Mean age was 50.6 years, 62.8% were female, and most patients were White (90.8%) (). Demographic and sociodemographic characteristics were similar between patients reporting QD versus BID use, with the exception that BID users were more likely to be single compared with QD users (13.4% vs. 9.3%, p = 0.033).
Table 1. Demographic and clinical characteristics of patients with asthma.
Patients with asthma reporting QD use appeared to have less severe asthma and better overall health than those reporting BID use across both claims and survey measures of health. Mean claims-based Charlson comorbidity scores were higher in BID users than QD users (1.3 vs. 1.1, p < 0.001) (). Additionally, average self-reported BMI was lower among QD users when compared with BID users (28.5 vs. 29.5, p = 0.012). QD users were more likely to be nonsmokers living in a smoke-free household (63.4% vs. 57.8%, p = 0.050), whereas, BID users were more likely to be current smokers (7.0% vs. 2.8%, p = 0.002). Mean general self-rated health status scores were 62.0, with higher scores reported by those with QD use compared with BID use (65.4 vs. 60.4, p < 0.001), indicating better perceived health (). Compared with BID users, the QD cohort had better asthma control based on mean ACT scores (21.1 vs. 19.8, p < 0.001). Intentional QD users were more likely to be classified as controlled (ACT >19) and report no use of rescue medication over the “past 4 weeks,” while BID users tended to be poorly controlled (ACT <16) and report rescue medication usage of “3 or more times per day over the past 4 weeks.”
Table 2. Asthma control, adherence, and treatment beliefs and satisfaction.
Utilization and costs
Compared with QD users, a greater proportion of BID users had ≥1 all-cause ER visit (32.6% vs. 27.3%, p = 0.046) and ≥1 ambulatory visit (99.3% vs. 97.5%, p = 0.009) during the baseline period (). BID users also had more ambulatory visits, on average, than QD users (19.2 vs. 14.5, p < 0.001). Compared with QD users, patients reporting BID use were more likely to have ≥1 asthma-related ER (11.3% vs. 6.9%, p = 0.012) or ambulatory visit (90.4% vs. 83.8%, p < 0.001) and required more asthma-related ER (0.2 vs. 0.1, p = 0.004) and ambulatory visits (4.1 vs. 2.8, p < 0.001). Compared with QD users, BID users had higher counts of unique medications dispensed (10.5 vs. 8.2, p < 0.001) and number of unique medication dispensings (39.8 vs. 30.4, p < 0.001) during the baseline period. BID users were more likely to be prescribed an antidepressant compared with QD users (26.5% vs. 18.2%, p < 0.001). Consistent with self-reported rescue medication usage, BID users were more likely to have ≥1 fill for rescue medications in the claims data than QD users (83.9% vs. 74.8%, p < 0.001).
Table 3. Baseline healthcare resource utilization.
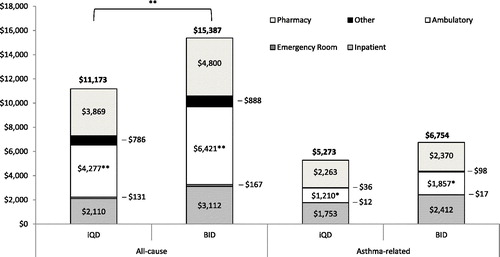
Mean all-cause annualized healthcare costs were $14 085 per patient, with $6297 attributable to asthma. Compared with QD users, BID users had higher all-cause costs, including higher total costs ($15 387 vs. $11 173, p = 0.009), medical costs ($10 587 vs. $7305, p = 0.018), and ambulatory costs ($6421 vs. $4277, p = 0.002) (). BID users also had higher asthma-related ambulatory costs compared with QD users ($1857 vs. $1210, p = 0.032).
Patients with QD use were more likely to be prescribed fluticasone propionate/salmeterol (68.8% vs. 57.2%, p < 0.001) or fluticasone propionate (13.2% vs. 8.4%, p = 0.007) and less likely to be prescribed mometasone/formoterol (6.0% vs. 13.7%, p < 0.001) than BID users (). Almost one-third (32.9%) of all patients reported taking a break of a week or longer from their inhaled controller. Based on responses to the AAAQ, QD users were more at risk for nonadherence to their asthma medication plan than BID users ().
Table 4. Use of prescribed inhaled medication overall and by medication usage.
Reasons for once daily use
The majority of patients reported the main reason for QD inhaled controller use was the perceived lack of need to take more than one daily dose, with 44.1% reporting “I do not need to take it more than once per day” and 34.0% reporting “My doctor told me to take it once per day” (). Of patients who reported physician-directed QD dosing, 70.1% confirmed the pharmacy prescription was written for QD use. Patients with QD use were more likely to agree with the AAAQ statement “My asthma is mild and does not require regular preventative treatment” and 44.8% had “need” as a barrier compared with 29.4% of BID users (p < 0.001) (). Patients with BID use indicated a greater need for their inhaled controller than QD users, per the average Adherence Estimator Necessity Domain scores ().
Table 5. Main reasons for intentional QD usage of BID-intended inhaled medications.
Discussion
Previous studies have reported low rates of adherence to inhaled controllers among patients with asthma, with estimates ranging from 30% to 70% of the prescribed dose [Citation15–19]. In this study, 69% of respondents reported inhaled controller use that followed the recommended BID schedule, while 31% of patients reported QD use. Studies investigating intentional nonadherence of inhaled controllers are limited. In a Danish study, intentional nonadherence (non-BID use a few times per week) was reported by 24% of patients with prevalent ICS use [Citation20], while Gadkari et al. reported 34% of patients currently on therapy for chronic disease, including asthma, were nonadherent (intentionally skipped or altered doses) [Citation21].
The Health Belief Model suggests patients are more likely to be adherent to treatment if they perceive their illness as significant and the proposed treatment as effective with minimal side effects, cost, or lifestyle modifications [Citation22,Citation23]. We found patients most commonly reported a lack of perceived need to take their inhaled controller more than QD (44%) as the main reason to use the twice daily regimen once daily. Three quarters of the QD users reported their asthma was controlled as measured by the ACT, suggesting that most experienced mild or nonexistent asthma symptoms when surveyed. This was further supported by the fact that QD users tended to agree with the AAAQ statement “My asthma is mild and does not require regular preventative treatment.” In contrast, patients reporting BID use were more likely to agree with the Adherence Estimator statement “I am convinced of the importance of my prescription medication,” indicating a low risk of not taking their medication as prescribed. Several studies have reported the association of patient doubts about medication necessity and concerns regarding adverse events with nonadherence to inhaled controller regimens [Citation6,Citation24–28]. In Danish patients with asthma who were prescribed ICS therapy for at least 1 year, 90% of those intentionally nonadherent reported the main reason for nonadherence was a lack of perceived symptoms, and 60% reported they would prefer to take more rescue medications instead of increasing their inhaled controller dose [Citation20]. Similarly, Partridge et al. observed that 70% of patients preferred to adjust their inhaled controller needs to changes in their asthma (i.e. take less medication when well, more when symptomatic) [Citation29]. A symptom-directed approach to asthma treatment in patients with mild-to-moderate asthma in Greaves et al. produced similar outcomes compared with patients who were adherent to their ICS treatment, whereas adherent patients with more severe asthma had better outcomes than those with variable ICS use [Citation30].
Intentional QD users had better controlled asthma compared with BID users, as evidenced by higher ACT scores and lower need for rescue medications. Patients with QD use also had fewer all-cause and asthma-related ER and ambulatory visits, thus lower healthcare costs at baseline compared with BID users. This is further supported by the observed mean general health status scores being 5 points higher in QD versus BID users and almost 25 points higher when compared to mean scores from data collected among a normative sample of patients with asthma aged 40 and older [Citation15]. Compared with BID users, more QD users had no comorbidities (14% vs. 7%) and lower Charlson comorbidity scores (1.1 vs. 1.3). Nonadherence (according to the medication label) has been shown to increase in cases of mild and severe disease [Citation31], which may explain why healthier patients with better controlled asthma in this survey were more likely to use their BID-indicated controller QD, and is in line with a lower perceived need for treatment. Additionally, for patients on low-dose maintenance ICS/LABA (Global Initiative for Asthma, GINA, Step 3) who have achieved asthma control for at least 3 months, GINA guidelines recommend a step-down in therapy to QD to find the minimum effective dose to maintain control. For patients on a moderate or high dose of ICS/LABA (GINA Step 4), guidelines recommend a 50% reduction in the ICS component only [Citation1]. Given that patients who chose QD use were more likely to have better asthma control, less severe asthma, and greater health compared to patients taking twice-daily therapy, it is possible that they were directed by their physician to step-down their therapy per the recommended guidelines; however, we found that only one-third (34%) of patients reported that their main reason for QD use was per the physician’s orders, and of those patients, 70.1% confirmed the prescription was written for QD use. Overall, only 11% of the 1401 patients in the analysis were prescribed once daily use by their healthcare provider. Prescribers seem to be following recommended product labeling; however, this may not match a patient’s perceived need. Physicians should consider routinely assessing patient’s health status using measures such as the ACT, as well as their medication taking beliefs and behaviors, to better assess appropriate therapy.
Limitations
Results of this study are based on survey and claims data and should be interpreted under the context of certain limitations. Response to the patient survey was voluntary and respondents may not be representative of the general population (e.g. sample bias toward patients interested in research, healthier, more engaged in their health). Asthma diagnosis was self-reported and not confirmed by medical records or physician contact. Additionally, data was not collected on whether patients had an asthma action plan which may have indicated a step-down in asthma therapy during times of asthma control. Lastly, the results are from a managed care population with prescription drug benefits and may not be applicable to other populations.
Conclusions
In adult patients with asthma, perceptions regarding health and the perceived necessity of inhaled controllers to control or treat asthma are the main drivers of medication-taking behavior. In this study, most of the intentional QD controller use was by patient’s decision, and not by the prescriber, mainly due to a perceived lack of need for more than once-daily therapy. Patients who chose once-daily use were more likely to have better asthma control, less severe asthma, and greater health compared to patients taking BID therapy. Examination of long-term outcomes associated with intentional QD use of inhaled controllers among asthma patients with similar severity is warranted to examine if asthma control can be maintained with less frequent use of controllers.
Declaration of Interest
C.B. and R.S. are employees of GlaxoSmithKline. A.N. was a GlaxoSmithKline employee at the time this work was completed and current affiliation is Shire. P.J., M.J., and A.W. are employees of Optum (Eden Prairie, MN). All authors participated in study design, analysis, interpretation of data, writing of this manuscript, and the decision to submit this article for publication.
Acknowledgements
Medical writing assistance was provided by Deja Scott-Shemon, MPH, Optum.
Additional information
Funding
References
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2018. Available from: www.ginasthma.org.
- Corsico AG, Cazzoletti L, de Marco R, Janson C, Jarvis D, Zoia MC, Bugiani M, et al. Factors affecting adherence to asthma treatment in an international cohort of young and middle-aged adults. Respir Med 2007;101:1363–1367.
- National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol 2007;120:S94–S138.
- Horne R, Price D, Cleland J, Costa R, Covey D, Gruffydd-Jones K, Haughney J, et al. Can asthma control be improved by understanding the patient's perspective? BMC Pulm Med 2007;7:8.
- Wroe AL. Intentional and unintentional nonadherence: a study of decision making. J Behav Med 2002;25:355–372.
- Horne R, Weinman J. Self-regulation and self-management in asthma: exploring the role of illness perceptions and treatment beliefs in explaining nonadherence to preventer medication. Psychol Health 2002;17:17–32.
- Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol 2004;113:59–65.
- McHorney CA. The Adherence Estimator: a brief, proximal screener for patient propensity to adhere to prescription medications for chronic disease. Curr Med Res Opin 2009;25:215–238.
- Schatz M, Zeiger RS, Yang S-J, Weinstein AG, Chen W, Saris-Baglama RN, Turner-Bowker DM, et al. Development and preliminary validation of the Adult Asthma Adherence Questionnaire. J Allergy Clin Immunol Pract 2013;1:280–288.
- Atkinson MJ, Sinha A, Hass SL, Colman SS, Kumar RN, Brod M, Rowland CR. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes 2004;2:12.
- Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel J-M, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011;173:676–682.
- Bayliss EA, Ellis JL, Shoup JA, Zeng C, McQuillan DB, Steiner JF. Association of patient-centered outcomes with patient-reported and ICD-9-based morbidity measures. Ann Fam Med 2012;10:126–133.
- US Department of Labor. Bureau of Labor Statistics. Consumer Price Index - Chained Consumer Price Index [cited 2016 July 22]. Available from: http://data.bls.gov/cgi-bin/surveymost.
- AAPOR. Standard definitions: final dispositions and case codes and outcome rates for surveys. Deerfield, IL: AAPOR; 2008.
- Gamble J, Stevenson M, McClean E, Heaney LG. The prevalence of nonadherence in difficult asthma. Am J Respir Crit Care Med 2009;180:817–822.
- Williams LK, Pladevall M, Xi H, Peterson EL, Joseph C, Lafata JE, Ownby DR, et al. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. J Allergy Clin Immunol 2004;114:1288–1293.
- Williams LK, Peterson EL, Wells K, Campbell J, Wang M, Chowdhry VK, Walsh M, et al. A cluster-randomized trial to provide clinicians inhaled corticosteroid adherence information for their patients with asthma. J Allergy Clin Immunol 2010;126:225–231, 31 e1-4.
- Bender B, Milgrom H, Rand C. Nonadherence in asthmatic patients: is there a solution to the problem? Ann Allergy Asthma Immunol 1997;79:177–185, quiz 85–86.
- Onyirimba F, Apter A, Reisine S, Litt M, McCusker C, Connors MLou, ZuWallack R, et al. Direct clinician-to-patient feedback discussion of inhaled steroid use: its effect on adherence. Ann Allergy Asthma Immunol 2003;90:411–415.
- Ulrik CS, Backer V, Søes-Petersen U, Lange P, Harving H, Plaschke PP. The patient's perspective: adherence or nonadherence to asthma controller therapy? J Asthma 2006;43:701–704.
- Gadkari AS, McHorney CA. Unintentional nonadherence to chronic prescription medications. How unintentional is it really? BMC Health Serv Res 2012;12:98.
- Becker MH, Maiman LA. Sociobehavioral determinants of compliance with health and medical care recommendations. Med Care 1975;13:10–24.
- Rosenstock IM. Why people use health services. Milbank Mem Fund Q 1966;44:94–127.
- Chambers CV, Markson L, Diamond JJ, Lasch L, Berger M. Health beliefs and compliance with inhaled corticosteroids by asthmatic patients in primary care practices. Respir Med 1999;93:88–94.
- Menckeberg TT, Bouvy ML, Bracke M, Kaptein AA, Leufkens HG, Raaijmakers JAM, Horne R, et al. Beliefs about medicines predict refill adherence to inhaled corticosteroids. J Psychosom Res 2008;64:47–54.
- Emilsson M, Berndtsson I, Lötvall J, Millqvist E, Lundgren J, Johansson Å, Brink E, et al. The influence of personality traits and beliefs about medicines on adherence to asthma treatment. Prim Care Respir J 2011;20:141–147.
- Horne R, Weinman J. Patients' beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res 1999;47:555–567.
- Byer B, Myers LB. Psychological correlates of adherence to medication in asthma. Psychol Health Med 2000;5:389–393.
- Partridge MR, van der Molen T, Myrseth SE, Busse WW. Attitudes and actions of asthma patients on regular maintenance therapy: the INSPIRE study. BMC Pulm Med 2006;6:13–21.
- Greaves CJ, Hyland ME, Halpin DMG, Blake S, Seamark D. Patterns of corticosteroid medication use: nonadherence can be effective in milder asthma. Prim Care Resp J 2005;14:99–105.
- Spilker B. Methods of assessing and improving patient compliance in clinical trials. Patient compliance in medical practice and clinical trials. New York (NY): Raven Press; 1991.