Abstract
To address the critical need for improving the chemical characterization of the organic composition of ambient particulate matter, we introduce a combined thermal desorption aerosol gas chromatograph—aerosol mass spectrometer (TAG-AMS). The TAG system provides in-situ speciation of organic chemicals in ambient aerosol particles with hourly time resolution for marker compounds indicative of sources and transformation processes. However, by itself the TAG cannot separate by particle size and it typically speciates and quantifies only a fraction of the organic aerosol (OA) mass. The AMS is a real-time, in-situ instrument that provides quantitative size distributions and mass loadings for ambient fine OA and major inorganic fractions; however, by itself the AMS has limited ability for identification of individual organic compounds due to the electron impact ionization detection scheme used without prior molecular separation.
The combined TAG-AMS system provides real-time detection by AMS followed by semicontinuous analysis of the TAG sample that was acquired during AMS operation, achieving simultaneous and complementary measurements of quantitative organic mass loading and detailed organic speciation. We have employed a high-resolution time-of-flight mass spectrometer (HR-ToF-MS) to enable elemental-level determination of OA oxidation state as measured on the AMS, and to allow improved compound identification and separation of unresolved complex mixtures (UCM) measured on the TAG. The TAG-AMS interface has been developed as an upgrade for existing AMS systems. Such measurements will improve the identification of organic constituents of ambient aerosol and contribute to the ability of atmospheric chemistry models to predict ambient aerosol composition and loadings.
Copyright 2014 American Association for Aerosol Research
1. INTRODUCTION
Organic aerosol (OA) is well known to constitute a large fraction of the total tropospheric particulate burden (Kanakidou et al. Citation2005; Jimenez et al. Citation2009), and as such has important implications for human health (Jang et al. Citation2006; Mauderly and Chow Citation2008; Pope et al. Citation2009) and the earth's climate (Liu and Wang Citation2010; Solomon et al. 2007). In particular, the effects of aerosols represent the single largest source of uncertainty in our understanding of global radiative forcing (Solomon et al. 2007). The characterization of sources and fates of OA in the atmosphere is crucial to our understanding of the relationship between anthropogenic activity and global climate. This includes primary OA (POA), emitted to the atmosphere directly, and secondary OA (SOA), formed by gas-to-particle conversion processes. Because atmospheric particulate matter may be composed of thousands of individual organics, only a fraction of which have been identified thus far (Hamilton et al. Citation2004; Goldstein and Galbally Citation2007), the detailed characterization of OA represents a substantial experimental challenge. The development of instrumentation for the rapid characterization of the composition of OA is thus an important priority in atmospheric research.
Detailed chemical analysis of individual organic molecules has been an important method for determining major OA sources to the atmosphere (Fraser et al. Citation2000; Schauer and Cass Citation2000; Yue and Fraser Citation2004a,b; Jaeckels et al. Citation2007; Williams et al. Citation2007, Citation2010a) and for determining chemical reaction pathways from atmospheric oxidation (Jang et al. Citation2003; Arey et al. Citation2009; Paulot et al. Citation2009). Traditionally, particle samples are collected for 12–24 h onto filters and returned to the lab for solvent extraction or direct thermal desorption and molecular identification and quantification by gas chromatography-mass spectrometry techniques (Schauer and Cass Citation2000; Ho et al. Citation2008). This technique can determine the presence of key source-marking compounds that have been previously characterized in source chemical profiles (Rogge et al. Citation1991, Citation1993a,Citationb,c, Citation1994, Citation1997a, Citationb, 1998). The first fully automated in situ GC/MS analysis was introduced by Williams et al. (Citation2006), the thermal desorption aerosol gas chromatograph (TAG). Here, air is collected for 30 min and automatically desorbed and transferred to a GC/MS for identification and quantification of key organic marker compounds. With the higher time resolution of the in situ TAG system (1-h), it is now possible to use statistical tools such as positive matrix factorization (PMF), a tool to determine covarying groups of species which can often be interpreted as sources or atmospheric processes, on shorter sampling periods than was previously possible with 24-h time resolution, e.g., 1.5 weeks (Williams et al. Citation2010a) instead of years (Jaeckels et al. Citation2007).
Recent improvements in OA characterization have been made through advanced factor analysis techniques (Ulbrich et al. Citation2009; Ulbrich et al. Citation2012) applied to volatility-resolved (Huffman et al. Citation2008) and/or bulk high-resolution mass spectral data (Docherty et al. Citation2011). Aerodyne Research, Inc. (ARI) has developed a novel aerosol mass spectrometer (AMS) allowing for real-time chemically- and size-resolved mass loading measurements of atmospheric aerosol particles (Jayne et al. Citation2000; Jimenez Citation2003; DeCarlo et al. Citation2006). The utility of the AMS has been demonstrated in many major field deployments across the globe (Zhang et al. Citation2007; Ng et al. Citation2010; Spracklen et al. Citation2011).
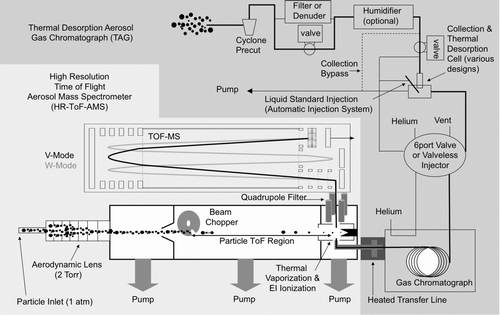
As outlined by Hallquist et al. (Citation2009), there is no single instrument capable of fully quantifying total OA with molecular level separation. Near the two extremes of quantification and separation capabilities are the AMS, capable of total OA quantification but with limited chemical resolution, and the TAG, capable of molecular level separation, but only quantifies a fraction of the total OA mass. Here, we introduce a combined TAG-AMS system () to provide highly time-resolved, detailed organic speciation of ambient aerosol particles along with quantitative size-resolved total organic mass loadings. A high-resolution time-of-flight mass spectrometer (HR-ToF-MS) is utilized to incorporate the recent advances of HR-ToF-AMS (highlighted in Section 1.2) and to extend the capabilities of the traditional quadrupole-MS TAG instrument (e.g., potential to build molecular structure of compounds not available in mass spectral databases, and separation of unresolved complex mixtures (UCM) at the elemental level).
FIG. 1 Schematic of the combined thermal desorption aerosol gas chromatograph—aerosol mass spectrometer (TAG-AMS). TAG samples and AMS samples are alternately delivered to the high-resolution time-of-flight mass spectrometer. The TAG inlet delivers individual organic marker compounds and the AMS inlet delivers size-resolved fine aerosol that is separated into total organics, sulfate, nitrate, ammonium, and chloride fractions. Elemental ratios of O:C and H:C are attainable from both sample inlets using the high-resolution mass spectral capacity of the ToF-MS detector.
1.1. The Thermal Desorption Aerosol Gas Chromatograph
In the TAG system, ambient atmospheric PM2.5 (particulate matter <2.5 μm in diameter) or PM1 (depending on the particle cyclone employed) is collected by means of humidification and inertial impaction. The sample is then thermally desorbed, separated by a gas chromatograph (GC), and identified and quantified at the molecular level using electron impact ionization quadrupole mass spectrometry (MS). The TAG system offers around the clock measurements to determine diurnal, weekly, and seasonal patterns in OA composition.
The TAG system has been used to determine major OA components in a variety of environments, including remote (Williams et al. Citation2007), urban (Williams et al. Citation2010a), and forested (Worton et al. Citation2011). TAG measurements have been used to apportion black carbon sources in an urban setting (Lambe et al. Citation2009). Additionally, ambient phase-transitioning of semivolatile organic compounds has been estimated from TAG measurements (Williams et al. Citation2010b), and recently, a semivolatile TAG instrument has been developed to separately quantify the gas-phase and particle-phase fraction of semivolatile organic compounds (Zhao et al. Citation2013). Finally, comprehensive two-dimensional (GCxGC) gas chromatography and time-of-flight mass spectrometry (ToFMS) has been added to the TAG collector to allow for greater molecular separation (i.e., greater than an order of magnitude more species separated) and increased sensitivity (Goldstein et al. Citation2008; Worton et al. Citation2012). This 2D-TAG instrument has recently been used in laboratory-based controlled chamber experiments (Isaacman et al. Citation2011a) and ambient air sampling during the CalNex 2010 campaign (Hayes et al. Citation2013).
The TAG system has undergone extensive quantification development (Kreisberg et al. Citation2009) and has been compared to traditional filter collection techniques (Lambe et al. Citation2010). TAG calibration is performed through injection of known quantities of liquid standards. These standards are composed of a wide variety of organic compounds with varying polarity, volatility, and functionality. In addition to external standards applied at varying concentrations to determine calibration curves (Kreisberg et al. Citation2009), recent efforts to fully automate the calibration system (Isaacman et al. Citation2011b) have made it possible to apply deuterated internal standards in each ambient sample to correct for possible matrix effects (Lambe et al. Citation2010). Additional developments on the TAG collector include: (1) the option for a metal-fiber filter collection-thermal-desorption (CTD) cell in place of the inertial impaction CTD cell (Zhao et al. Citation2013), (2) a valveless injection system (Zhao et al. Citation2013), and (3) the option to use a high-efficiency denuder instead of a filter to alternate between sampling modes that preserve the particle signal in each sample () (Zhao et al. Citation2013).
One limitation of the TAG technique is that it does not detect the total organic mass loading. In the TAG, the total OA mass is equal to the sum of the compounds that are resolved (R), not resolved (NR), and not eluting (NE) through the gas chromatography column. The NR fraction can be further explored using the HR-ToF-MS of the TAG-AMS system to determine the oxidation state of these complex mixtures and to determine if multiple categories of complex mixtures exist in single samples (a finding that could lead to attributing this mass to specific sources in a similar way to how resolved compounds are handled in the analysis procedures). The NE fraction may be composed of very high polarity and/or extremely nonvolatile species that are either not able to be desorbed at our maximum temperature, are desorbed but not efficiently transferred through the GC column, or are thermally decomposed. It is important to note that the resolved fraction identified by the TAG is often less than 20% of the total OA mass as measured by the AMS. While TAG can offer critical information for the determination of particle sources through select organic marker compounds (which is not currently possible with the AMS), TAG does not offer total quantification of the entire organic mass concentrations as is obtained by AMS measurement. An analysis of the division of OA amongst R, NR, and NE for a variety of sources will be possible with the TAG-AMS system and is the focus of future work.
1.2. The Aerodyne Aerosol Mass Spectrometer
The AMS allows real-time, in situ measurements of the size distributions and chemical composition of aerosol particles (Jayne et al. Citation2000). The AMS operates by using an aerodynamic lens (Liu et al. Citation1995; Zhang et al. Citation2002) to sample submicron particles into vacuum where they are aerodynamically sized, thermally vaporized on a heated surface, and chemically analyzed via electron impact ionization time-of-flight mass spectrometry. The AMS is a rapidly evolving instrument which has been undergoing improvements in hardware, instrument control and signal processing and analysis software since its inception.
The AMS has been described in detail previously (Jayne et al. Citation2000; Allan Citation2003; Jimenez Citation2003; DeCarlo et al. Citation2006; Drewnick et al. Citation2009) so only a brief overview is given here. The AMS samples particles into high vacuum, aerodynamically sizes the particles via particle time-of-flight (PToF) and analyzes particle composition via high-resolution MS following thermal particle vaporization. The instrument consists of three main sections: (1) an aerosol sampling chamber (inlet + aerodynamic lens), (2) a PToF sizing chamber, and (3) a particle composition detection chamber (vaporization, ionization and detection), as shown in . Each chamber is separated by small apertures and is differentially pumped. Quantification of nonrefractory aerosol mass is possible due to the use of standard electron impact ionization and the separation of the vaporization and ionization steps in the detection region.
The AMS has been deployed in numerous field campaigns (Jimenez et al. Citation2009; Ng et al. Citation2010). Taken together, the worldwide measurements with the AMS provide a unique picture of the variability and evolution of aerosol properties. Zhang et al. (Citation2007) and Ng et al. (Citation2010) have recently compiled AMS measurements from dozens of field campaigns conducted in the Northern Hemisphere. Although the sampling locations in these compilations cover a range of urban, industrial, marine, and rural environments, a common feature of almost all sites is the significant contribution of organic species to the total aerosol mass. On average 42% (17–70%) of the nonrefractory submicron aerosol mass is apportioned to organic species (Zhang et al. Citation2007). Thus, in recent years an important goal of the AMS community has involved developing means of improving the characterization of the OA component measured by the AMS. This goal has been approached through advances in instrumentation as well as improved data analysis tools such as PMF (Ulbrich et al. Citation2009, Citation2012).
In parallel with field studies, the AMS has been used to characterize the yields and mechanisms of SOA formation in smog chamber experiments. The AMS is in use in many photoreaction chambers worldwide, including Teflon laboratory chambers (Anttila et al. Citation2005; Bahreini et al. Citation2005; Alfarra et al. Citation2006; Trainer et al. Citation2006; Chhabra et al. Citation2011) and steady-state flow tubes (George et al. Citation2007; Kang et al. Citation2011; Lambe et al. Citation2011a,b). Recent studies have attempted to directly compare lab results and ambient OA observations, using AMS mass spectral components as the reference (George et al. Citation2007; Grieshop et al. Citation2007).
The incorporation of a time-of-flight mass spectrometer into the AMS (ToF-AMS) represents a significant instrumental advance over the previous quadrupole AMS (Q-AMS) instrument because of its increased sensitivity, its ability to provide complete mass spectra on a single particle basis, and its capability to provide size distributions for all m/z (or alternatively complete size-resolved mass spectra). The high-resolution version (HR-ToF-AMS) is capable of mass resolution ranging from 3000 (in V-mode) to 4500–5000 (in W-mode) (DeCarlo et al. Citation2006), with the newest detectors achieving above this published range. The high mass resolution allows the separation of each unit mass peak into separate contributions for specific elemental compositions (i.e., C, H, O, N, and S content) based on small differences, or defect, in the exact mass (Aiken et al. Citation2007). Information about the elemental compositions of organic-containing particulate matter provides valuable insight into its sources as well as transformations (Aiken et al. Citation2008; Ng et al. Citation2010, Citation2011).
Although the organic AMS mass spectra can be used to quantify organic mass, they are too complex to enable the identification of most individual organic molecules. Multivariate methods have been developed that allow for the improved separation of the organic ambient aerosol component into distinct chemical classes (Zhang et al. Citation2005; Ulbrich et al. Citation2009, Citation2012). Early multivariate analysis results of organic AMS spectra focused on two dominant types of OA groups worldwide: hydrocarbon-like OA (HOA) and oxygenated organic aerosol (OOA). More recent PMF analyses have discovered additional OA separation amongst HOA, biomass burning OA (BBOA), cooking organic aerosol (COA), semivolatile OOA (SV-OOA), and low-volatility OOA (LV-OOA). The components SV-OOA and LV-OOA were initially termed based on observed volatility differences using thermal denuder sampling (Huffman et al. Citation2008) and mass spectral differences between these components are largely distinguished by differing ratios of m/z 43:m/z 44 (Ng et al. Citation2010). While PMF analysis and elemental ratio analysis of HR-ToF-AMS data has proven to be a quantitative approach to characterizing bulk OA properties and separating OA into major components, there is insufficient chemical separation to identify all individual specific source contributions. Source marker compounds available with the TAG-AMS will provide critically needed information for the interpretation of the organic components extracted from AMS data.
2. TAG-AMS DESIGN AND DEVELOPMENT
Further details of each individual instrument can be found in existing literature, TAG: (Williams et al. Citation2006; Kreisberg et al. Citation2009; Williams et al. Citation2010a), AMS: (Jayne et al. Citation2000; Allan Citation2003; Jimenez Citation2003; DeCarlo et al. Citation2006; Drewnick et al. Citation2009). The technical approach to a combined TAG-AMS system involves introducing the output of the TAG GC column into the ionizing region of a high-resolution time-of-flight mass spectrometer (HR-ToFMS) in the AMS (). Frequent switching (every 1 or 2 h) between acquisition of online thermal vaporization MS data (AMS data) and time-averaged GC-resolved thermal desorption of collected aerosol (TAG data), utilizing a single high-resolution mass spectrometer, provides complementary measurements of individual organic species from the TAG and size specific OA mass loadings from the AMS. Also included from the AMS are size specific sulfate, nitrate, ammonium, and chloride mass concentrations, as well as elemental abundance O:C, H:C, and N:C ratios. Elemental ratios are acquired through TAG-mode as a function of GC retention time, and will be explored in Section 3.4. With 1-h (2-h) time resolution, the TAG system collects aerosol at 9 LPM for 30 min (90 min), resulting in a sample volume of 0.27 m3 (0.81 m3).
The TAG inlet is capable of collecting a fraction of the gas-phase component of semivolatile organic compounds. This fraction has been used to determine phase transitions for common compound classes in previous work (Williams et al. Citation2010b). The Semi-Volatile TAG collection system has been designed to improve the quantitation of both the gas fraction and particle fraction of semivolatile organic compounds (Zhao et al. Citation2013). The TAG-AMS system developed here does not utilize the recently developed semivolatile collection cell and is instead operated with a denuder on the inlet of the system to ensure the TAG system does not collect additional gas-phase sample compared to the AMS system. It can also operate with a rotation between denuded and undenuded sampling to characterize phase transitioning of compound classes observed by the TAG-mode.
2.1. Transfer Line
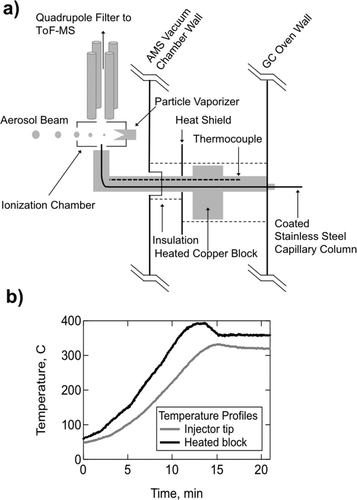
A custom heated transfer line was produced to allow for efficient transfer of GC effluent to the ionization region of the AMS (). The GC column is connected to a 15" length × 1/32" OD Inertium® coated stainless steel capillary which travels through a heated (300°C) copper rod, through a modified rear flange of the AMS, and is directed into the bottom of the ion cage inside the AMS. The transfer line between TAG and AMS is heated using a cartridge heater located outside the AMS and controlled by PID with the feedback thermocouple located at the end of the copper rod inside the AMS vacuum chamber (i.e., tip of the stainless capillary). Tests showed that the internal thermocouple location (end of copper rod) took approximately 20 min to stabilize temperature, and the final temperature displayed a 40°C temperature gradient (cooler) compared to the location of the external cartridge heater ().
FIG. 2 Design and performance of a heated GC-MS interface. (a) The GC column is connected to a transfer line (coated stainless steel capillary) that travels through a heated copper block, delivering the sample to the ionization chamber prior to mass spectral detection. A quadrupole filter is used to deflect the large abundance of carrier helium gas prior to delivery to the ToF-MS. (b) Temperature profiles of heated transfer line system. For a 350°C set point temperature at the copper block, there is approximately a 40°C deviation as measured at the tip of the injector. Heat up time to reach stable operating temperature is about 20 min.
2.2. Quadrupole High-Pass Filter
Following sample delivery through the custom transfer line, separated gas molecules are ionized and directed through a quadrupole high-pass filter (Tofwerk AG, Thun, Switzerland), to eliminate transmission of all ions below a pre-determined mass-to-charge ratio, and the remaining ions are finally sent into the HR-ToFMS for identification and quantification (Tofwerk AG, Thun, Switzerland). For our purposes, we set the quadrupole high-pass filter to deflect ions below m/z 10 in order to eliminate the transmission of ions from our high-concentration carrier helium gas (m/z 4). Our initial testing employed the traditional ion focusing optics to direct ions into the detector instead of a quadrupole high-pass filter. The quadrupole high-pass filter was added to eliminate m/z baseline issues and rapid detector degradation that were resulting from detector saturation from large quantities of helium ions. Existing AMS systems can be upgraded to accept TAG sample by replacing the rear vacuum chamber flange and associated internal chamber cylinder with the new flange and internal cylinder that incorporates the heated transfer line. It is also recommended to replace the existing ion optics lenses with a quadrupole high-pass filter for extended use.
2.3. Software
AMS data acquisition and analysis software was updated to accommodate GC collection and analysis. GC analysis from the TAG-AMS system is performed using a custom built software panel based in Igor (Wavemetrics, Inc., Portland, OR, USA). GC data are collected as HDF5 files (The_HDF_Group), similar to AMS data acquisition but collected in Fast MS mode (0.5 s averaging) instead of AMS General Alteration mode (1 min typical averaging) (Kimmel et al. Citation2011). Data files are imported to Igor using the AMS software package SeQUential Igor data RetRiEvaL (SQUIRREL) for data preprocessing, then imported as separate chromatograms into a new GC analysis panel for compound identification and integration. High mass resolution analysis is performed using the AMS software package Peak Integration by Key Analysis (PIKA). The Igor TAG panel is still being actively developed. More detail on this software will be presented in a future publication.
3. INITIAL TESTING
Mass concentration calibrations are performed separately for the two inlets (e.g., hundreds of individual organic compounds injected into the collection cell for TAG calibration and aerosolized test particles collected by AMS for traditional AMS calibration).
Particle sizing is also performed on the AMS system and is determined by the PToF between the chopper and the vaporizer. PToF distributions of size selected ammonium nitrate with and without helium indicated no systematic change in the distribution upon adding helium.
3.1. Ionization Efficiency (IE)
IE is a measure of the number of parent molecules that get ionized compared the number of parent molecules that were available in the ionization region. It was of interest to observe how the addition of helium into the AMS vacuum chamber/ionization cage would affect the IE of the AMS, since helium flow from the GC into the AMS is continuous regardless of analysis mode. During the TAG analysis mode, the AMS chopper is closed to block the AMS particle and gas beam, and GC effluent carried by helium is analyzed. During AMS analysis mode, the helium carrier gas from the GC is left flowing, but does not transfer any sample since the oven is cooled and secluded from the TAG sample cell. Using ammonium nitrate test particles, AMS IE decreased by ∼35% in the presence of helium gas, displaying an IE = (1.4 ± 0.1) × 10−7 with no helium flow and an IE = (0.90 ± 0.18) × 10−7 with 1 ml min−1 helium flow (typical flow during operation) into the ionization chamber. Since the number of He atoms present in the ionization region is insufficient to deplete the current of ionizing electrons, the lower IE is likely due to the presence of high numbers of He+ ions that repel some of the ions from other analytes away from the region that is efficiently extracted into the mass spectrometer. It is thus recommended that the TAG GC column should be operated in a “constant flow” mode as opposed to the optional “constant pressure” mode to keep the concentration of He+ ions constant throughout the measurement cycle.
An investigation on the possibility of helium flow affecting the AMS particle beam was performed using test ammonium nitrate particles. It is observed that under typical GC column flows (∼1 to 2 ml min−1) the helium flow does not disrupt the particle beam. At helium flows beyond what is used in our operation (e.g., ∼5 ml min−1), the AMS particle beam focus on the vaporizer can be altered, but only if the particle beam is already misaligned to the edge of the vaporizer. It is thus important to ensure good beam alignment when using the TAG-AMS and to operate with helium flows less than 3 ml min−1.
3.2. Chemical Standards
Initial TAG-AMS laboratory testing was performed using a traditional TAG collection system and Agilent 6890 GC (Williams et al. Citation2006), the custom transfer line described previously, and an HR-ToFMS (V-mode) with electron impact ionization and traditional ion focusing lenses (DeCarlo et al. Citation2006). Soon after initial testing, the ion focusing lenses were replaced with a miniature quadrupole high-pass filter as described above.
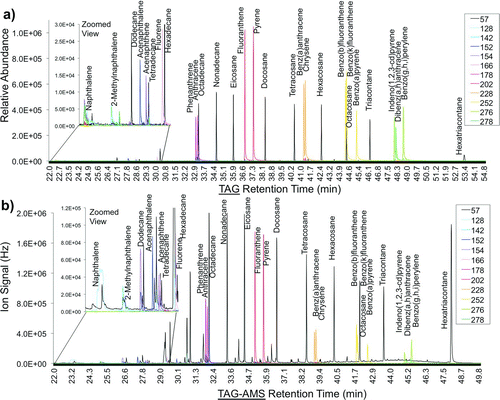
The TAG-AMS system was first tested using liquid standards of known composition and quantities. Liquid standards were injected through the injection port on the TAG collection and thermal desorption cell, transferred through the GC system and into the AMS. shows the results of a combined alkane/PAH standard that covers a wide range of volatilities. shows the results from a standalone TAG system with quadrupole MS detection (Agilent 5973), and is the result of the same standard (composition and quantities) analyzed using the combined TAG-AMS, where the HR-ToFMS on the AMS is now the GC detector. The same range of species is detected by both versions of the TAG system. The PAH ion signals are of the same scale or larger than the m/z 57 signal of alkanes on the quad-TAG (), where the PAH ion signals on the TAG-AMS chromatogram () have a relatively smaller response than m/z 57 from alkanes. This may be an indication of differences between ionization conditions and detectors. To ensure maximum detection for a wide range of organic compounds, the HR-ToF-MS used in the TAG-AMS system and accompanying quadrupole high-pass filter should both be tuned to maximize the throughput of a high bandwidth (wide range of ions).
FIG. 3 Chromatograms of alkane/PAH standards. (a) 12.5 ng of each compound were first analyzed using a standalone TAG system (with quadrupole MS detection, Agilent 5973). (b) The same standard (12.5 ng of each species) was analyzed using the combined TAG-AMS system (with HR-ToF-MS, TofWerk). The same range of species are observed in both samples.
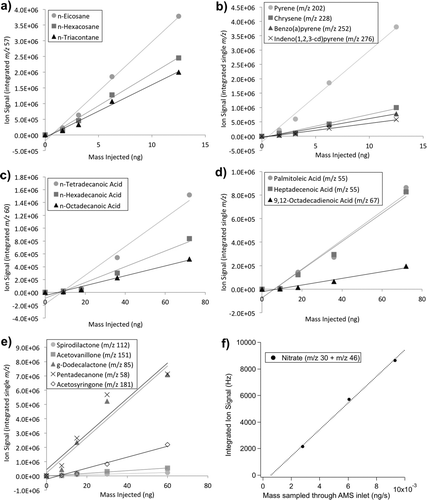
shows the response to single injections of a range of standards (alkanes, PAHs, saturated and unsaturated acids, and ketones) at multiple concentrations from the TAG-AMS system. While the responses to multilevel standard injections are slightly better explained by a power law fit, due to the response becoming nonlinear near the detection limit (Kreisberg et al. Citation2009), we report linear fits here, since it holds true above the detection limit. Intercept is the only parameter that is largely skewed due to this simplification. This explains why most compounds have a negative intercept (see Table S1 in the online supplementary information [SI]). Also reported here is an ammonium nitrate calibration curve for the AMS inlet of the TAG-AMS system ().
FIG. 4 TAG-AMS calibration curves for select chemical classes. TAG figures (a–e) display an integrated ion signal (counts) for each species (y-axis) per ng of species injected (x-axis): (a) alkanes, (b) PAHs, (c) carboxylic acids, (d) various acids, (e) various ketones, and AMS calibration using (f) ammonium nitrate (NH4NO3). Compounds were separated through TAG chromatography except for ammonium nitrate that was sampled through the AMS inlet.
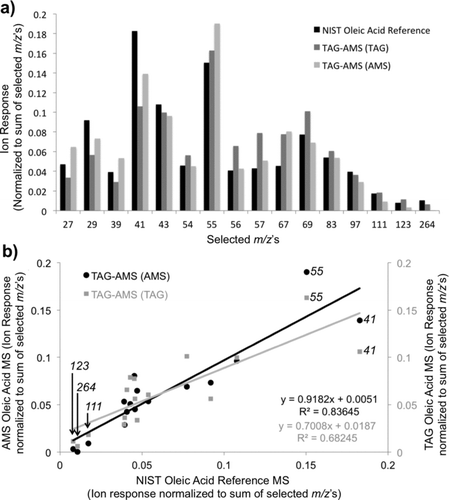
3.3. Mass Spectra
Mass spectral signatures of an example compound (oleic acid) are plotted in , comparing mass spectra from the NIST standard reference (NIST Chemistry Webbook) to the TAG inlet on the TAG-AMS and the AMS inlet on the TAG-AMS. Oleic acid was aerosolized and analyzed using both TAG and AMS modes on the TAG-AMS system. shows select m/z ratios (large response ions and distinct heavy m/z's) versus normalized ion signal (normalized to largest response ion for each method). The overall mass spectral trend is very similar for each method, and is easily identifiable as oleic acid. Some minor differences are observed. Here, it is observed that while the NIST reference displays the most abundant fragment at m/z 41 and m/z 55 as the second most abundant, the TAG and AMS modes both observe the largest fragment at m/z 55, with m/z 41 as the second most abundant. Comparing TAG and AMS signals, the AMS has slightly higher response than TAG for m/z ratios <41, where TAG has relatively higher response for most m/z ratios >41. This observation is highlighted further in . This slight difference likely results from temperature differences in the molecules being ionized, where AMS desorbs at 600°C prior to ionization and TAG molecules travel through a cooler 300°C transfer line, so the higher temperature of sample from the AMS leads to more fragmentation after ionization, consistent with other work on temperature dependent ionization (Amirav et al. Citation2008; Isaacman et al. Citation2012).
FIG. 5 Comparisons of oleic acid mass spectra. (a) Oleic acid mass spectra as obtained by the NIST reference library (black), by the TAG inlet on the TAG-AMS system (dark grey), and by the AMS inlet on the TAG-AMS system (light grey). Only select ions (largest contributors to total signal) are included. Individual ion responses have been normalized to the sum of all selected ions. (b) AMS and TAG mass spectra (MS) of oleic acid (y-axes) verses NIST MS reference (x-axis). Compared to TAG, here it is seen that AMS has a relatively higher response for smaller m/z's (41 and 55 labeled here), and a lower response larger m/z's (111, 123, and 264 shown here).
3.4. High Mass Resolution GC/MS
With the addition of the TAG system, volatility resolution is now observable that was not previously detected in the bulk aerosol mass spectra of the AMS. Some volatility resolution can be achieved with the AMS using an external thermal denuder system, but chromatography provides molecular level separation. By exploring the high mass resolution GC/MS data obtained on the TAG-AMS system, new information can be obtained on the chemical structure of chromatograms, including the fraction of the signal that is an UCM which is most often ignored in traditional GC analysis of atmospheric samples. A large test sample was collected from Aerodyne Research laboratory air (collected January 15, 2009 for 13-h at 9 LPM, analyzed with HR-ToF-MS in W-mode) was analyzed using modified AMS software programs as described above. This sample was dominated by laboratory pump oil as evidenced by the large hydrocarbon UCM signal eluting in the second half of the chromatogram (), with smaller contributions likely from various sources. A variety of individual compounds are identified in this sample, many of which are listed in the SI (Table S2). Observed retention times are shown to agree well with retention times from a previous TAG study where the same GC column and temperature ramp rate was applied (Figure S1). For samples containing compounds that do not return a match to NIST mass spectral database species, having high-resolution mass spectral data on individual fragments will assist in reconstructing potential molecular structures. This will be particularly helpful for the many biogenic compounds and oxygenated biogenic compounds that are not currently available in NIST reference libraries.
highlights two common unit mass ions present in many TAG chromatograms (m/z 43 and 57). Utilizing the high mass resolution of the TAG-AMS ToF detector, unit mass ions can be further divided into exact mass peaks (). It is noticed in that early in the chromatogram, both the oxygenated and hydrocarbon peaks are present (represented here by m/z 43 peaks C3H7 + and C2H3O+, and by m/z 57 peaks C4H9 + and C3H5O+), while shows that late in the chromatogram, only the hydrocarbon peaks are observed (C3H7 + and C4H9 +). This is partially due to the fact that the lowest volatility polar species that could be present later in the chromatogram are the same species that are difficult to elute through the GC column without prior chemical derivatization. Also, this sample has a very large hydrocarbon contribution in the second half of the chromatogram that makes contributions from oxidized species appear small. A typical outdoor sample would not have such a large hydrocarbon UCM signal, unless sampling in the near-field of an emission source.
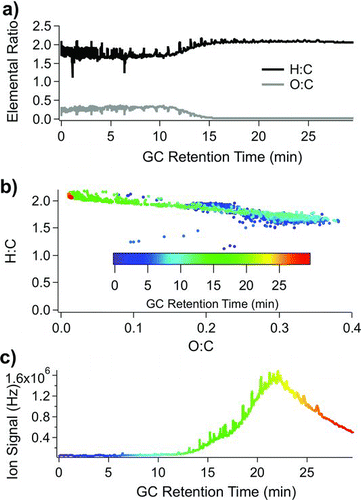
Using the AMS software package Analytic Procedure for Elemental Separation (APES) to separate high-resolution ions into their elemental components (Aiken et al. Citation2008), we can further observe this trend, where the O:C ratio decreases midway through the chromatogram, and the H:C ratio increases midway through the chromatogram (). Further, comparing H:C ratios to O:C ratios in a van Krevelen diagram (), it can be seen that O:C ratios are higher for early retention times (as high as O:C = 0.4 for material eluting near 8 min) and dramatically decreases toward the end of the chromatogram. Retention times are color coded according to retention times displayed in . O:C ratios approach zero for compounds eluting after 16 min. It can be seen that there is little change in the H:C and O:C ratio for this large quantity of material eluting after 16 min, indicating that there is similar chemical composition for much of the late UCM mass. It is observed that many of the individual compounds eluting prior to 16 min have oxygen content, while only two compounds (phthalates) eluting after 16 min contain oxygen (Table S2). Using high-resolution MS analysis on this chromatogram shows that the eluting material can be roughly separated into oxygenated UCM + oxygenated resolved species in the early section of the chromatogram and hydrocarbon UCM + hydrocarbon resolved species in the later section of the chromatogram.
FIG. 6 TAG chromatogram from Aerodyne Research Inc. laboratory air (collected overnight on 15 January 2009). (a) Single ion chromatograms for unit mass m/z 43 and m/z 57, (b) single high-resolution ion chromatograms for C2H3O, C3H7, C3H5O, and C4H9 shown to separate the hydrocarbon peaks from oxygenated peaks, (c) example high-resolution mass spectra focused around the m/z 43 and m/z 57 range for an early retention period in the chromatogram, showing both oxidized and hydrocarbon signals, and (d) example high-resolution mass spectra focused around the m/z 43 and m/z 57 range for a late retention period in the chromatogram, showing the dominance of the hydrocarbon signal. (e) A zoomed image of the early retention time section of the high-resolution ion chromatograms shown in (b). Here, it can be seen that both oxygenated ions and hydrocarbon ions contribute to species observed in this section of the sample.
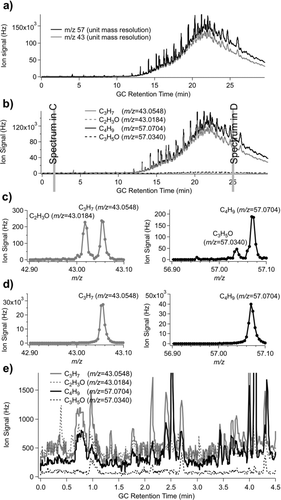
FIG. 7 TAG chromatogram from Aerodyne Research Inc. laboratory air (collected overnight on 15 January 2009). (a) Elemental analysis across chromatogram to show O:C ratios and H:C ratios as a function of chromatography volatility. Low-volatility oxygenated OA does not transfer efficiently through a 30 m GC column, partially explaining the decreased O:C ratio at higher column temperatures. (b) van Krevelen diagram outlining the H:C ratio vs. O:C ratio color coded by GC retention time. Maximum O:C ratios are reached around retention times = 8 min, and minimum O:C ratios are reached for retention times >16 min. (c) TAG total ion signal displayed with the same retention time color code as (b).
A limited comparison was made using single-species aerosol (oleic acid at a relatively high concentration and repeated for tetracosane at a relatively lower concentration). High-resolution analysis of TAG data yielded an O:C (H:C) for oleic acid of 0.20 (1.63), and an O:C (H:C) for tetracosane of 0.09 (2.18). Based on molecular structures, O:C (H:C) ratios for oleic acid should be 0.11 (1.89) and for tetracosane should be 0.00 (2.08). While the trend between species is correct, the absolute elemental ratios are offset from the expected values. Since the Aiken et al. high-resolution elemental parameterization was established for AMS measurements obtained at 600°C, there may be a temperature dependence that would change the parameterization for TAG measurements. It will be useful to derive new coefficients at different temperatures in future work.
It is also observed that uncalibrated raw high-resolution peaks (V-mode) are slightly broader for TAG samples compared to AMS samples, with a slight m/z calibration offset showing TAG peaks slightly shifted to a lighter mass (average on the order of 0.015 amu earlier than AMS peaks) (Figure S2). The cause of these observed differences is not currently understood, but physical differences include the previously mentioned difference in ionization temperature as well as ion concentration differences. While the TAG sample represents a smaller fraction of the total aerosol mass compared to the AMS, the TAG sample is concentrated during collection and compounds are focused through the GC column, yielding much larger ion signal throughout the course of sample detection. Further analysis needs to be performed over a range of particle types, concentrations, and temperatures to determine if the observations here represent a systematic difference between the two techniques, insight that will lead to a TAG-specific elemental analysis parameterization. Currently, TAG and AMS high-resolution mass spectral data are calibrated and analyzed independently.
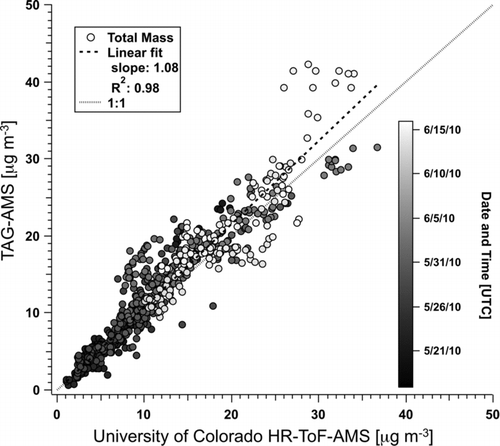
3.5. Intercomparison with Conventional HR-ToF-AMS Measurements
shows a scatter plot of ambient nonrefractory PM1.0 (NR-PM1) concentrations obtained by co-located TAG-AMS and HR-ToF-AMS measurements during the 2010 CalNex campaign in Pasadena, CA, USA (Hayes et al. Citation2013). Here, NR-PM1 represents the sum of organic, sulfate, nitrate, ammonium, and chloride species. The TAG-AMS and HR-ToF-AMS data shown represent 10 min averages of 1 min measurements. shows that measurements from the two instruments on an average agree to within ∼10% (slope = 1.08), and are well correlated (r 2 = 0.98). This agreement indicates there is no significant difference in collection efficiency (CE) of the AMS due to the added helium in the TAG-AMS system. Details of this study will be reported in a future manuscript. These intercomparison results suggest that the TAG-AMS is appropriate as a standalone technique for both molecular level speciation of organic compounds (TAG) and bulk speciation of organic and inorganic species (AMS).
4. CONCLUSIONS AND DISCUSSION
The combined TAG-AMS system takes advantage of the strengths of the individual systems: separation and identification of specific compounds by the TAG, and mass spectral analysis of total organic loading in the AMS, while time-sharing the most expensive component (the HR-ToFMS detector). Speciated organics from the TAG will assist in identifying primary PMF factors from AMS analysis, and will also help to define the transformation processes leading to SOA production and oxidative evolution (Williams et al. Citation2010a). New value-added capability of the coupled system will become apparent in analysis of the UCM mass spectra in the TAG. Detection with the HR-ToFMS will enable more complete analysis of UCM in TAG chromatograms, as is routine in the AMS mass spectral analysis (DeCarlo et al. Citation2006). This will enable a more direct analysis of the chemical mass balance for TAG PMF components, as was previously estimated through comparison to AMS OA mass (Williams et al. Citation2010a). This TAG mass balance will be particularly informative for the highly oxygenated fraction of SOA, which is not efficiently elutable via GC analysis. For nonpolar and lightly oxidized species that do elute from the GC, the incorporation of resolved marker compounds and volatility resolved UCM from the TAG will further resolve the complex mass spectral components identified with the AMS and aid in the interpretation of AMS component composition, source attributions, or formation mechanisms. Elemental analysis (e.g., through van Krevelen diagrams) of TAG chromatograms is a promising path to gain understanding of the chemical nature of OA sources and oxidative formation and transformation pathways in the atmosphere.
Continued improvements to the TAG-AMS system are currently under development to make the instrument more compact and provide simplified operation and data analysis (e.g., miniaturization of the GC system, alternative particle collection techniques, alternative chromatography techniques, simplified controls, automated calibration, analysis software development). TAG-specific elemental analysis parameterization will be derived through analysis of high-resolution mass spectral TAG data over a wide range of known chemical species and at ionization temperatures specific to TAG operation. Further application will test the TAG-AMS system's ability to provide a continuous chemical mass balance of OA and its elemental composition correlated with speciated molecular components and volatility-resolved UCM critical for the evaluation of aerosol sources and transformation processes.
FUNDING
TAG-AMS was developed through a Phase I and Phase II small business innovation research (SBIR) grant from the U.S. Department of Energy (contract# DE-FG02-08ER85160). Patrick L. Hayes and Jose L. Jimenez were supported by CARB 08-319/11-305 and DOE (BER/ASR) DE-SC0006035.
SUPPLEMENTAL MATERIAL
Supplemental data for this article can be accessed on.
Williams_et_al_TAG-AMS-AS_T-2013_supplemental-final-v2.zip
Download Zip (522.3 KB)REFERENCES
- Aiken , A. C. , DeCarlo , P. F. and Jimenez , J. L. 2007 . Elemental Analysis of Organic Species with Electron Ionization High-Resolution Mass Spectrometry . Anal. Chem. , 79 : 8350 – 8358 .
- Aiken , A. C. , Decarlo , P. F. , Kroll , J. H. , Worsnop , D. R. , Huffman , J. A. Docherty , K. S. 2008 . O/C and OM/OC Ratios of Primary, Secondary, and Ambient Organic Aerosols with High-Resolution Time-of-Flight Aerosol Mass Spectrometry . Environ. Sci. Technol. , 42 : 4478 – 4485 .
- Alfarra , M. R. , Paulsen , D. , Gysel , M. , Garforth , A. A. , Dommen , J. Prévôt , A. S. H. 2006 . A Mass Spectrometric Study of Secondary Organic Aerosols Formed from the Photooxidation of Anthropogenic and Biogenic Precursors in a Reaction Chamber . Atmos. Chem. Physics , 6 : 5279 – 5293 .
- Allan , J. D. 2003 . Quantitative Sampling using an Aerodyne Aerosol Mass Spectrometer 1. Techniques of Data Interpretation and Error Analysis . J. Geophys. Res. , 108 ( D3 ) doi: 10.1029/2002JD002358
- Amirav , A. , Gordin , A. , Poliak , M. and Fialkov , A. B. 2008 . Gas Chromatography-Mass Spectrometry with Supersonic Molecular Beams . J. Mass Spectrom. , 43 : 141 – 163 .
- Anttila , P. , Rissanen , T. , Shimmo , M. , Kallio , M. , Hyotylainen , T. Kulmala , M. 2005 . Organic Compounds in Atmospheric Aerosols from a Finnish Coniferous Forest . Boreal. Environ. Res. , 10 : 371 – 384 .
- Arey , J. , Obermeyer , G. , Aschmann , S. M. , Chattopadhyay , S. , Cusick , R. D. and Atkinson , R. 2009 . Dicarbonyl Products of the OH Radical-Initiated Reaction of a Series of Aromatic Hydrocarbons . Environ. Sci. Technol. , 43 : 683 – 689 .
- Bahreini , R. , Keywood , M. D. , Ng , N. L. , Varutbangkul , V. , Gao , S. Flagan , R. C. 2005 . Measurements of Secondary Organic Aerosol from Oxidation of Cycloalkenes, Terpenes, Andm-Xylene Using an Aerodyne Aerosol Mass Spectrometer . Environ. Sci. Technol. , 39 : 5674 – 5688 .
- Chhabra , P. S. , Ng , N. L. , Canagaratna , M. R. , Corrigan , A. L. , Russell , L. M. Worsnop , D. R. 2011 . Elemental Composition and Oxidation of Chamber Organic Aerosol . Atmos. Chem. Phys. , 11 : 8827 – 8845 .
- DeCarlo , P. F. , Kimmel , J. R. , Trimborn , A. , Northway , M. J. , Jayne , J. T. Aiken , A. C. 2006 . Field-Deployable, High-Resolution, Time-of-Flight Aerosol Mass Spectrometer . Anal. Chem. , 78 : 8281 – 8289 .
- Docherty , K. S. , Aiken , A. C. , Huffman , J. A. , Ulbrich , I. M. , DeCarlo , P. F. Sueper , D. 2011 . The 2005 Study of Organic Aerosols at Riverside (SOAR-1): Overview, Instrumental Intercomparisons, and Fine Particle Composition . Atmos. Chem. Phys. , 11 : 12387 – 12420 .
- Drewnick , F. , Hings , S. S. , Alfarra , M. R. , Prevot , A. S. H. and Borrmann , S. 2009 . Aerosol Quantification with the Aerodyne Aerosol Mass Spectrometer: Detection Limits and Ionizer Background Effects . Atmos. Meas. Tech. , 2 : 33 – 46 .
- Fraser , M. P. , Kleeman , M. J. , Schauer , J. J. and Cass , G. R. 2000 . Modeling the Atmospheric Concentrations of Individual Gas-Phase and Particle-Phase Organic Compounds . Environ. Sci. Technol. , 34 : 1302 – 1312 .
- George , I. J. , Vlasenko , A. , Slowik , J. G. , Broekhuizen , K. and Abbatt , J. P. D. 2007 . Heterogeneous Oxidation of Saturated Organic Aerosols by Hydroxyl Radicals: Uptake Kinetics, Condensed-Phase Products, and Particle Size Change . Atmos. Chem. Phys. , 7 : 4187 – 4201 .
- Goldstein , A. H. and Galbally , I. E. 2007 . Known and Unexplored Organic Constituents in the Earth's Atmosphere . Environ. Sci. Technol. , 41 : 1514 – 1521 .
- Goldstein , A. H. , Worton , D. R. , Williams , B. J. , Hering , S. V. , Kreisberg , N. M. Panic , O. 2008 . Thermal Desorption Comprehensive Two-Dimensional Gas Chromatography for in-situ Measurements of Organic Aerosols . J. Chromatogr. A. , 1186 : 340 – 347 .
- Grieshop , A. P. , Donahue , N. M. and Robinson , A. L. 2007 . Is the Gas-Particle Partitioning in Alpha-Pinene Secondary Organic Aerosol Reversible? . Geophys. Res. Lett., , : 34
- Hallquist , M. , Wenger , J. C. , Baltensperger , U. , Rudich , Y. , Simpson , D. Claeys , M. 2009 . The Formation, Properties and Impact of Secondary Organic Aerosol: Current and Emerging Issues . Atmos. Chem. Phys. , 9 : 5155 – 5236 .
- Hamilton , J. F. , Webb , P. J. , Lewis , A. C. , Hopkins , J. R. , Smith , S. and Davy , P. 2004 . Partially Oxidised Organic Components in Urban Aerosol using GCXGC-TOF/MS . Atmos. Chem. Phys. , 4 : 1279 – 1290 .
- Hayes , P. L. , Ortega , A. M. , Cubison , M. J. , Froyd , K. D. , Zhao , Y. Cliff , S. S. 2013 . Organic Aerosol Composition and Sources in Pasadena, California, During the 2010 CalNex Campaign . J. Geophys. Res.,: Atmos. , 118 ( 16 ) : 9233 – 9257 .
- Ho , S. S. H. , Yu , J. Z. , Chow , J. C. , Zielinska , B. , Watson , J. G. Sit , E. H. L. 2008 . Evaluation of an in-Injection Port Thermal Desorption-Gas Chromatography/Mass Spectrometry Method for Analysis of Non-Polar Organic Compounds in Ambient Aerosol Samples . J. Chromatogr. A. , 1200 : 217 – 227 .
- Huffman , J. A. , Ziemann , P. J. , Jayne , J. T. , Worsnop , D. R. and Jimenez , J. L. 2008 . Development and Characterization of a Fast-Stepping/Scanning Thermodenuder for Chemically-Resolved Aerosol Volatility Measurements . Aerosol Sc. Technol. , 42 : 395 – 407 .
- Isaacman , G. , Kreisberg , N. M. , Worton , D. R. , Hering , S. V. and Goldstein , A. H. 2011b . A Versatile and Reproducible Automatic Injection System for Liquid Standard Introduction: Application to in-situ Calibration . Atmos. Meas. Tech. , 4 : 1937 – 1942 .
- Isaacman , G. , Wilson , K. R. , Chan , A. W. H. , Worton , D. R. , Kimmel , J. R. Nah , T. 2012 . Improved Resolution of Hydrocarbon Structures and Constitutional Isomers in Complex Mixtures Using Gas Chromatography-Vacuum Ultraviolet-Mass Spectrometry . Anal. Chem. , 84 : 2335 – 2342 .
- Isaacman , G. , Worton , D. R. , Kreisberg , N. M. , Hennigan , C. J. , Teng , A. P. Hering , S. V. 2011a . Understanding Evolution of Product Composition and Volatility Distribution Through in-situ GC × GC Analysis: A Case Study of Longifolene Ozonolysis . Atmos. Chem. Phys. , 11 : 5335 – 5346 .
- Jaeckels , J. M. , Bae , M. S. and Schauer , J. J. 2007 . Positive Matrix Factorization (PMF) Analysis of Molecular Marker Measurements to Quantify the Sources of Organic Aerosols . Environ. Sci. Technol. , 41 : 5763 – 5769 .
- Jang , M. S. , Carroll , B. , Chandramouli , B. and Kamens , R. M. 2003 . Particle Growth by Acid-Catalyzed Heterogeneous Reactions of Organic Carbonyls on Preexisting Aerosols . Environ. Sci. Technol. , 37 : 3828 – 3837 .
- Jang , M. , Ghio , A. J. and Cao , G . 2006 . Exposure of BEAS-2B Cells to Secondary Organic Aerosol Coated on Magnetic Nanoparticles . Chem. Res. Toxicol. , 19 : 1044 – 1050 .
- Jayne , J. T. , Leard , D. C. , Zhang , X. F. , Davidovits , P. , Smith , K. A. Kolb , C. E. 2000 . Development of an Aerosol Mass Spectrometer for Size and Composition Analysis of Submicron Particles . Aerosol Sci. Technol. , 33 : 49 – 70 .
- Jimenez , J. L. 2003 . Ambient Aerosol Sampling using the Aerodyne Aerosol Mass Spectrometer . J. Geophys. Res., , : 108
- Jimenez , J. L. , Canagaratna , M. R. , Donahue , N. M. , Prevot , A. S. H. , Zhang , Q. Kroll , J. H. 2009 . Evolution of Organic Aerosols in the Atmosphere . Science , 326 : 1525 – 1529 .
- Kanakidou , M. , Seinfeld , J. H. , Pandis , S. N. , Barnes , I. , Dentener , F. J. Facchini , M. C. 2005 . Organic Aerosol and Global Climate Modelling: A Review . Atmos. Chem. Phys. , 5 : 1053 – 1123 .
- Kang , E. , Toohey , D. W. and Brune , W. H. 2011 . Dependence of SOA Oxidation on Organic Aerosol Mass Concentration and OH Exposure: Experimental PAM Chamber Studies . Atmos. Chem. Phys. , 11 : 1837 – 1852 .
- Kimmel , J. R. , Farmer , D. K. , Cubison , M. J. , Sueper , D. , Tanner , C. Nemitz , E. 2011 . Real-Time Aerosol Mass Spectrometry with Millisecond Resolution . Int. J. Mass Spectrom. , 303 : 15 – 26 .
- Kreisberg , N. , Hering , S. , Williams , B. , Worton , D. and Goldstein , A. 2009 . Quantification of Hourly Speciated Organic Compounds in Atmospheric Aerosols, Measured by an In-Situ Thermal Desorption Aerosol Gas Chromatograph (TAG) . Aerosol Sci. Technol. , 43 : 38 – 52 .
- Lambe , A. , Chacon-Madrid , H. , Nguyen , N. , Weitkamp , E. , Kreisberg , N. Hering , S. 2010 . Organic Aerosol Speciation: Intercomparison of Thermal Desorption Aerosol GC/MS (TAG) and Filter-Based Techniques . Aerosol Sci. Technol. , 44 : 141 – 151 .
- Lambe , A. T. , Ahern , A. T. , Williams , L. R. , Slowik , J. G. , Wong , J. P. S. Abbatt , J. P. D. 2011a . Characterization of Aerosol Photooxidation Flow Reactors: Heterogeneous Oxidation, Secondary Organic Aerosol Formation and Cloud Condensation Nuclei Activity Measurements . Atmos. Meas. Tech. , 4 : 445 – 461 .
- Lambe , A. T. , Logue , J. M. , Kreisberg , N. M. , Hering , S. V. , Worton , D. R. Goldstein , A. H. 2009 . Apportioning Black Carbon to Sources Using Highly Time-Resolved Ambient Measurements of Organic Molecular Markers in Pittsburgh . Atmos. Environ. , 43 : 3941 – 3950 .
- Lambe , A. T. , Onasch , T. B. , Massoli , P. , Croasdale , D. R. , Wright , J. P. Ahern , A. T. 2011b . Laboratory Studies of the Chemical Composition and Cloud Condensation Nuclei (CCN) Activity of Secondary Organic Aerosol (SOA) and Oxidized Primary Organic Aerosol (OPOA) . Atmos. Chem. Phys. , 11 : 8913 – 8928 .
- Liu , P. , Ziemann , P. J. , Kittelson , D. B. and McMurry , P. H. 1995 . Generating Particle Beams of Controlled Dimensions and Divergence: I. Theory of Particle Motion in Aerodynamic Lenses and Nozzle Expansions . Aerosol Sci. Technol. , 22 : 293 – 313 .
- Liu , X. and Wang , J. 2010 . How Important is Organic Aerosol Hygroscopicity to Aerosol Indirect Forcing . Environ. Res. Lett. , 5 : 044010 doi: 10.1088/1748-9326/5/4/044010
- Mauderly , J. L. and Chow , J. C. 2008 . Health Effects of Organic Aerosols . Inhal. Toxicol. , 20 : 257 – 288 .
- Ng , N. L. , Canagaratna , M. R. , Jimenez , J. L. , Zhang , Q. , Ulbrich , I. M. and Worsnop , D. R. 2011 . Real-Time Methods for Estimating Organic Component Mass Concentrations from Aerosol Mass Spectrometer Data . Environ. Sci. Technol. , 45 : 910 – 916 .
- Ng , N. L. , Canagaratna , M. R. , Zhang , Q. , Jimenez , J. L. , Tian , J. Ulbrich , I. M. 2010 . Organic Aerosol Components Observed in Northern Hemispheric Datasets from Aerosol Mass Spectrometry . Atmos. Chem. Phys. , 10 : 4625 – 4641 .
- Paulot , F. , Crounse , J. D. , Kjaergaard , H. G. , Kurten , A. , St. Clair , J. M. Seinfeld , J. H. 2009 . Unexpected Epoxide Formation in the Gas-Phase Photooxidation of Isoprene . Science , 325 : 730 – 733 .
- Pope , C. A. , Ezzati , M. and Dockery , D. W. 2009 . Fine-Particulate Air Pollution and Life Expectancy in the United States . New Engl. J. Med. , 360 : 376 – 386 .
- Rogge , W. F. , Hildemann , L. M. , Mazurek , M. A. and Cass , G. R. 1994 . Sources of Fine Organic Aerosol .6. Cigarette-Smoke in the Urban Atmosphere . Environ. Sci. Technol. , 28 : 1375 – 1388 .
- Rogge , W. F. , Hildemann , L. M. , Mazurek , M. A. , Cass , G. R. and Simonelt , B. R. T. 1991 . Sources of Fine Organic Aerosol .1. Charbroilers and Meat Cooking Operations . Environ. Sci. Technol. , 25 : 1112 – 1125 .
- Rogge , W. F. , Hildemann , L. M. , Mazurek , M. A. , Cass , G. R. and Simoneit , B. R. T. 1993a . Sources of Fine Organic Aerosol .4. Particulate Abrasion Products from Leaf Surfaces of Urban Plants . Environ. Sci. Technol. , 27 : 2700 – 2711 .
- Rogge , W. F. , Hildemann , L. M. , Mazurek , M. A. , Cass , G. R. and Simoneit , B. R. T. 1993b . Sources of Fine Organic Aerosol .5. Natural-Gas Home Appliances . Environ. Sci. Technol. , 27 : 2736 – 2744 .
- Rogge , W. F. , Hildemann , L. M. , Mazurek , M. A. , Cass , G. R. and Simoneit , B. R. T. 1993c . Sources of Fine Organic Aerosol .3. Road Dust, Tire Debris, and Organometallic Brake Lining Dust - Roads as Sources and Sinks . Environ. Sci. Technol. , 27 : 1892 – 1904 .
- Rogge , W. F. , Hildemann , L. M. , Mazurek , M. A. , Cass , G. R. and Simoneit , B. R. T. 1993d . Sources of Fine Organic Aerosol .2. Noncatalyst and Catalyst-Equipped Automobiles and Heavy-Duty Diesel Trucks . Environ. Sci. Technol. , 27 : 636 – 651 .
- Rogge , W. F. , Hildemann , L. M. , Mazurek , M. A. , Cass , G. R. and Simoneit , B. R. T. 1997a . Sources of Fine Organic Aerosol .8. Boilers Burning No. 2 Distillate Fuel Oil . Environ. Sci. Technol. , 31 : 2731 – 2737 .
- Rogge , W. F. , Hildemann , L. M. , Mazurek , M. A. , Cass , G. R. and Simoneit , B. R. T. 1997b . Sources of Fine Organic Aerosol .7. Hot Asphalt Roofing Tar Pot Fumes . Environ. Sci. Technol. , 31 : 2726 – 2730 .
- Rogge , W. F. , Hildemann , L. M. , Mazurek , M. A. , Cass , G. R. and Simoneit , B. R. T. 1998 . Sources of Fine Organic Aerosol. 9. Pine, Oak and Synthetic Log Combustion in Residential Fireplaces . Environ. Sci. Technol. , 32 : 13 – 22 .
- Schauer , J. J. and Cass , G. R. 2000 . Source Apportionment of Wintertime Gas-Phase and Particle Phase Air Pollutants Using Organic Compounds as Tracers . Environ. Sci. Technol. , 34 : 1821 – 1832 .
- Solomon , S. , Qin , D. , Manning , M. , Alley , R. B. , Berntsen , T. , Bindoff , N. L. , Chen , Z. , Chidthaisong , A. , Gregory , J. M. , Hegerl , G. C. , Heimann , M. , Hewitson , B. , Hoskins , B. J. , Joos , F. , Jouzel , J. , Kattsov , V. , Lohmann , U. , Matsuno , T. , Molina , M. , Nicholls , N. , Overpeck , J. , Raga , G. , Ramaswamy , V. , Ren , J. , Rusticucci , M. , Somverville , F. , Stocker , T. F. , Whetton , P. , Wood , R. A. and Wratt , D. 2007 . “ Technical Summary ” . In Climate Change 2007: The Physical Science Basis , Edited by: Solomon , S. , Qin , D. , Manning , M. , Chen , Z. , Marquis , M. , Averyt , K. B. , Tignor , M. and Miller , H. L. Cambridge, UK, and New York , , USA : Cambridge University Press . Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change
- Spracklen , D. V. , Jimenez , J. L. , Carslaw , K. S. , Worsnop , D. R. , Evans , M. J. Mann , G. W. 2011 . Aerosol Mass Spectrometer Constraint on the Global Secondary Organic Aerosol Budget . Atmos. Chem. Phys. , 11 : 12109 – 12136 .
- The_HDF_Group . 2013 . Hierarchical data format version 5, 2000–2010 . Available online at: http://www.hdfgroup.org/HDF5
- Trainer , M. G. , Pavlov , A. A. , DeWitt , H. L. , Jimenez , J. L. , McKay , C. P. Toon , O. B. 2006 . Inaugural Article: Organic Haze on Titan and the Early Earth . Proc. Natl. Acad. Sci. , 103 : 18035 – 18042 .
- Ulbrich , I. , Canagaratna , M. R. , Ng , N. L. , Zhang , Q. and Jimenez , J. L. 2012 . Three-Dimensional Factorization of Size-Resolved Organic Aerosol Mass Spectra from Mexico City . Atmos. Meas. Tech. , 5 : 195 – 224 .
- Ulbrich , I. M. , Canagaratna , M. R. , Zhang , Q. , Worsnop , D. R. and Jimenez , J. L. 2009 . Interpretation of Organic Components from Positive Matrix Factorization of Aerosol Mass Spectrometric Data . Atmos. Chem. Phys. , 9 : 2891 – 2918 .
- Williams , B. , Goldstein , A. , Kreisberg , N. and Hering , S. 2006 . An In-Situ Instrument for Speciated Organic Composition of Atmospheric Aerosols: Thermal Desorption Aerosol GC/MS-FID (TAG) . Aerosol Sc. Technol. , 40 : 627 – 638 .
- Williams , B. J. , Goldstein , A. H. , Kreisberg , N. M. and Hering , S. V. 2010b . Atmospheric Chemistry Special Feature: In situ Measurements of Gas/Particle-Phase Transitions for Atmospheric Semivolatile Organic Compounds . Proc. Natl. Acad. Sci. , 107 : 6676 – 6681 .
- Williams , B. J. , Goldstein , A. H. , Kreisberg , N. M. , Hering , S. V. , Worsnop , D. R. Ulbrich , I. M. 2010a . Major Components of Atmospheric Organic Aerosol in Southern California as Determined by Hourly Measurements of Source Marker Compounds . Atmos. Chem. Phys. , 10 : 11577 – 11603 .
- Williams , B. J. , Goldstein , A. H. , Millet , D. B. , Holzinger , R. , Kreisberg , N. M. Hering , S. V. 2007 . Chemical Speciation of Organic Aerosol During the International Consortium for Atmospheric Research on Transport and Transformation 2004: Results from in situ Measurements . J. Geophys. Res. Atmos. , 112 ( D10 ) doi: 10.1029/2006JD007601
- Worton , D. R. , Goldstein , A. H. , Farmer , D. K. , Docherty , K. S. , Jimenez , J. L. Gilman , J. B. 2011 . Origins and Composition of Fine Atmospheric Carbonaceous Aerosol in the Sierra Nevada Mountains, California . Atmos. Chem. Phys. , 11 : 10219 – 10241 .
- Worton , D. R. , Kreisberg , N. M. , Isaacman , G. , Teng , A. P. , McNeish , C. Górecki , T. 2012 . Thermal Desorption Comprehensive Two-Dimensional Gas Chromatography: An Improved Instrument for In-Situ Speciated Measurements of Organic Aerosols . Aerosol Sci. Technol. , 46 : 380 – 393 .
- Yue , Z. and Fraser , M. P. 2004a . Polar Organic Compounds Measured in Fine Particulate Matter During TexAQS 2000 . Atmos. Environ. , 38 : 3253 – 3261 .
- Yue , Z. W. and Fraser , M. P. 2004b . Characterization of Nonpolar Organic Fine Particulate Matter in Houston, Texas Special Issue of Aerosol Science and Technology on Findings from the Fine Particulate Matter Supersites Program . Aerosol Sci. Technol. , 38 : 60 – 67 .
- Zhang , Q. , Jimenez , J. L. , Canagaratna , M. R. , Allan , J. D. , Coe , H. Ulbrich , I. 2007 . Ubiquity and Dominance of Oxygenated Species in Organic Aerosols in Anthropogenically-Influenced Northern Hemisphere Midlatitudes . Geophys. Res. Lett., , : 34
- Zhang , Q. , Worsnop , D. R. , Canagaratna , M. R. and Jimenez , J. L. 2005 . Hydrocarbon-Like and Oxygenated Organic Aerosols in Pittsburgh: Insights into Sources and Processes of Organic Aerosols . Atmos. Chem. Phys. , 5 : 3289 – 3311 .
- Zhang , X. , Smith , K. A. , Worsnop , D. R. , Jimenez , J. L. , Jayne , J. T. and Kolb , C. E. 2002 . A Numerical Characterization of Particle Beam Collimation by an Aerodynamic Lens-Nozzle System. Part 1, An Individual Lens or Nozzle . Aerosol Sc. Technol. , 36 : 617 – 631 .
- Zhao , Y. , Kreisberg , N. M. , Worton , D. R. , Teng , A. P. , Hering , S. V. and Goldstein , A. H. 2013 . Development of an In Situ Thermal Desorption Gas Chromatography Instrument for Quantifying Atmospheric Semi-Volatile Organic Compounds . Aerosol Sci. Technol. , 47 : 258 – 266 .