Abstract
Objectives
The purpose of this study was to examine the number of exacerbations, counts of eosinophils, and asthma-related symptoms 1 year before and after initiating benralizumab for the treatment of severe eosinophilic asthma.
Methods
Patients with prior exacerbations and newly initiating benralizumab were identified in the claims-based Healthcare Integrated Research Database. Claims were used to assess benralizumab treatment patterns, exacerbations, healthcare resource utilization, and other asthma medication used. Among a subset of patients, medical records were abstracted for Asthma Control Test (ACT) scores and asthma symptoms.
Results
There were 506 patients meeting inclusion/exclusion criteria for claims-based analyses and 123 for medical-record analyses. The number of patients experiencing exacerbations significantly decreased from baseline to follow-up (40% reduction, McNemar’s χ2 = 204.00, p < .001). The mean number of exacerbations also decreased from 3.2 (1.5) to 1.2 (1.4) (paired t = 24.45, p < .001; Cohen’s D = 1.09). The effects were larger among patients with eosinophils ≥300 cells/µL. Among patients with an ACT available for baseline and follow-up (n = 47), there was a significant reduction in the number of patients with scores <19 (72% vs. 45%, p < .01).
Conclusions
Treatment with benralizumab resulted in fewer exacerbations, reduced utilization, and improved ACT scores. This study demonstrates that benralizumab is an effective treatment option for patients with severe eosinophilic asthma.
Introduction
Asthma, a chronic inflammatory respiratory airway disease, is usually characterized by variable airflow obstruction, persistent symptoms such as wheeze, cough, shortness of breath, chest tightness, and airway hyper-responsiveness (Citation1). Asthma is a global problem, estimated as affecting more than 5% of the worldwide population and more than 8% of the US population (Citation2,Citation3). Severe asthma is defined as asthma that remains uncontrolled in patients who are adherent, are treated with optimized high-dose inhaled corticosteroid (ICS) and long-acting beta agonist (LABA) treatment, use proper inhaler technique, and have managed comorbid and environmental contributors to their asthma (Citation4). Severe asthma affects an estimated 3% to 14% of adult patients with asthma (Citation5–10). Typified by airway inflammation and the presence of a high concentration of eosinophils in sputum or blood, severe eosinophilic asthma is the most common phenotype of severe asthma (Citation11–13).
By consensus, the American Thoracic Society (ATS) and the European Respiratory Society (ERS) guidelines have been combined to consolidate diagnostic criteria for severe eosinophilic asthma (Citation11). Severe eosinophilic asthma is diagnosed by major objective measures, including diagnosis of severe asthma, persistently elevated sputum or serum eosinophil counts, frequent exacerbations, and dependence on oral corticosteroids (OCS) (Citation11). Minor criteria include late onset of disease, upper airway disease, other biomarkers (exhaled nitric oxide fraction, serum periostin, dipeptidyl peptidase-4), fixed airway obstruction, and air trapping or presence of mucus plugs (Citation11,Citation14). Spirometry, biomarkers, and self-reported clinical response using survey tools such as the Asthma Control Test (ACT) or the Asthma Control Questionnaire are among the tools used to monitor response to therapeutic interventions (Citation11). Severe eosinophilic asthma is difficult to control, has a high disease burden, and can result in frequent asthma exacerbations requiring urgent treatment (Citation15,Citation16). According to ATS/ERS guidelines as well as Global Initiative for Asthma (GINA) standards, a step-wise approach to treatment includes optimizing ICS-LABA therapy, management of contributing factors, and assessment of severe asthma phenotype (Citation1,Citation17). Agents which are considered prior to the addition of biologic add-on treatment include as-needed short-acting beta agonists (SABAs), long-acting antimuscarinic antagonists (LAMA), leukotriene modifiers/leukotriene receptor antagonists, low dose theophylline, low dose azithromycin, and low dose OCS (as a last resort due to adverse effects) (Citation1,Citation17,Citation18). However, severe eosinophilic asthma may be poorly controlled despite these treatments. For patients who require an advanced therapy, a biologic agent which targets the (interleukin) IL-5/IL-5R pathway, such as benralizumab, mepolizumab, or reslizumab, may be appropriate (Citation1,Citation16,Citation17).
Benralizumab has been available in the United States since November 2017 and is indicated for patients 12 and older with severe asthma with eosinophilic phenotype (Citation19). In randomized clinical trials (RCTs), benralizumab has been shown to reduce exacerbations and improve lung function in patients with moderate-to-severe eosinophilic asthma (Citation20). However, the real-world treatment patterns and impact on healthcare utilization and costs of benralizumab in the United States have not been thoroughly assessed. This study builds on previous real-world evidence studies which found that benralizumab reduced exacerbation rates and healthcare utilization and costs among patients with severe eosinophilic asthma (Citation21,Citation22). While these studies found benralizumab to be clinically and economically beneficial, they were limited to claims data observations and lacked symptom assessment data. The purpose of this study was to examine the number of exacerbations, eosinophil counts, and asthma-related symptoms before and after initiation of benralizumab.
Methods
Data sources
The Healthcare Integrated Research Database (HIRD) is a large healthcare database curated by Carelon Research (formerly HealthCore) for uses including health economics, outcomes, and pharmacoepidemiologic research. Data within the HIRD are updated monthly and a 3-month lag is imposed to account for claims adjudication processing. The HIRD is built on a broad, clinically rich, and geographically diverse spectrum of longitudinal medical and pharmacy claims data from health plan members across the United States affiliated with Elevance Health. Members may be enrolled in Commercial, Medicare Advantage, and Medicare supplement (Medigap) plans. Member enrollment, medical care (professional and facility claims), outpatient prescription drug use (pharmacy claims), and healthcare utilization may be tracked for health plan members in the database dating back to January 2006. The laboratory data were obtained from large, outpatient laboratories and abstracted electronic medical records via data feeds in the HIRD. Claims data and medical records for this study were obtained from providers under a waiver of authorization from the IRB.
Study sample
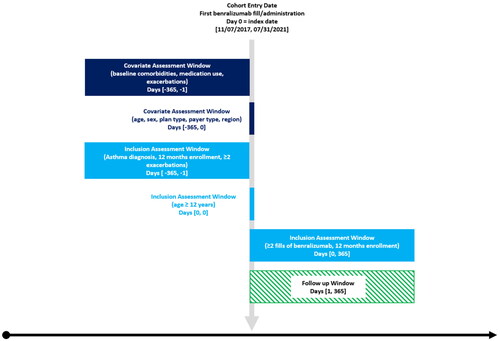
The study was a single cohort pre-post design, using retrospective data between November 01, 2016 and July 31, 2021. The earliest date of benralizumab initiation (from medical and pharmacy claims) was the index date. Medical claims were prioritized over pharmacy claims in cases of suspected duplication. The baseline period was 12 months before the index date. The follow-up period was 12 months after the index date.
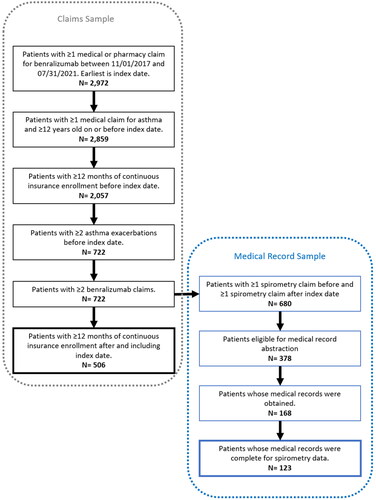
Patients meeting the following criteria were included in the study (see ): aged ≥12 years on index date, having ≥365 days of continuous insurance enrollment before and after the index date, having ≥2 asthma exacerbations (defined in Outcomes section below) before the index date (in line with prescribing guidelines), and having ≥2 claims for benralizumab (rationale in Exposure section below). For a subset of patients, we were able to obtain medical records to supplement the claims and laboratory result data (see ). However, to obtain a sufficiently powered sample, the required follow-up time was allowed to be less than 12 months for medical record sub-sample, while still requiring ≥2 doses of benralizumab.
Exposure
The exposure was ≥2 doses of benralizumab to account for the dosing schedule. Benralizumab is administered day 1, week 4, and week 8 for the first three doses and every 8 weeks thereafter.
Outcomes
The primary study outcome was asthma exacerbation, which was defined as (all ICD-10 codes in the Appendix):
an inpatient visit with an asthma-related primary diagnosis code (ICD-10: J45%) and without a diagnosis code for chronic obstructive pulmonary disease (COPD) acute myocardial infarction (MI), or congestive heart failure (CHF) during the same visit; or
an emergency room (ER) visit with an asthma diagnosis code without a diagnosis code for COPD, acute MI, CHF, or autoimmune diseases (see Appendix) during the same visit plus a claim of systemic corticosteroid (3 to 30 days supplied) within 5 days of the ER visit; or
an outpatient visits with an asthma diagnosis code without a diagnosis code for COPD, acute MI, CHF , or autoimmune diseases during the same visit plus a claim of systemic corticosteroid (3 to 30 days supplied) within 5 days of the outpatient visit.
If a patient experienced a new exacerbation ≤14 days of a previous exacerbation, they were combined into a single exacerbation episode with the hierarchy inpatient > ER > outpatient.
The secondary outcomes included (a) treatment patterns (adherence, discontinuation, switching), (b) documented symptoms, (c) healthcare resource utilization (HCRU) related to exacerbations, and (d) asthma medication use. Adherence was measured by proportion of days covered ≥0.80 during the follow-up period using imputed days’ supply of 28 days for doses 1 and 2 and 56 days for doses 3 to 8 from pharmacy claims. Discontinuation was defined as having a > 90-day gap after benralizumab last claim date plus 28 or 56 days supplied plus 90 days. Restarts were considered when a patient had a medical or pharmacy claim for benralizumab after a discontinuation. Switching to another biologic occurred when a patient had a claim for another biologic (i.e. omalizumab, mepolizumab, reslizumab, dupilumab) after discontinuing benralizumab. Utilization was assessed for exacerbation related care (i.e. occurring between start and end of an exacerbation) for number and duration of inpatient hospitalizations, number of ER visits, and number of outpatient office visits. Asthma symptoms documented by providers in the medical record were assessed up to 12 months before and after starting benralizumab among a subset of patients with available medical records; symptoms included coughing, wheezing, shortness of breath, chest tightness/pressure/pain, nasal congestion, nasal discharge, post-nasal drip, itching, and sneezing.
Other variables
Other variables were assessed to help describe the patient population including index date demographics: age, sex, payor, US Census region, year of index date, and prescriber specialty on index date. Other baseline characteristics from medical claims included the Quan-Charlson Comorbidity Index (QCI) Score (Citation23), other related comorbidities (e.g. allergic rhinitis, atopic dermatitis, autoimmune disorders, obesity, eosinophilic disorders, and infections), and eosinophil lab values (i.e. closest to index for baseline or furthest from index for follow-up) for the subset of patients with linked outpatient laboratory result data.
Additionally, medication use in the baseline and follow-up periods was assessed for common asthma treatments using pharmacy claims. These medications, alone or in combination, included ICS, OCS, long-acting beta agonists (LABAs), long-acting muscarinic antagonists (LAMAs), biologics (omalizumab, mepolizumab, reslizumab, dupilumab), and short-acting beta agonists (SABAs).
Analysis
The outcomes were assessed using inferential statistics for pre-post one-cohort design including paired t-tests (for continuous outcomes), McNemar’s (for categorical outcomes), binomial (for two group proportions), and repeated ANOVAs (for continuous outcomes by categories), as appropriate. Otherwise, only descriptive statistics were provided. The analyses were conducted as an intention-to-treat (ITT) design given than all patients had at least a loading dose.
Results
Baseline demographic and clinical characteristics
The 506 benralizumab patients included in the main analysis were mostly between 40 and 64 years of age (68%), with a mean (SD) age of 52.2 (14.3) years and mostly female (69%). Patients were seen by allergist/immunologists (39%) and pulmonologists (34%). During the baseline patients had a mean QCI score of 1.6 (1.1) and high proportions of comorbidities (15–78%). Among patients with available blood eosinophil count (n = 217), 135 had baseline counts ≥300 cells/uL, 82 had baseline counts < 300 cells/uL. Of the 217 patients, 171 had baseline counts ≥150 cells/uL ().
Benralizumab treatment patterns
The mean and median number of fills/administrations of benralizumab were both 5. The mean and median proportional of days covered were 0.60, with 36% of patients reaching a proportion of days covered (PDC) of ≥80%, indicating adherence throughout follow-up. About 52% of patients discontinued benralizumab during follow-up with a median time to discontinuation of 116 days. Of the discontinuers, 52% restarted on benralizumab after a median of 114 days. About one-quarter of discontinuers switched to another biologic medication. We did not assess switching back to benralizumab after starting a new biologic.
Exacerbations
The number of patients experiencing exacerbations significantly decreased from baseline to follow-up (McNemar’s χ2 = 204.00, p < .001). Over 40% of patients had no exacerbations during follow-up. The mean number of exacerbations also significantly decreased from 3.2 (1.5) to 1.2 (1.4) (paired t = 24.45, p < .001; Cohen’s D = 1.09), which was primarily driven by reductions in outpatient exacerbations ().
In patients with baseline eosinophil counts ≥300 cells/uL, 49% had no follow-up exacerbations relative to 38% of patients with eosinophil counts <300 cells/uL. A similar pattern was observed for patients with baseline eosinophils ≥150/uL (45% had no follow-up exacerbations) and 41% without follow-up exacerbations among patients with <150 cells/uL. The reduction in mean number of exacerbations was significant in patients with ≥300 and <300 cells/uL, though a larger reduction occurred in patients with ≥300 cells/uL (53% vs 72%). A similar pattern was observed for patients with baseline eosinophils ≥150/uL, with a69% reduction in number of follow-up exacerbations and 53% reduction among patients with <150 cells/uL.
Medical record data
There were 123 patients with available medical record data for analysis (see ). Provider-documented symptoms all showed numeric reductions in the number of each between baseline and follow-up. Statistical tests were not performed due to unequal observation time. Among patients with an ACT available for baseline and follow-up (n = 47), there was a significant reduction in the number of patients with scores <19 (72% vs. 45%, p < .01).
Healthcare utilization
The proportion of patients with an inpatient stay decreased significantly from baseline to follow-up (9.9% vs. 3.8%, McNemar’s χ2 =17.65, p < .001). The proportion of patients with an ER visit decreased by over half (20.4% vs. 8.5%, McNemar’s χ2 =36.26, p < .001) from baseline to follow-up. The proportion of patients seen for evaluation and management office visits also decreased significantly from 98% to 56% (McNemar’s χ2 =201.37, p < .001) and the mean number of visits decreased from 3.6 to 1.5 (paired t = 18.62, p < .001).
Among patients with both baseline and follow-up eosinophil labs (n = 113), the mean (SD) number of cells decreased significantly (paired t = 8.44, p < .001) from 508.2 (547.6) to 59.0 (91.1) cells/uL. The median eosinophil levels decreased from 400 cell/uL during baseline to 0 cells/uL during follow-up. Only 12 of 113 patients had eosinophil counts ≥150 cells/UL during follow-up.
Medications
Asthma medications were used frequently within this population (see ). OCS were the most frequently used medication with >99% of patients using them during baseline, which declined to 75% of patients using them during follow-up (p < .001). The mean number of fills was reduced by roughly half from baseline to follow-up (6.0 vs. 3.1, paired t = 17.70, p < .001). SABAs were also common, with 91.5% of patients having a prescription filled during the baseline and 75% with a fill during follow-up (McNemar’s χ2 =60.31 p < .001). The mean number of fills for SABAs decreased by 27% (5.1 vs. 3.7; paired t = 8.46, p < .001). Other common baseline medications included ICS + LABA combinations (85%), LAMAs (46%), ICS + LAMA + LABA combination (36%), and ICS (36%). Fills of LAMAs and ICS decreased significantly from baseline to follow-up (paired t = −2.49, p < .013 and paired t = 3.04, p < .001).
Discussion
This study demonstrates real world effectiveness and value consistent with RCTs (Citation24,Citation25) and previous real-world studies (Citation21,Citation22) of benralizumab for the treatment of severe eosinophilic asthma. We observed significant reductions in the number of patients having exacerbations and the reductions in the number of exacerbations overall. The magnitudes of reduction were nearly the same as observed in previous Zephyr studies (Citation21,Citation22) using non-overlapping patient populations, demonstrating a robust treatment effect. Importantly, around 40% of patients may experience a remission of severe asthma symptoms, leading to lower disease burden. The reductions in exacerbations may also lead to a better quality of life and less concern over respiratory problems about respiratory problems.
The reductions in exacerbations led to lower exacerbation-related HCRU across healthcare settings. Patients also used fewer asthma maintenance and rescue medications, including systemic corticosteroids. Taken together with the improved ACT scores and lower number of patients experiencing symptoms during follow-up, benralizumab appears to reduce the disease burden for patients and for payers. The changes in ACT scores also indicates that patients may have an improved quality of life after initiating benralizumab. The reductions were observed across different levels of baseline eosinophils, with greater reductions in exacerbations with higher levels of eosinophils (≥300 cells/uL). The magnitude of reduction suggests that benralizumab is effective in patients with more severe eosinophilic asthma, as indicated by the higher number serum eosinophils.
Half of patients using benralizumab discontinued use, yet about half of those (25% of all patients) restarted benralizumab after an average of 4 months, suggesting that patients are cycling on and off benralizumab, potentially due to the seasonality of environmental triggers of asthma during the spring and summer. We did not assess for this seasonality, but future work should examine it directly. Another quarter of discontinuers switched to another biologic medication. Future studies should explore the effectiveness of other biologics after switching to determine whether biologics are effective for all patients with severe asthma. The proportion of patients discontinuing was much higher in our sample than observed in other studies for biologics generally (10%) (Citation26), potentially indicating a difference in the sample population or in definition of discontinuation. It was also noted that a high proportion of patients continued to use SABAs before and after initiation of benralizumab; this is a function of our US-based sample for which there is no approved maintenance and reliever therapy, so SABAs continue to be the standard of care in the United States, in contrast to 2023 GINA guidelines (Citation27).
Limitations
Only patients with 24 months of continuous eligibility were included, which could induce selection and survivor biases. However, this design was necessary to observe the treatment patterns before and after initiation of benralizumab. To mitigate against this, an exploratory analysis of patients with variable follow-up was conducted and patient characteristics were similar between groups. The study analysis of exacerbations was performed using an ITT type analysis because each patient had at least 2 doses of benralizumab. Therefore, the results should be interpreted carefully considering discontinuations (24.7% permanent discontinuations) and switching (13%). Data derived from medical and pharmacy claims are subject to undetected coding errors. In addition, the presence of a claim for a filled prescription does not indicate that the medication was consumed or that it was taken as prescribed. Any medications provided as samples by the physician will not be observed in the claims data. Finally, all patients included in this study were enrolled in commercial or Medicare health insurance plans in the United States and satisfy all the inclusion/exclusion criteria. The results are generalizable to patients with commercial insurance across the US meeting similar inclusion/exclusion criteria but may not be generalizable to patients not meeting inclusion/exclusion criteria, or with other types of health insurance or who are uninsured, or to those outside the United States.
Conclusion
Patients with severe eosinophilic asthma and who initiated benralizumab treatment saw reductions in the number of asthma exacerbations with a substantial proportion having none. Patients also experienced lower HCRU, fewer symptoms, and reduced asthma controller or rescue medication use, including systemic corticosteroids. This study adds to the growing literature supporting the use of benralizumab as an effective treatment for the long-term control of severe eosinophilic asthma.
Declaration of interest
JLS, JB, and BN are employees and shareholders of Elevance Health, supporting Carelon Research, which received funding from AstraZeneca for this research. YC, TB, and DC are employees of AstraZeneca.
Additional information
Funding
References
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2022. Available from: www.ginasthma.org; https://ginasthma.org/gina-reports/.
- Song P, Adeloye D, Salim H, Dos Santos JP, Campbell H, Sheikh A, Rudan I. Global, regional, and national prevalence of asthma in 2019: a systematic analysis and modelling study. J Glob Health. 2022;12:04052. doi:10.7189/jogh.12.04052.
- Pate CA, Zahran HS, Qin X, Johnson C, Hummelman E, Malilay J. Asthma surveillance – United States, 2006–2018. MMWR Surveill Summ. 2021;70(5):1–32. doi:10.15585/mmwr.ss7005a1.
- Levy ML, Bacharier LB, Bateman E, Boulet L-P, Brightling C, Buhl R, Brusselle G, Cruz AA, Drazen JM, Duijts L, et al. Key recommendations for primary care from the 2022 Global Initiative for Asthma (GINA) update. NPJ Prim Care Respir Med. 2023;33(1):7. doi:10.1038/s41533-023-00330-1.
- Bleecker ER, Gandhi H, Gilbert I, Murphy KR, Chupp GL. Mapping geographic variability of severe uncontrolled asthma in the United States: management implications. Ann Allergy Asthma Immunol. 2022;128(1):78–88. doi:10.1016/j.anai.2021.09.025.
- Domingo C, Sicras-Mainar A, Sicras-Navarro A, Sogo A, Mirapeix RM, Engroba C. Prevalence, T2-biomarkers and cost of severe asthma in the era of biologics: the BRAVO-1 study. J Investig Allergol Clin Immunol (online ahead of print on November 22, 2022).
- Hansen S, von Bülow A, Sandin P, Ernstsson O, Janson C, Lehtimäki L, Kankaanranta H, Ulrik C, Aarli BB, Fues Wahl H, et al. Prevalence and management of severe asthma in the Nordic countries: findings from the NORDSTAR cohort. ERJ Open Res. 2023;9(2):00687–2022. doi:10.1183/23120541.00687-2022.
- Hardtstock F, Krieger J, Wilke T, Lukas M, Ultsch B, Welte R, Quinzler R, Maywald U, Timmermann H. Epidemiology, treatment and health care resource use of patients with severe asthma in Germany – a retrospective claims data analysis. J Asthma. 2023;60:1280–1289.
- Hekking PW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902. doi:10.1016/j.jaci.2014.08.042.
- Wang E, Wechsler ME, Tran TN, Heaney LG, Jones RC, Menzies-Gow AN, Busby J, Jackson DJ, Pfeffer PE, Rhee CK, et al. Characterization of severe asthma worldwide: data from the International Severe Asthma Registry. Chest. 2020;157(4):790–804. doi:10.1016/j.chest.2019.10.053.
- Buhl R, Humbert M, Bjermer L, Chanez P, Heaney LG, Pavord I, Quirce S, Virchow JC, Holgate S, Expert group of the European Consensus Meeting for Severe Eosinophilic Asthma Severe eosinophilic asthma: a roadmap to consensus. Eur Respir J. 2017;49(5):1700634. doi:10.1183/13993003.00634-2017.
- Heaney LG, Perez de Llano L, Al-Ahmad M, Backer V, Busby J, Canonica GW, Christoff GC, Cosio BG, FitzGerald JM, Heffler E, et al. Eosinophilic and noneosinophilic asthma: an expert consensus framework to characterize phenotypes in a Global Real-Life Severe Asthma Cohort. Chest. 2021;160(3):814–830. doi:10.1016/j.chest.2021.04.013.
- Pavlidis S, Takahashi K, Ng Kee Kwong F, Xie J, Hoda U, Sun K, Elyasigomari V, Agapow P, Loza M, Baribaud F, et al. “T2-high” in severe asthma related to blood eosinophil, exhaled nitric oxide and serum periostin. Eur Respir J. 2019;53(1):1800938. doi:10.1183/13993003.00938-2018.
- Wagener AH, de Nijs SB, Lutter R, Sousa AR, Weersink EJM, Bel EH, Sterk PJ. External validation of blood eosinophils, FE(NO) and serum periostin as surrogates for sputum eosinophils in asthma. Thorax. 2015;70(2):115–120. doi:10.1136/thoraxjnl-2014-205634.
- Hiles SA, Gibson PG, McDonald VM. Disease burden of eosinophilic airway disease: comparing severe asthma, COPD and asthma-COPD overlap. Respirology. 2021;26(1):52–61. doi:10.1111/resp.13841.
- Davila Gonzalez I, Moreno Benitez F, Quirce S. Benralizumab: a new approach for the treatment of severe eosinophilic asthma. J Investig Allergol Clin Immunol. 2019;29(2):84–93. doi:10.18176/jiaci.0385.
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi:10.1183/09031936.00202013.
- Gibson PG, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, Jenkins C, Peters MJ, Marks GB, Baraket M, et al. Efficacy of azithromycin in severe asthma from the AMAZES randomised trial. ERJ Open Res. 2019;5(4):00056–2019. doi:10.1183/23120541.00056-2019.
- Food and Drug Administration. Fasenra (benralizumab) injection, for subcutaneous use. 2017. Available from: www.accessdata.fda.gov/drugsatfda_docs/label/2017/761070s000lbl.pdf. [last accessed May 2, 2023].
- Farne HA, Wilson A, Powell C, Bax L, Milan SJ. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9(9):CD010834. doi:10.1002/14651858.CD010834.pub3.
- Chung Y, Katial R, Mu F, Cook EE, Young J, Yang D, Betts KA, Carstens DD. Real-world effectiveness of benralizumab: results from the ZEPHYR 1 Study. Ann Allergy Asthma Immunol. 2022;128(6):669–676.e6. doi:10.1016/j.anai.2022.02.017.
- Carstens D, Maselli DJ, Mu F, Cook EE, Yang D, Young JA, Betts KA, Genofre E, Chung Y. et al. Largest real-world effectiveness study of benralizumab for severe eosinophilic asthma: ZEPHYR 2. J. Allergy Clin. Immunol. Pract. 2023;11(7):2150–2161.e4.
- Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel J-M, Sundararajan V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi:10.1093/aje/kwq433.
- Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, Sproule S, Gilmartin G, Aurivillius M, Werkström V, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta(2)-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2115–2127. doi:10.1016/S0140-6736(16)31324-1.
- FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, Ferguson GT, Busse WW, Barker P, Sproule S, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–2141. doi:10.1016/S0140-6736(16)31322-8.
- Menzies-Gow AN, McBrien C, Unni B, Porsbjerg CM, Al-Ahmad M, Ambrose CS, Dahl Assing K, von Bülow A, Busby J, Cosio BG, et al. Real World biologic use and switch patterns in severe asthma: data from the International Severe Asthma Registry and the US CHRONICLE Study. J Asthma Allergy. 2022;15:63–78. doi:10.2147/JAA.S328653.
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2023. Available from: www.ginasthma.org; https://ginasthma.org/gina-reports/.
Appendix A:
Diagnosis code
Figure 3. Changes in asthma exacerbations before and after starting benralizumab. McNemar’s χ2 for counts of patients and paired t-test for mean exacerbations.
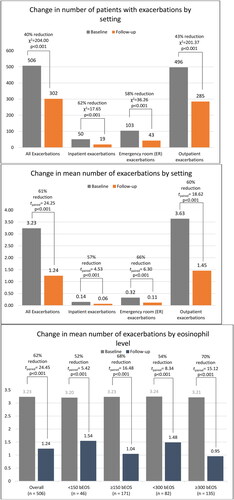
Table 1. Patient demographic and clinical characteristics during baseline and on index date for the claims sample and the medical record sample.
Table 2. Asthma Control Test and symptoms before and after starting benralizumab.
Table 3. Asthma medication use before and after starting benralizumab.