Abstract
Objective
Lung function testing is used in diagnosing asthma and assessing asthma control. Spirometry is most commonly used, but younger children can find performing this test challenging. Non-volitional tests such as airwave oscillometry (AOS) may be helpful in that population. We compared the success of spirometry and AOS in assessing bronchodilator responsiveness in children.
Methods
AOS was conducted alongside routine lung function testing. Resistance at 5 Hz (R5), the difference between the resistance at 5 and 20 Hz (R5-20) and the area under the reactance curve (AX) were assessed. Patients between 5 and 16 years old attending clinic with wheeze or asthma were assessed. Patients performed AOS, followed by spirometry and were then given 400 µg salbutamol; the tests were repeated 15 minutes later.
Results
Lung function testing was performed in 47 children of whom 46 (98%) and 32 (68%) performed acceptable baseline oscillometry and spirometry, respectively (p < 0.001). Children unable to perform acceptable spirometry were younger (7.35, range: 5.4–10.3 years) than those who could (10.4, range: 5.5–16.9 years), p < 0.001. The baseline z-scores of AOS R5 correlated with FEV1 (r = 0.499, p = 0.004), FEF75 (r = 0.617, p < 0.001), and FEV1/FVC (r = 0.618, p < 0.001). There was a positive bronchodilator response assessed by spirometry (change in FEV1 ≥ 12%) in eight children which corresponded to a change in R5 of 36% (range: 30%–50%) and a change in X5 of 39% (range: 15%–54%).
Conclusions
Oscillometry is a useful adjunct to spirometry in assessing young asthmatic children’s lung function. The degree of airway obstruction, however, might affect the comparability of the results of the two techniques.
Introduction
Asthma is the most common chronic respiratory disease worldwide (Citation1). In the UK, one in 11 children has a diagnosis of asthma (Citation2), which is associated with significant morbidity as indicated by 2.8 million school days lost annually (Citation3). The most frequent signs are cough, wheeze, and breathlessness (Citation4). Reversible airflow obstruction, as assessed by spirometry, is an objective assessment to support a diagnosis of asthma in children and young people aged 5–16 years with suggestive signs (Citation5). The European Respiratory Society (ERS) Task Force for pediatric asthma recommends to achieve asthma control with minimization of asthma manifestations (Citation6,Citation7), the key monitoring tools are symptomology and lung function testing (Citation7). The Global Initiative for Asthma recommends spirometry as the gold standard lung function test (Citation8). Spirometry, however, can be challenging in younger children because of the requirements to make a maximal effort and to follow instructions to generate acceptable maneuvers (Citation9). A paucity of trained respiratory technicians to supervise spirometry measurements is another obstacle to its use (Citation10). An alternative to spirometry which may be particularly useful in younger children is the non-volitional forced oscillation technique (FOT), in which oscillations are superimposed on passive tidal breathing to assess respiratory impedance (Citation11). As well as a lower requirement for patient comprehension and operator-training, FOT is a non-aerosol generating procedure, which minimizes the potential transmission of SARS-CoV-2 (Citation12). The oscillations of FOT can be delivered as pulses [impulse oscillometry (IOS)] or sinusoidal waves (Citation13). Several groups have used FOT to successfully assess bronchodilator responsiveness and asthma control in children and young people (Citation14–18). FOT could be a useful adjunct to spirometry in younger asthmatic children, who are unable to perform acceptable spirometry.
Developments in FOT technology have led to small, portable, and hand-held devices such as airwave oscillometry (AOS), which uses a vibrating mesh to generate a multifrequency sinusoidal pseudorandom noise (PRN) signal. The portability of AOS has benefits over other FOT devices as it can be used for home and clinic-based lung function testing (Citation19). Importantly, more successful AOS than spirometry measurements were made in 5–17-year-old children with Down syndrome, likely due to it being a non-volitional test (Citation20). The AOS markers, resistance at 5 Hz (R5), the difference between the resistance at 5 and 20 Hz (R5-20) and the area under the reactance curve (AX), measure peripheral airway disease in asthma (Citation13,Citation21). R5 correlated well with forced expiration volume in 1 s (FEV1), another measure of airflow obstruction, in asthmatic children (Citation22). In adult asthmatic patients, AOS was more sensitive than spirometry at detecting bronchodilator reversibility (Citation23). Furthermore, changes in IOS results were more sensitive than changes in FEV1 in diagnosing children with “asthmatic cough” (Citation24). In vivo and in vitro studies have demonstrated differences in resistance (Rrs) and reactance (Xrs) results between AOS and IOS devices (Citation25,Citation26). The differences between AOS and IOS were seen in resistances greater or equal to 10 Hz and reactance in asthmatic children (Citation27). The ERS, however, recently proposed a universal bronchodilator response threshold for all FOT devices of 40% of R5 results and 50% of reactance at 5 Hz (X5) results in children and adults (Citation28). It should be noted that large reference datasets of healthy children have identified lower bronchodilator thresholds of a change of 32% at R5 (Citation29).
The aim of our study was to compare the success of spirometry and AOS in assessing bronchodilator responsiveness in children with asthma or wheeze. Our secondary aims were to assess the correlation of spirometry and AOS results assessing airway obstruction and to review the ERS proposed bronchodilator response thresholds for the AOS device.
Methods
AOS assessments were conducted alongside routine lung function testing at the Amanda Smith Pulmonary Function Laboratory, King’s College Hospital NHS Foundation Trust (KCH), London, UK. Patients attending clinic aged between 5 and 16 years old, presenting with wheeze or asthma, were assessed. “Asthma” was defined as a clinical diagnosis of asthma before referral. “Wheezing” was defined as individuals with wheeze, who had not been given a clinical diagnosis at the time of referral. They were asked to withhold inhalers on the day of lung function testing until the assessments had taken place. Patients performed AOS, followed by spirometry and were then given 400 µg salbutamol via a spacer. Both tests were repeated in the same order 15 minutes after the bronchodilator challenge. AOS was performed before spirometry to avoid the potential impact of forced respiratory maneuvers on AOS results. Individuals who could not perform acceptable baseline spirometry did not undergo bronchodilator challenge. This study was approved as a quality improvement audit by KCH’s Pediatric Department (ref: CH119).
Airwave oscillometry
The Thorasys Tremoflo-C100 Airwave Oscillometry System™ (Thorasys, Montreal, QC, Canada) was used with Thorasys software. The device has a vibrating mesh to generate a multifrequency composite sinusoidal waveform from 5 to 37 Hz. Resistance (R5) and reactance (X5) at 5 Hz, the difference of resistance at 5 and 20 Hz (R5-20) and the area under the reactance curve (AX) were recorded. Verification of the device’s results was performed daily using a standard 15 cm H20 calibration load.
Patients were assessed in a seated upright position with a nose clip in place and supporting their cheeks with both hands. Children who could not tolerate the nose clip had their nose held by their accompanying parent/guardian. The participants breathed through a standard mouthpiece filter. A custom made 30-s template of pseudorandom noise was used, which is longer than the standard 20 s minimum duration of data acquisition (Citation29). Testing was repeated until a minimum of three tests were obtained with coherence of variance (CoV) equal or less than 15% at R5, (Citation29) up to a maximum of five trials, to be considered a “successful” result. The results were then expressed as z-scores using reference equations from a healthy population of children and young people which took into account the individual’s height, sex, and age (Citation30,Citation31). A positive bronchodilator response was defined as a decrease in R5 of 40% or an increase in X5 of 50% (Citation28).
Spirometry
Spirometry was performed using the MasterScreen (MS-PFT PRO) spirometer and analyzed with SentrySuite software. Testing was performed with the participant in an upright seated position, with nose clip in place. Patients were coached to inhale to total lung capacity and then to undertake a forced, complete expiration. Verbal and visual prompts were used. Spirometry curves were reviewed by a pediatric respiratory physiologist and considered “successful” if three acceptable forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) maneuvers were achieved as per the American Thoracic Society (ATS) and ERS guidelines (Citation32). A maximum of 10 trials were attempted. Calibration of the device was performed twice daily using a 3 L syringe. The results were converted to z-scores using an appropriate age, sex, height, and ethnic appropriate reference range (Citation33–35). FEV1, the ratio of FEV1 to FVC (FEV1/FVC), and the forced expiratory flow at 75% of the vital capacity (FEF75) results were recorded. A positive bronchodilator response was defined as an increase in FEV1 of equal or greater than 12% (Citation36).
Sample size
A previous study comparing lung function measurements in 7-year-old children found that 66.7% and 92.5% were able to perform acceptable spirometry and oscillometry testing, respectively (Citation37). To detect such a difference (25.8%) in successful measurements with 90% power at the 5% significance level, a sample size of 47 patients was required (Citation38).
Analysis
The data were tested for normality using the Shapiro–Wilk test and found not to be normally distributed. The chi-squared test was used to assess whether the proportions of successful measurements with spirometry or AOS differed significantly. The ages of children who could and could not perform spirometry were assessed for statistical significance using the Mann–Whitney U test. The agreement between R5 and FEV1 was assessed using a Bland/Altman analysis (Citation39). The data in the Bland Altman plot were analyzed by linear regression. Pearson’s two-tailed correlation coefficients were calculated to assess the strengths of correlations between spirometry and AOS results. Statistical analysis was conducted via SPSS software Version 28.
Results
Forty-seven children completed lung function testing (). Most patients presented with asthma (74%) and the remainder with wheeze (26%). Eighty-nine percent of the children were currently being treated with an inhaler: salbutamol (n = 41), a long-acting beta agonist (n = 42), and/or a corticosteroid (n = 39). In the past year, 16 of the children (34%) had received oral corticosteroids. A greater proportion of acceptable results were obtained with AOS (98%, 46/47) than with spirometry (68%, 32/47), p < 0.001. The median age of children who failed spirometry was lower (7.35, range: 5.4–10.3 years) than those who could perform spirometry (10.4, range: 5.5–16.9 years), p < 0.001. One patient was unable to perform either test.
Table 1. Demographics of study population.
There was a significant regression slope for the z-scores of FEV1 and R5 (mean Bland–Altman bias: −0.573 z-scores, p = 0.003; ). Linear regression analysis of the Bland-Altman data demonstrated a relatively good correlation (r = 0.503) and low variance (r2 = 0.252), p = 0.003. Pre-bronchodilator R5 results correlated with FEV1 (p = 0.004, r = 0.488), FEV1/FVC (p < 0.001, r = 0.618), and FEF75 (p < 0.001, r = 0.617) (). Baseline R5-20 correlated significantly with FEV1 (p = 0.015, r = 0.419), FEV1/FVC (p < 0.001, r = 0.568), and FEF75 (p = 0.002, r = 0.529). Pre-bronchodilator AX results correlated with FEV1 (p < 0.001, r = 0.68), FEV1/FVC (p = 0.007, r = 0.574), and FEF75 (p = 0.003, r = 0.618).
Figure 1. Bland-Altman plot for FEV1 z-scores paired with R5 z-scores, Regression equation: y = −0.73 + 1.6x.
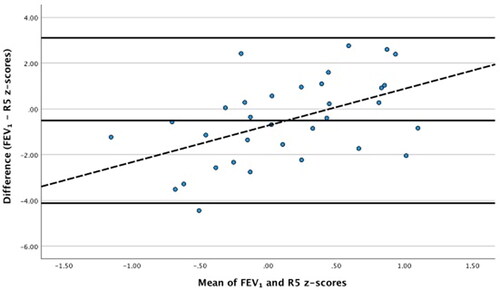
Table 2. Correlation of AOS parameters and spirometry parameters at baseline.
Eight children (17%) had a positive bronchodilator response as determined by the results of spirometry. They had a median age of 8.75 years (6.8–16.9 years). The eight children had median changes in R5 of −36% (range: −30% to −50%) and in X5 of 39% (15%–54%). Only three children (6.4%) had a positive bronchodilator response as assessed by AOS using the ERS definition. All three children had positive bronchodilator responses as assessed by spirometry. The bronchodilator ΔR5 correlated with ΔFEV1 (p < 0.001, r = −0.574), ΔFEV1/FVC (p < 0.001, r = 0.–0.577), and ΔFEF75 (p = 0.015, r = −0.424). The bronchodilator ΔAX correlated with ΔFEV1 (p = 0.003, r = −0.512), ΔFEV1/FVC (p = 0.005, r = 0.–0.487), and ΔFEF75 (p = 0.034, r = −0.376; ).
Table 3. Correlation of delta change in AOS and delta change in spirometry results.
Discussion
We have demonstrated that more children had acceptable AOS than spirometry results. The children who failed spirometry were significantly younger than those who produced acceptable results. AOS parameters of peripheral airway obstruction (R5, R5-20 and AX) correlated significantly with spirometry parameters of obstructive airflow disease (FEV1, FEF75, and FEV1/FVC) at baseline, further suggesting AOS assessments are useful in asthmatic children.
Our finding that oscillometry gave more technically acceptable results than spirometry is consistent with other studies in which oscillometry and spirometry were compared in children with Down syndrome (Citation20,Citation40) and those born prematurely (Citation37,Citation41,Citation42). In this study, the children unable to perform spirometry were younger than those who could produce acceptable results. AOS perhaps then may be most useful in the youngest children. Indeed, oscillometry, has been used as a tool to investigate preschool wheeze (Citation14,Citation15,Citation43). Technically acceptable AOS results were defined as three results with a coherence of variance (CoV) equal to or less than 15% at R5 (30). Cottee et al. have suggested a quality grading system (A–F) for oscillometry, with an “A” grade awarded to at least three trials with CoV equal or less than 10% at R5 (Citation44), which is stricter than the criteria we applied. A grading system would be similar to the spirometry standard, in which an “A” grade is awarded to three acceptable trials within 0.15 L for people over 6 years old and 0.1 L for people 6 years old and younger (Citation32).
Assessments of peripheral airway resistance, R5 and R5-20, correlated significantly with spirometry parameters of airflow obstruction, FEV1, FEV1/FVC, and FEF75, at baseline. The Bland-Altman regression slope of z-scores of FEV1 and R5 was significant, with a mean bias of −0.573 z-scores. Linear regression analysis of the Bland-Altman data showed a significant slope with greater discrepancy at more extreme values suggesting the degree of airway obstruction might affect the comparability of the results of the two techniques. Oscillometry, therefore, might underestimate severe airway disease and this should be considered when defining the upper and lower limits of “normal” for AOS. The bronchodilator changes in spirometry parameters most strongly correlated with ΔR5, rather than ΔR5-20. In peripheral airway obstruction, resistance is highest at low oscillation frequencies of 5 Hz (R5) and falls with increasing frequency; therefore, elevated R5 reflects peripheral obstructive airway disease (Citation13). Computational modeling has supported R5-20 as a marker of small airway dysfunction in asthma (Citation21). In a study of three to 6-year old asthmatic children, FEV1 more strongly correlated with R5 (p = 0.006, r = −0.51) than R20 (p = 0.015, r = −0.46) (Citation22). In that study, the authors reported that R5 had good between-test variability (0.92 for baseline-placebo) and within-test repeatability (4.1%). Further, in a study of prematurely born children who had bronchopulmonary dysplasia when assessed at 6–9 years old, FEV1 better correlated with R5 and R10 (p < 0.002, r = −0.43–079) than values of reactance (Citation45). We found the strongest correlation of baseline R5 with FEF75 (R = 0.617, p < 0.001) and FEV1/FVC (R = 0.618, p < 0.001). FEV1/FVC has previously been shown to be more sensitive marker than FEV1 in the detection of mild asthma in children (Citation46). FEF75 is a better marker of smaller peripheral airway caliber than FEV1, which more assesses the larger central airway (Citation47). A retrospective analysis of over 2000 spirometry results performed by asthmatic children aged 6–18 years found that FEF75 was more sensitive than FEV1 in detecting mild (33% versus 6.8%, p < 0.0001) and severe (71% versus 14.8%, p = 0.0001) airway obstruction (Citation48).
We also observed significant correlations at baseline and following the bronchodilator challenge between spirometry measures of airway obstruction and the reactance measure of AX, a summative marker of peripheral airway obstruction, (Citation13). The Pediatric Asthma Controller Trial (PACT) used IOS and spirometry in a double-blind randomized trial of three treatments for asthmatic children aged between 6 and 14 years (Citation8). They found the changes in AX demonstrated continued improvement over a prolonged period. The improvements in FEV1 and FEF25–75 were limited to the first 12 weeks of therapy, whereas improvements in AX were seen for a further 36 weeks in the group receiving fluticasone. Subjects more likely to respond with improvements in AX had a higher level of peripheral blood eosinophils, a marker of severe asthma (Citation49), which supports the idea that AX may be more sensitive to peripheral airway dysfunction than spirometry in asthmatic patients. Recently, a group showed that abnormality in the reactance parameters of X5 and AX correlated with poor asthma control in adults (p < 0.001 and p < 0.001, respectively) (Citation50). This suggests that both resistance and reactance markers might be useful in detecting peripheral airway obstruction in asthmatic patients.
Our final aim was to investigate the bronchodilator response assessed by these two devices. Significant correlations were found between the post-bronchodilator changes in ΔR5 with ΔFEV1 (p < 0.001, r = −0.574), ΔFEV1/FVC (p < 0.001, r = 0.–0.577), and ΔFEF75 (p = 0.015, r = −0.424) Short et al. compared the magnitude of beta blocker-induced bronchoconstriction and bronchodilation as assessed by IOS and spirometry in a randomized placebo-controlled crossover study of asthmatic adults (Citation51). All the IOS changes were of larger magnitude than the changes in spirometry, the greatest changes were in R5-20 and AX. This suggests that oscillometry may be more sensitive than spirometry in detecting reversible airway obstruction.
A positive bronchodilator response was defined as an increase in FEV1 of equal or greater than 12% (Citation36). In our study, only eight children (17%) met this criteria with corresponding changes in R5 of −36% (−30% to −50%) and X5 of 39% (15%–54%). These are lower than the threshold values suggested by the ERS, of at least −40% in R5 and 50% in X5, which were based on data sets from healthy children (Citation30,Citation52–56). In the largest dataset (n = 508) of children aged between 2 and 13 years old, however, the cut off for the lowest frequency of resistance (R6) was −32%, and closer to our findings (Citation30). Furthermore, two other studies used cutoff values for change in R5 of 29% (Citation53) and 37% (Citation54). In older children and young people, the highest threshold for adults was 32% (Citation57). It should be noted that those studies investigated IOS, rather than AOS.
Previous work has demonstrated lower resistance values and more negative reactance values measured by AOS in comparison to IOS devices (Citation25). Dandurand et al. assessed AOS and IOS devices using mechanical loads of known impedance (Citation58). They found large variation between devices when higher impedance loads were used, which was greater with regards to reactance. Lundblad et al. performed a similar study using a reference respiratory phantom, healthy individuals and patients with COPD (Citation59). They showed systematic differences between devices in vivo which were especially marked for measures of AX, but were significantly lower with IOS than AOS devices (p < 0.001). Testing using the respiratory phantom found close correlation between AOS results and test loads, whilst IOS results deviated significantly at higher loads (p < 0.0028). The authors suggested that varying calibration procedures might account for those differences. In view of their results, we suggest that device- and age-specific bronchodilator thresholds might be beneficial for deciding bronchodilator response assessed by oscillometry.
Our findings have clinical implications in that they suggest oscillometry could be used as a lung function test for children unable to perform acceptable spirometry. Schulze et al. conducted a post-hoc analysis of a pediatric asthma study with a 1-year follow up. In that study, using an area under the curve (AUC) analysis, R5 (AUC 0.8, p < 0.001) was superior to FEV1 (AUC 0.62) in predicting adverse events in asthma patients, defined as the occurrence of asthma symptoms and the use of salbutamol (Citation60). In another study, adults were classified to have mild, moderate persistent and severe persistent asthma according to NHLBI guidelines (Citation61) and their lung function was then assessed using both oscillometry and spirometry (Citation62). There were significant differences in the percentage predicted R5 (108.1% versus 130.1% versus 171.5% p < 0.05) and R5-R20 (1.2 versus 2.4 versus 3.3 hPa/l/s, p < 0.05) in the mild, moderate, and severe groups, respectively. This suggests that oscillometry can also be used to differentiate between clinically defined asthma severity.
Our study benefits from including a range of younger and older children. It was conducted in a real-world lung function clinic and so not subject to the possible biases of a research study. One of the limitations was that children who were unable to perform acceptable baseline spirometry did not go on to have bronchodilator challenge because of clinic time constraints. Therefore, children with positive bronchodilator responses as assessed by AOS might have been missed. Another limitation is that the reference datasets of Polish (Citation31), Australian and Italian (Citation30) children might not adequately represent the multi-ethnic population of children who were included in this report. Previous studies have also used other FOT device-based reference ranges (Citation20) in accordance with ERS guidance (Citation29). Unfortunately, until multi-ethnic reference datasets are available as they are for spirometry (Citation33), little can be done to account for this shortcoming. Children with wheeze (probable asthma) and physician-diagnosed asthma were included in this report. Although this would give a more comprehensive dataset of AOS in healthy and disease states, it perhaps reduced the power of our study in detecting the bronchodilator response threshold for the AOS device, as only eight patients had positive responses. Our conclusions regarding the comparison of spirometry and AOS in assessing bronchodilator responsiveness are limited by the low proportion of children (17%) who had a positive bronchodilator response. That low rate could be due to the influence of the treatments the children were receiving, but they were asked withhold their inhalers on the morning of testing.
In conclusion, we have shown that oscillometry could be a useful adjunct to spirometry in lung function testing of asthmatic children. The benefits were most evident in younger children who were unable to perform spirometry. Future work should aim to correlate the bronchodilator response as assessed by AOS with the clinical severity of asthma. We also suggest that device- and age-specific bronchodilation thresholds may be useful when using oscillometry.
Authors’ contributions
SG collected the AOS data, analyzed the data and drafted the manuscript. MT collected the spirometry data. LW was involved in conceptualization of the project. JC was involved in conceptualization of the project. CH conceptualized the project and analyzed the data. AG conceptualized the project and edited the manuscript. All authors approved the final manuscript.
Ethics
This study was approved as a quality improvement audit by KCH’s Pediatric Department (ref: CH119).
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Collaborators GBDCRD. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5:691–706.
- UK A. Asthma facts and statistics. 2021. Available from: https://www.asthma.org.uk/advice/understanding-asthma/what-is-asthma/.
- Mukherjee M, Stoddart A, Gupta RP, Nwaru BI, Farr A, Heaven M, Fitzsimmons D, Bandyopadhyay A, Aftab C, Simpson CR, et al. The epidemiology, healthcare and societal burden and costs of asthma in the UK and its member nations: analyses of standalone and linked national databases. BMC Med. 2016;14(1):113. doi:10.1186/s12916-016-0657-8.
- Bisgaard H, Szefler S. Prevalence of asthma-like symptoms in young children. Pediatr Pulmonol. 2007;42(8):723–728. doi:10.1002/ppul.20644.
- NICE. Asthma: diagnosis, monitoring and chronic asthma management. 2021. Available from nice.org.uk/ng80.
- National Asthma E, Prevention P. Expert Panel Report 3 (EPR-3). Guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94–S138.
- Pijnenburg MW, Baraldi E, Brand PL, Carlsen KH, Eber E, Frischer T, Hedlin G, Kulkarni N, Lex C, Mäkelä MJ, et al. Monitoring asthma in children. Eur Respir J. 2015;45(4):906–925. doi:10.1183/09031936.00088814.
- 2022 GINA Report. Global strategy for asthma management and prevention. Available from: https://ginasthma.org/reports/.
- Seed L, Wilson D, Coates AL. Children should not be treated like little adults in the PFT lab. Respir Care. 2012;57(1):61–70. doi:10.4187/respcare.01430.
- Kaminsky DA, Marcy TW, Bachand M, Irvin CG. Knowledge and use of office spirometry for the detection of chronic obstructive pulmonary disease by primary care physicians. Respir Care. 2005;50(12):1639–1648.
- Dubois AB, Brody AW, Lewis DH, Burgess BF.Jr. Oscillation mechanics of lungs and chest in man. J Appl Physiol. 1956;8(6):587–594. doi:10.1152/jappl.1956.8.6.587.
- Kouri A, Gupta S, Yadollahi A, Ryan CM, Gershon AS, To T, Tarlo SM, Goldstein RS, Chapman KR, Chow CW. Addressing reduced laboratory-based pulmonary function testing during a pandemic. Chest. 2020;158(6):2502–2510. doi:10.1016/j.chest.2020.06.065.
- HJ, Smith, PR, MD, Goldmant. European Respiratory Monograph. Forced oscillation technique and impulse oscillometry. 2005.
- Shin YH, Jang SJ, Yoon JW, Jee HM, Choi SH, Yum HY, Han MY. Oscillometric and spirometric bronchodilator response in preschool children with and without asthma. Can Respir J. 2012;19(4):273–277. doi:10.1155/2012/560323.
- Starczewska-Dymek L, Bozek A, Mielnik M. The sensitivity and specificity of the forced oscillation technique in the diagnosis of bronchoconstriction in children. J Asthma. 2021;58(3):334–339. doi:10.1080/02770903.2019.1702054.
- Robinson PD, Brown NJ, Turner M, Van Asperen P, Selvadurai H, King GG. Increased day-to-day variability of forced oscillatory resistance in poorly controlled or persistent pediatric asthma. Chest. 2014;146(4):974–981. doi:10.1378/chest.14-0288.
- Lee E, Yoon J, Cho HJ, Hong SJ, Yu J. Respiratory reactance in children aged three to five years with postinfectious bronchiolitis obliterans is higher than in those with asthma. Acta Paediatr. 2017;106(1):81–86. doi:10.1111/apa.13632.
- Larsen GL, Morgan W, Heldt GP, Mauger DT, Boehmer SJ, Chinchilli VM, Lemanske RF, Jr, Martinez F, Strunk RC, Szefler SJ, Childhood Asthma Research and Education Network of the National Heart, Lung, and Blood Institute, et al. Impulse oscillometry versus spirometry in a long-term study of controller therapy for pediatric asthma. J Allergy Clin Immunol. 2009;123(4):861–867 e1. doi:10.1016/j.jaci.2008.10.036.
- Wong A, Hardaker K, Field P, Huvanandana J, King GG, Reddel H, Selvadurai H, Thamrin C, Robinson PD. Home-based forced oscillation technique day-to-day variability in pediatric asthma. Am J Respir Crit Care Med. 2019;199(9):1156–1160. doi:10.1164/rccm.201809-1659LE.
- Vielkind ML, Hamlington KL, Wolter-Warmerdam K, Meier MR, Liu AH, Hickey FJ, Brown MA, DeBoer EM. Airwave oscillometry to measure lung function in children with Down syndrome. Pediatr Res. 2022;91(7):1775–1780. doi:10.1038/s41390-021-01664-7.
- Foy BH, Soares M, Bordas R, Richardson M, Bell A, Singapuri A, Hargadon B, Brightling C, Burrowes K, Kay D, et al. Lung computational models and the role of the small airways in asthma. Am J Respir Crit Care Med. 2019;200(8):982–991. doi:10.1164/rccm.201812-2322OC.
- Olaguibel JM, Alvarez-Puebla MJ, Anda M, Gomez B, Garcia BE, Tabar AI, Arroabarren E. Comparative analysis of the bronchodilator response measured by impulse oscillometry (IOS), spirometry and body plethysmography in asthmatic children. J Investig Allergol Clin Immunol. 2005;15(2):102–106.
- Kuo CR, Lipworth B. Airwave oscillometry and patient-reported outcomes in persistent asthma. Ann Allergy Asthma Immunol. 2020;124(3):289–290. doi:10.1016/j.anai.2019.12.017.
- Taniuchi N, Hino M, Yoshikawa A, Miyanaga A, Tanaka Y, Seike M, Gemma A. Usefulness of simultaneous impulse oscillometry and spirometry with airway response to bronchodilator in the diagnosis of asthmatic cough. J Asthma. 2022. [Epub ahead of print].
- Soares M, Richardson M, Thorpe J, Owers-Bradley J, Siddiqui S. Comparison of forced and impulse oscillometry measurements: a clinical population and printed airway model study. Sci Rep. 2019;9(1):2130. doi:10.1038/s41598-019-38513-x.
- Zimmermann SC, Watts JC, Bertolin A, Jetmalani K, King GG, Thamrin C. Discrepancy between in vivo and in vitro comparisons of forced oscillation devices. J Clin Monit Comput. 2018;32(3):509–512. doi:10.1007/s10877-017-0050-y.
- Ducharme FM, Jroundi I, Jean G, Lavoie Boutin G, Lawson C, Vinet B. Interdevice agreement in respiratory resistance values by oscillometry in asthmatic children. ERJ Open Res. 2019;5(1):00138-2018. doi:10.1183/23120541.00138-2018.
- Thamrin C, Robinson PD, Farah CS, King GG. Authors of the ERS technical standards for respiratory oscillometry: technical standards for respiratory oscillometry and bronchodilator response cut-offs. Eur Respir J. 2022;59(3):2102663. doi:10.1183/13993003.02663-2021.
- King GG, Bates J, Berger KI, Calverley P, de Melo PL, Dellaca RL, Hall GL, Ioan I, Irvin CG, Kaczka DW, et al. Technical standards for respiratory oscillometry. Eur Respir J. 2020;55(2):1900753. doi:10.1183/13993003.00753-2019.
- Calogero C, Simpson SJ, Lombardi E, Parri N, Cuomo B, Palumbo M, de Martino M, Shackleton C, Verheggen M, Gavidia T, et al. Respiratory impedance and bronchodilator responsiveness in healthy children aged 2-13 years. Pediatr Pulmonol. 2013;48(7):707–715. doi:10.1002/ppul.22699.
- Nowowiejska B, Tomalak W, Radliński J, Siergiejko G, Latawiec W, Kaczmarski M. Transient reference values for impulse oscillometry for children aged 3-18 years. Pediatr Pulmonol. 2008;43(12):1193–1197. doi:10.1002/ppul.20926.
- Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, Hallstrand TS, Kaminsky DA, McCarthy K, McCormack MC, et al. Standardization of spirometry 2019 update. An official American thoracic society and European respiratory society technical statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. doi:10.1164/rccm.201908-1590ST.
- Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, ERS Global Lung Function Initiative, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi:10.1183/09031936.00080312.
- Rosenthal M, Cramer D, Bain SH, Denison D, Bush A, Warner JO. Lung function in white children aged 4 to 19 years: II–single breath analysis and plethysmography. Thorax. 1993;48(8):803–808. doi:10.1136/thx.48.8.803.
- Rosenthal M, Bain SH, Cramer D, Helms P, Denison D, Bush A, Warner JO. Lung function in white children aged 4 to 19 years: I-spirometry. Thorax. 1993;48(8):794–802. doi:10.1136/thx.48.8.794.
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi:10.1183/09031936.05.00035205.
- Durlak W, Klimek M, Wroński M, Trybulska A, Kwinta P. Multimodal longitudinal respiratory function assessment in very low birth weight 7-year-old children. Adv Med Sci. 2021;66(1):81–88. doi:10.1016/j.advms.2020.12.006.
- Wang HaC S-C. Sample Size Calculation for Comparing Proportions. 2007.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310.
- Fernandez-Plata R, Perez-Padilla R, Del Rio-Hidalgo R, Garcia-Sancho C, Gochicoa-Rangel L, Rodriguez-Hernandez C, Torre-Bouscoulet L, Martínez-Briseño D. Quality of pulmonary function tests in participants with down syndrome. Arch Bronconeumol (Engl Ed). 2019;55(10):513–518. doi:10.1016/j.arbr.2019.02.018.
- Bjorn L, Erik M, Per T, Mikael N, Jenny H. Agreement between spirometry and impulse oscillometry for lung function assessment in 6-year-old children born extremely preterm and at term. Pediatr Pulmonol. 2020;55(10):2745–2753. doi:10.1002/ppul.24976.
- Thunqvist P, Tufvesson E, Bjermer L, Winberg A, Fellman V, Domellof M, Melen E, Norman M, Hallberg J. Lung function after extremely preterm birth-A population-based cohort study (EXPRESS). Pediatr Pulmonol. 2018;53(1):64–72. doi:10.1002/ppul.23919.
- Ortiz G, Menendez R. The effects of inhaled albuterol and salmeterol in 2- to 5-year-old asthmatic children as measured by impulse oscillometry. J Asthma. 2002;39(6):531–536. doi:10.1081/JAS-120004923.
- Cottee AM, Thamrin C, Farah CS, Seccombe LM. Quality assessment pathway for respiratory oscillometry. ERJ Open Res. 2022;8(1):00569-2021. doi:10.1183/23120541.00569-2021.
- Brostrom EB, Thunqvist P, Adenfelt G, Borling E, Katz-Salamon M. Obstructive lung disease in children with mild to severe BPD. Respir Med. 2010;104(3):362–370. doi:10.1016/j.rmed.2009.10.008.
- Lukic KZ, Coates AL. Does the FEF25-75 or the FEF75 have any value in assessing lung disease in children with cystic fibrosis or asthma? Pediatr Pulmonol. 2015;50(9):863–868. doi:10.1002/ppul.23234.
- McFadden ER, Jr., Linden DA. A reduction in maximum mid-expiratory flow rate. A spirographic manifestation of small airway disease. Am J Med. 1972;52(6):725–737. doi:10.1016/0002-9343(72)90078-2.
- Francisco B, Ner Z, Ge B, Hewett J, Konig P. Sensitivity of different spirometric tests for detecting airway obstruction in childhood asthma. J Asthma. 2015;52(5):505–511. doi:10.3109/02770903.2014.984842.
- Pavlidis S, Takahashi K, Ng Kee Kwong F, Xie J, Hoda U, Sun K, Elyasigomari V, Agapow P, Loza M, Baribaud F, et al. “T2-high” in severe asthma related to blood eosinophil, exhaled nitric oxide and serum periostin. Eur Respir J. 2019;53(1):1800938. doi:10.1183/13993003.00938-2018.
- Cottee AM, Seccombe LM, Thamrin C, King GG, Peters MJ, Farah CS. Oscillometry and asthma control in patients with and without fixed airflow obstruction. J Allergy Clin Immunol Pract. 2022;10(5):1260–1267 e1. doi:10.1016/j.jaip.2021.12.026.
- Short PM, Williamson PA, Lipworth BJ. Sensitivity of impulse oscillometry and spirometry in beta-blocker induced bronchoconstriction and beta-agonist bronchodilatation in asthma. Ann Allergy Asthma Immunol. 2012;109(6):412–415. doi:10.1016/j.anai.2012.09.010.
- Hellinckx J, De Boeck K, Bande-Knops J, van der Poel M, Demedts M. Bronchodilator response in 3-6.5 years old healthy and stable asthmatic children. Eur Respir J. 1998;12(2):438–443. doi:10.1183/09031936.98.12020438.
- Nielsen KG, Bisgaard H. Discriminative capacity of bronchodilator response measured with three different lung function techniques in asthmatic and healthy children aged 2 to 5 years. Am J Respir Crit Care Med. 2001;164(4):554–559. doi:10.1164/ajrccm.164.4.2006119.
- Malmberg LP, Pelkonen A, Poussa T, Pohianpalo A, Haahtela T, Turpeinen M. Determinants of respiratory system input impedance and bronchodilator response in healthy Finnish preschool children. Clin Physiol Funct Imaging. 2002;22(1):64–71. doi:10.1046/j.1475-097X.2002.00396.x.
- Thamrin C, Gangell CL, Udomittipong K, Kusel MM, Patterson H, Fukushima T, Schultz A, Hall GL, Stick SM, Sly PD. Assessment of bronchodilator responsiveness in preschool children using forced oscillations. Thorax. 2007;62(9):814–819. doi:10.1136/thx.2006.071290.
- Oostveen E, Dom S, Desager K, Hagendorens M, De Backer W, Weyler J. Lung function and bronchodilator response in 4-year-old children with different wheezing phenotypes. Eur Respir J. 2010;35(4):865–872. doi:10.1183/09031936.00023409.
- Oostveen E, Boda K, van der Grinten CPM, James AL, Young S, Nieland H, Hantos Z. Respiratory impedance in healthy subjects: baseline values and bronchodilator response. Eur Respir J. 2013;42(6):1513–1523. doi:10.1183/09031936.00126212.
- Kim CW, Kim JS, Park JW, Hong CS. Clinical applications of forced oscillation techniques (FOT) in patients with bronchial asthma. Korean J Intern Med. 2001;16(2):80–86. doi:10.3904/kjim.2001.16.2.80.
- Dandurand RJ, Lavoie J-P, Lands LC, Hantos Z, the Oscillometry Harmonisation Study Group. Comparison of oscillometry devices using active mechanical test loads. ERJ Open Res. 2019;5(4):00160-2019. doi:10.1183/23120541.00160-2019.
- Lundblad LKA, Miletic R, Piitulainen E, Wollmer P. Oscillometry in chronic obstructive lung disease: in vitro and in vivo evaluation of the impulse oscillometry and tremoflo devices. Sci Rep. 2019;9(1):11618. doi:10.1038/s41598-019-48039-x.
- Schulze J, Biedebach S, Christmann M, Herrmann E, Voss S, Zielen S. Impulse oscillometry as a predictor of asthma exacerbations in young children. Respiration. 2016;91(2):107–114. doi:10.1159/000442448.
- National Heart LaBI. NIH Expert Panel report II: guidelines for the diagnosis and maangement of asthma. 1997.

