Abstract
Exposure to atmospheric particulate matter (PM) is a leading global health risk. Despite extensive studies, it remains unclear as to what components or characteristics of PM best account for its toxicity. In vitro assays, including acellular and cellular assays, are widely used for PM toxicity evaluation. Acellular assays typically aim at assessing the oxidative potential (OP) of PM and linking OP to health endpoints. Cellular assays allow for elucidating the mechanisms of cellular signaling, response, and damage upon exposure to PM and linking cellular readouts to PM properties. Given the extraordinary chemical complexity and diversity of PM, there is a pressing need to efficiently evaluate OP and cellular response for PM emitted from different sources and formed under a variety of environmental conditions. Yet, current technologies are still not capable of high‐throughput, high‐content, and high time-resolution analysis, as well as mimicking physiologically relevant conditions. Microfluidic techniques are a valuable alternative technology to address some of the current challenges. In this article, we review the recent advances in applying microfluidic techniques for both cellular and acellular assays and discuss their advantages compared to conventional formats. Finally, we provide a prospective outlook on the future directions and challenges of using microfluidic techniques for in vitro toxicity studies of atmospheric PM.
Copyright © 2021 American Association for Aerosol Research
EDITOR:
1. Introduction
Airborne particulate matter (PM) has been recognized as a large health risk for humans for decades (Dedoussi et al. Citation2020; Donaldson et al. Citation2001; Lelieveld et al. Citation2019; Pope, Ezzati, and Dockery Citation2009). A recent study by Burnett et al. (Citation2018) even estimated that globally 8.9 million premature deaths per year are associated with exposure to outdoor PM, which is several folds larger than previous calculations (Forouzanfar et al. Citation2016; Lelieveld et al. Citation2015). It suggests that outdoor PM is an even more important health risk factor than previously thought. Although connections between PM pollution and disease and mortality have been uncovered, it is still unclear as to what components or characteristics of PM best account for its overall toxicity due to the complexity of ambient PM mixture.
In vitro assays including acellular and cellular assays, are the most common approaches aiming at linking PM exposure with various health endpoints and elucidating cellular response networks and toxicity pathways, as they are cost-effective compared to, for example, mouse models. A number of acellular assays (see a summary of acellular assays in recent review articles by Bates et al. [Citation2019] and Gao et al. [Citation2020]) have been developed to assess the oxidative potential (OP) of PM (Abrams et al. Citation2017; Bates et al. Citation2015; Weichenthal et al. Citation2016). These assays are currently faster and less resource‐intensive than cellular assays, but they do not offer detailed insights into biological mechanisms upon PM exposure. Acellular assays allow for efficient development of a large OP data set for use in source apportionment and health analyses of PM at different locations or formed under a variety of environmental conditions (Bates et al. Citation2019). Studies using acellular assays have found that most OP assays respond to copper and some OP assays respond to organics especially organics from biomass burning (Bates et al. Citation2019; Tuet et al. Citation2019; Verma et al. Citation2015). A few studies have shown that the association of certain cardiorespiratory endpoints such as asthma and congestive heart disease with OP measurements is stronger than with PM mass concentration (Abrams et al. Citation2017; Bates et al. Citation2019; Fang et al. Citation2016). Conventional acellular assays usually involve filter‐based PM collection at fixed locations, PM extraction, and the incubation of PM extract with chemical probes followed by response recording over specific time points (Gao et al. Citation2020). These assays may be insufficient to 1) provide high temporal resolution online monitoring of OP to fully capture the influence by atmospheric processing (e.g., aging) or episodes (e.g., rush hours); 2) evaluate OP exposure at a personal level. Further development of technologies is needed to overcome the abovementioned limitations.
In contrast, cellular assays allow for elucidating the mechanisms of cellular signaling, response, and damage and linking cellular readouts to PM properties. For example, a few previous studies have suggested that mitochondria are the primary organelles affected by changes in redox status from PM exposure. A change in redox homeostasis alters the activation of redox‐sensitive signaling pathways such as nuclear factor erythroid 2-related factor 2 (Nrf2). Nrf2 signaling is thereby suggested to coordinate the response linking mitochondrial signaling and cell fate (i.e., survival, damage, or death) following PM exposure (Pardo et al. Citation2020; Pardo et al. Citation2019). Current results from cellular assays have also suggested that metals and organic fractions play important roles in PM‐induced cellular responses, and atmospheric aging process may enhance the toxicity of PM (Chowdhury et al. Citation2019; Chowdhury et al. Citation2018; Li, Li et al. Citation2020; Pardo et al. Citation2020; Tuet, Chen, Fok, Gao et al. Citation2017). In general, current cellular assays involve exposing PM extract to cells and measuring cellular endpoints with microplate readers at a specific time point (Landreman et al. Citation2008; Pardo et al. Citation2019; Tuet et al. Citation2016). There are some limitations in cellular assays: 1) cell culture is labor‐intensive and therefore cellular assays are more time‐ and labor‐intensive compared to acellular assays; 2) most current assays are low resolution and low throughput, i.e., only report population averages and are endpoint measurement thus provide no dynamical information; 3) in vitro cellular investigations are usually performed using a monolayer of one cell type excluding interactions with neighboring cells (Wang et al. Citation2019). These limitations hinder our understanding of cellular response upon PM exposure and mechanisms of toxicity and damage. New technologies are therefore needed to enable high-resolution and high-throughput analysis of cellular response, as well as in building more physiologically relevant conditions for in vitro evaluation of PM toxicity.
Microfluidics is a versatile technology that has well‐recognized advantages such as flexibility of device design, low consumption of reagents, parallelization, and automation of assays (Vyawahare, Griffiths, and Merten Citation2010; Whitesides Citation2006). It has been developed for a wide range of applications, from biology and medicine to chemistry as well as atmospheric aerosol science (He, Ranganathan, and Li Citation2018; Metcalf, Narayan, and Dutcher Citation2018; Sun and Lu Citation2019; Theberge et al. Citation2010). In the past decade, microfluidic techniques have been introduced to address the abovementioned limitations in both cellular and acellular assays. There are a number of emerging studies (Liu, Whitley et al. Citation2020; Xu et al. Citation2020; Zheng et al. Citation2019; Zheng et al. Citation2017) demonstrating the great potential of microfluidic technologies to overcome the challenges associated with conventional in vitro assays for PM toxicity studies.
This article intends to introduce recent progress in the development and applications of microfluidic techniques for in vitro toxicity studies of PM exposure, with emphasis on how microfluidic platforms can be a valuable alternative compared to conventional in vitro assay formats. Finally, we also provide a prospective outlook on promising directions for future research.
2. Microfluidic techniques in acellular assays for evaluating PM oxidative potential
Oxidative potential is generally defined as the catalytic generation of reactive oxygen species (ROS) by PM components with simultaneous depletion of antioxidants. Common acellular OP assays include dithiothreitol (DTT) assay, ascorbic acid (AA) assay, glutathione (GSH) assay, and electron spin resonance (ESR) assay (an overview of these OP assays can refer to Bates et al. (Citation2019) and Gao et al. (Citation2020) and references therein). A summary of the current state of knowledge on the associations between OP and different PM chemical components, emission sources, and various health endpoints, is provided in recent review articles (Bates et al. Citation2019; Gao et al. Citation2020; Jiang, Ahmed et al. Citation2019; Pietrogrande, Russo, and Zagatti Citation2019; Shiraiwa et al. Citation2017). As OP assays are less resource‐intensive and faster to conduct than cellular assays and there is increasing evidence suggesting that OP is a plausible metric for PM toxicity, a rapid rise of OP measurements have been performed worldwide. In this section, we discuss the recent studies of using microfluidic techniques to overcome some limitations in conventional acellular OP assays.
2.1. Temporal resolution of online monitoring of OP
Although various acellular OP assays have been developed, most of the approaches require filter-based PM collection over a certain period of time (e.g., 24 hrs) and filter extraction processes prior to reacting with chemical probes (e.g., DTT) (Cho et al. Citation2005; Fang et al. Citation2015; Verma et al. Citation2009). These protocols pose limitations such as reducing the temporal resolution of the OP measurement and increasing the potential of chemical alteration of PM during sampling, storage, and extraction procedures. Several online systems, therefore, have been recently developed to overcome potential problems with filter collection and off-line analysis (Eiguren-Fernandez, Kreisberg, and Hering Citation2017; Puthussery, Zhang, and Verma Citation2018; Sameenoi et al. Citation2012). These systems usually involve directing PM into a particle-into-liquid sampler (PILS) (Weber et al. Citation2001) coupling to an automated analytical system, which can provide hourly resolution of OP measurement.
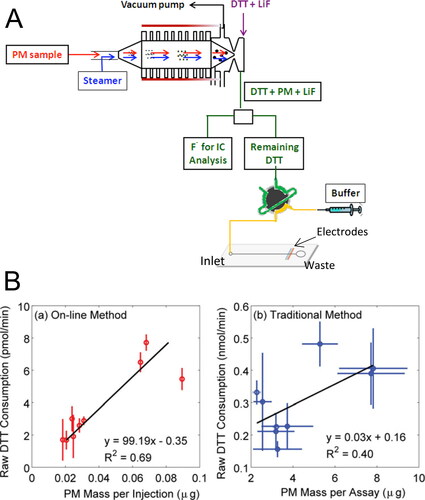
In the system developed by Sameenoi et al. (Citation2012), they used a PILS directly coupled to a microfluidic electrochemical sensor for measuring DTT activity of PM (). The electrode is built in cobalt(II) phthalocyanine (Co(II)‐PC) modified carbon paste, which presents good selectivity for the catalytic oxidation of thiol compounds such as DTT. Carbon paste electrodes have been widely used as electrochemical sensors in microfluidic devices because of their ease of fabrication and the ability to modify the electrode with a range of chemically selective dopants (Švancara et al. 2009). The advantages of using microfluidic electrochemical sensors for measuring DTT activity of PM compared to using UV-Vis detectors include the following: 1) it eliminates the need for quenching and developing reagents that are associated with UV-Vis detection and thus the noise associated, since following reacting with PM, the remaining DTT is directly analyzed by the sensor; 2) it reduces the required PM mass, which is three orders of magnitude lower than the amount needed for the traditional DTT assay (7-214 ng vs. 5-40 µg) (Cho et al. Citation2005; Kumagai et al. Citation2002; Li et al. Citation2003; Li, Wyatt, and Kamens Citation2009); 3) it provides higher temporal resolution measurement, reporting an independent measurement approximately every 3 min, while 1 hr time resolution is reported for the systems with UV-Vis detection (Eiguren-Fernandez, Kreisberg, and Hering Citation2017; Puthussery, Zhang, and Verma Citation2018). This system was first validated for its performance in OP measurement by comparing it with traditional DTT assay. This system has also been validated in the laboratory using simulated aerosol samples (Koehler et al. Citation2014). The results show that this online system provides a better correlation between DTT consumption rate and PM mass and increased precision at high temporal resolution, compared to the traditional method (). For online systems, a major advantage is the ability to run continuously in field studies with minimal maintenance. It is interesting to point out that to our knowledge, the long‐term stability (days to months) of this PILS-microfluidic sensor online system is yet to be evaluated. It is possible that unwanted air bubbles and channel clog are sometimes an issue in microfluidic device. The stability and maintenance of the microfluidic electrochemical sensor could be another issue hindering its application for long‐term measurements (Sameenoi et al. Citation2011). In addition, the fabrication of both electrode and microfluidic device needs expertise in a large set of equipment, slowing their application in aerosol OP studies (Berg et al. Citation2019).
Figure 1. Microfluidic electrochemical sensor for online monitoring of ambient OP. (A) Schematic overview of the online monitoring system of OP with a microfluidic electrochemical method. Adapted with permission from Sameenoi et al. (Citation2012). Copyright (2012) American Chemical Society. (B) Correlation between DTT consumption and PM mass. (a) Results from the online monitoring system. (b) Results from traditional DTT assay. From Koehler et al. (Citation2014), copyright ©2014 American Association for Aerosol Research, reprinted by permission of Taylor & Francis Ltd, http://www.tandfonline.com. on behalf of American Association for Aerosol Research.
2.2. Portable paper-based device for fast screening of OP
Another effort of using microfluidic techniques in OP measurement is developing microfluidic paper‐based analytical devices (µPADs) for personal exposure assessment, i.e., fast screening of OP from personal exposure to PM. The µPADs were originally developed for point‐of‐care medical diagnostics (Martinez et al. Citation2010) and now the applications of µPADs have been extended to different fields such as environmental analysis and food safety (Ozer, McMahon, and Henry Citation2020). The major advantages of µPAD over conventional methods include being portable, inexpensive, easy to perform, and providing fast analysis. Also, liquid transport in µPAD is driven via capillary force without the need for external pumps.
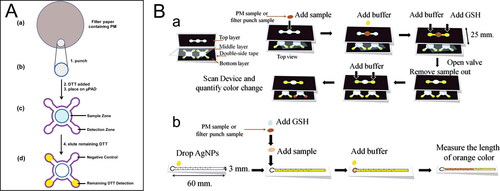
So far, µPADs have been introduced into two acellular OP assays, i.e., DTT and GSH assays (Dungchai et al. Citation2013; Sameenoi et al. Citation2013). Both µPADs methods have been validated with traditional assays, the results of which show no significant difference in OP measurement in comparison with traditional assays. In both assays, µPADs are fabricated by wax printing techniques, which is fast and inexpensive and does not require additional solvents or polymers compared to other fabrication techniques such as photolithography and inkjet printing (Cate et al. Citation2015; Yamada et al. Citation2015). There are some differences in the µPAD designs between the two assays (). In the µPAD of DTT assay, it only has one layer including a sample zone and four detection zones. The reaction between DTT and a filter sample of PM needs to be conducted off the device (in a Petri dish) followed by placing the DTT-treated filter in the sample zone () (Sameenoi et al. Citation2013). In contrast, the µPAD of GSH assay consists of three layers enabling all steps in the assay to be done on the device (a) (Dungchai et al. Citation2013). A top layer is for sample loading and reaction with GSH, a middle layer consisted of a polymer film is to provide a hydrophobic barrier that prevents sample from moving between layers until desired, and a bottom layer is a detection zone, where the remaining GSH reacts with silver nanoparticles to generate a reddish-brown color for GSH detection. Both µPADs use a scanner to capture the resulting color product and use an image processing software (e.g., ImageJ) to analyze color intensity for measuring DTT or GSH consumption. There is an alternative µPAD using distance‐based detection (b), in which the quantification can be achieved by reading the length of the color by the naked eye, eliminating the need for an external scanner. Note that colorimetric detection based on the differentiation of color intensity is semiquantitative, which can suffer from low sensitivity. Low‐cost fabrication methods for more sensitive electrochemical techniques are suggested to be a feasible alternative to colorimetric detection (Meredith et al. Citation2016).
Figure 2. Microfluidic paper-based analytical devices (µPAD) for ambient OP. (A) Steps to perform DTT assay in µPAD. Reprinted with permission from Sameenoi et al. (Citation2013). Copyright (2013) American Chemical Society. (B) Schematic diagram to perform GSH assay in µPAD. (a) A color intensity-based method. (b) A distance-based method. Reproduced from Dungchai et al. (Citation2013) with permission from The Royal Society of Chemistry.
One advantage of the µPAD systems is that they enable analyzing OP directly from a PM filter sample without substantial handling and extraction steps. Additionally, the µPAD methods require ∼10 times less PM mass than conventional methods, which can result in a much shorter PM collection time needed (Sameenoi et al. Citation2013). These improvements show the capability of µPAD systems for fast screening of OP. Combining with the use of personal PM filter samplers (Williams et al. Citation2000), the µPAD systems can be a promising tool for personal exposure studies, providing hundreds of individual OP measurements over long time periods. Another advantage is that the cost of µPAD device fabrication and analysis is extremely low, which can be orders of magnitude less than conventional methods, especially when using wax printing and colorimetric reagents (the cost is estimated to be less than $0.05 for each device) (Mentele et al. Citation2012; Sameenoi et al. Citation2013). These advantages can be especially useful for epidemiological studies evaluating the health effects of long-term PM exposure. To our knowledge, current epidemiological studies on analyzing the relationship between ambient OP and health outcomes only use OP data collected from a single location/limited locations of the study area (Abrams et al. Citation2017; Atkinson et al. Citation2016). The capability of µPAD systems in providing explicit OP measurements for individual exposure thus would improve the exposure assessment.
3. Microfluidic techniques in cellular assays for evaluating PM toxicity
In vitro cellular assays are another important format that is widely used to screen the toxic effects of PM (Cho et al. Citation2018; Pardo et al. Citation2020). Unlike acellular OP assays, which are specific to analyze the oxidative property of PM, cell-based assays not only can be used to evaluate the ability of PM to generate ROS and oxidative stress, but to elucidate the biological mechanisms or signaling pathways involved in specific cell damage or response. Such information can facilitate understanding downstream disease progression (Alfaro-Moreno et al. Citation2010). Compared to animal‐based assays, cell‐based assays are more cost-effective, experimentally easier to manipulate, and allow to analyze mechanisms of cellular response (e.g., apoptosis, inflammation) in detail (Pardo et al. Citation2020), with the caveat that they are not able to sufficiently model systemic and complex multi‐tissue effects that animal models offer.
Microfluidic systems have been utilized intensively in cellular assays (He, Ranganathan, and Li Citation2018; Mahto et al. Citation2015; Shembekar et al. Citation2016) due to their well-recognized advantages such as flexibility of device design and automation comparing to macroscopic devices (e.g., Petri dishes or well plates) and allowing for rapid and high-throughput analysis. It is also worth mentioning that the unique properties of polydimethylsiloxane (PDMS, a polymer that is widely used for the fabrication and prototyping of microfluidic devices) such as gas permeability, high flexibility, and optical transparency, open entirely new avenues for applying microfluidic systems in cellular assays. To date, a number of microfluidic-based cellular assays have been developed and demonstrated their utility in diverse research areas such as disease and biological studies (Demello Citation2006; Deng, Finck, and Fan Citation2019; Dittrich and Manz Citation2006; El-Ali, Sorger, and Jensen Citation2006; Prakadan, Shalek, and Weitz Citation2017; Vlassakis and Herr Citation2017). Many of these microfluidic-based cellular assays can be easily employed for PM toxicity studies. In this section, we focus on the developed microfluidic platforms that have been applied to study PM toxicity. We discuss how microfluidics can overcome the challenges that exist in conventional cellular assays, and/or what additional information can be obtained.
3.1. High-throughput and high-content screening of multiple endpoints
Microfluidics has prominent advantages in reduced reagent consumption and analysis time, ease of integration, and the potential for parallel processing and high-throughput analysis. These advantages make microfluidic systems an attractive solution for miniaturization and parallelization of biological assays. In contrast, conventional biological assays using macroscopic devices are usually labor-intensive (e.g., many pipetting steps into plates or wells), time‐consuming, require a larger volume of samples and reagents. A summary of the reported studies showing advantages of using microfluidic versus macroscopic systems for diverse biological assays is shown in Halldorsson et al. (Citation2015). For example, since large amounts of PM mass are needed in traditional protein analysis assays such as enzyme‐linked immunosorbent assay (ELISA, ∼0.08 mg PM per protein analysis [Zheng et al. Citation2017]), only a limited number of proteins can be analyzed. Cellular signaling pathways usually involve a number of proteins. Thus, to better unravel the cellular signaling pathways that are triggered upon PM exposure, approaches that can fulfill analyzing a larger number of proteins with minimal consumption of PM mass are needed. Microfluidic platforms have been increasingly developed to provide high-throughput and high-content analysis (Blay et al. Citation2020; Du, Fang, and den Toonder Citation2016; El-Ali, Sorger, and Jensen Citation2006; Whitesides Citation2006). In this section, we focus on discussing the ones that have been applied to study PM toxicity, while we envision that many other developed microfluidic systems are promising to expand their applications to PM toxicology.
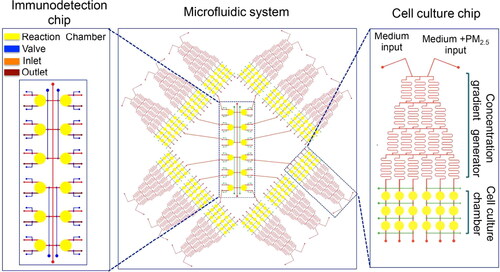
Recently, several microfluidic devices have been developed and applied for high-throughput and high-content analysis of multiple cellular endpoints upon PM exposure. Zheng et al. (Citation2017) designed and fabricated a microfluidic system consisting of concentration gradient generators, cell culture chambers, and an immune-detection chip. The microfluidic system enables the processes of PM dilution, cell culture, cell stimulation, and immunoassay to be done on-chip. shows a schematic of the microfluidic system, which is comprised of twelve uniform cell culture chips and an immunodetection chip. Each cell culture chip contains a concentration gradient generator and parallel cell culture chambers with a perfusion module. The immunodetection chip is used for protein microarray assays. A typical antibody “Sandwich” immunoassay is introduced to this system with all steps operated on the device. The protein analysis results of the on‐chip immunoassay show a good correlation with results from traditional ELISA. Importantly, the system requires a minimal amount of PM mass and could detect a large number of proteins. The authors demonstrated that the required PM mass for their system is around three orders of magnitude smaller than that for traditional ELISA (1.8 µg vs. 1.2 mg). Zhang et al. (Citation2017) fabricated a similar microfluidic system that integrates a cell culture chip and protein microarray chip to investigate the mechanisms of PM-mediated cytotoxicity in human skin cells. In the study of Cui et al. (Citation2015), they also developed a microfluidic system consisting of a concentration gradient generator and cell culture chambers, which is capable of conducting on-chip immunofluorescent analysis. Besides that, the cell culture chamber module is comprised of two layers, in which an upper layer is for introducing human alveolar macrophages and a lower layer is for introducing human bronchial cells (16HBE). This design is to enable studying the effect of PM-treated 16HBE cells on alveolar macrophage migration.
Figure 3. A representative microfluidic system for high-throughput on-chip immunoassay performance. The microfluidic system consists of twelve uniform structures and an immunodetection chip. The single structure unit consists of a concentration gradient generator and cell culture chambers. Reprinted with the permission from Zheng et al. (Citation2017). Copyright (2017) American Chemical Society.
These studies have demonstrated the capability of microfluidics for high-throughput and high-content analysis of multiple cellular endpoints induced by different mass concentrations of PM. This can improve our understanding on the dose‐dependent manner of cellular response and yield more insights into the triggered cellular signaling pathways upon PM exposure. Moreover, the high-throughput and high-content analysis provided by microfluidic systems can take a step further. For example, it can also provide subcellular information (e.g., nuclear, mitochondrial morphology) and large-scale genotypic or phenotypic screens (e.g., droplet‐based microfluidics) (Shembekar et al. Citation2016; Ye et al. Citation2007). For the concentration gradient generators, further studies may develop/apply concentration gradient generators spanning several orders of magnitude (Ahmed et al. Citation2013; Sugiura, Hattori, and Kanamori Citation2010) instead of using linear dilution series. Previous studies have found that the PM-induced dose-dependent cellular response exhibits a logarithmic manner (Chen et al. Citation2019; Kasurinen et al. Citation2018; Liu, Saavedra et al. Citation2020; Tuet, Chen, Fok, Champion et al. Citation2017; Tuet, Chen, Fok, Gao et al. Citation2017; Tuet et al. Citation2016; Tuet et al. Citation2019). A logarithmic dilution gradient generator equipped with the cell chamber chip will therefore be more practical for investigating cellular response upon PM exposure.
3.2. Microfluidic single-cell assay to study heterogeneity of cellular response
Cellular heterogeneity within isogenic cells has been well documented by numerous studies (Altschuler and Wu Citation2010; Elowitz et al. Citation2002). The heterogeneity could arise from intrinsically stochastic processes of gene expression and transcription. Studies have shown that conclusions could be biased by only using population averages, especially when the average is driven by outliers (Shalek et al. Citation2013; Toriello et al. Citation2008). This could also be true in cellular response to PM, though most current assays only report population averages. For example, Ardon-Dryer et al. (Citation2020) showed that when cells were exposed to ambient dust particles, drastically different cell death patterns were observed at the single‐cell level vs. population average level. The detection and analysis of cellular response at the single‐cell level is not only an essential way to demonstrate cell heterogeneity, but also an effective means to accurately characterize cellular response to stimuli and understand the underlying mechanisms or related signaling pathways in cells.
Flow cytometry is often the choice of technology for single‐cell analysis, as it is high‐throughput and can distinguish subpopulations of cells. However, this technology is neither capable of providing spatial information (e.g., unable to resolve subcellular components such as the mitochondria and the nuclei) nor monitoring the temporal change within the same cell. In comparison, a number of microfluidic techniques have been developed that allow for the analysis of cell heterogeneity and the tracking of single-cell temporal behavior (Chingozha et al. Citation2014; Chung et al. Citation2011; Di Carlo, Aghdam, and Lee Citation2006; Hosokawa et al. Citation2011; Kniss-James et al. Citation2017; Li, Motschman et al. Citation2020). Although the designs of these microfluidic platforms are different, they share some common purposes, i.e., efficiently capture single cells, retain them in a specific location, and control the environment surrounding them. These newly developed tools are suitable for investigating single‐cell response to PM, not only because they can provide high-resolution and high-content information, but because microfluidics is also a convenient method for controlling the exact cellular environment and experimental conditions over time.
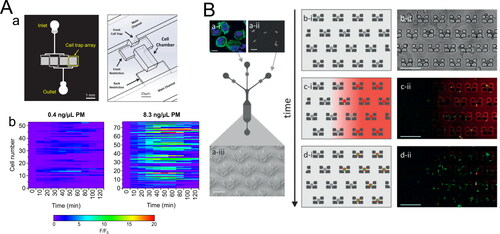
In a recently published study by our group (Liu, Whitley et al. Citation2020), we employed microfluidic single-cell trap arrays to image cellular ROS response upon PM exposure, at single-cell resolution and probe the temporal dynamics of the response on a per‐cell basis. The microfluidic cell trap array allows cells to be loaded and confined in a small chamber to maintain their identity during culture, stimulation, and imaging () (He et al. Citation2019). As a proof‐of‐principle study, cell‐to‐cell variability in macrophage ROS response to different mass concentrations of PM extract over time was evaluated. The results show that cellular ROS response is highly heterogeneous, and the dynamic behavior of single‐cell response is strongly dependent on PM mass concentrations () (Liu, Whitley et al. Citation2020). There are also some microfluidic platforms developed for evaluating engineered nanoparticles exposure (Cunha-Matos et al. Citation2016; Shah et al. Citation2016; Watson et al. Citation2014), and they undoubtedly can be introduced for PM toxicity studies. For example, Cunha-Matos et al. (Citation2016) developed a microfluidic device containing an array of microtraps (). Each trap consists of a low shear stress pocket (<0.05 dyn cm−2), avoiding damaging the cell membrane during the cell trapping. They employed the device to isolate single primary dendritic cells and monitor engineered nanoparticles uptake and intracellular processing across a range of molar concentrations over several hours on hundreds of single cells. The results reveal the heterogeneity in time and amplitude of cell response to similar stimuli (b, c, d).
Figure 4. Selected microfluidic techniques for analysis of single‐cell response (A) A microfluidic cell trap array that is applied to measure single‐cell ROS response from PM exposure. (a) Design of the microfluidic cell trap array. Adapted from He et al. (Citation2019). Copyright (2019) The American Association of Immunologist, Inc. (b) Representative heatmaps of single‐cell traces of ROS response induced by two different PM extracts. Adapted from Liu, Whitley et al. (Citation2020). Copyright (2020) American Chemical Society. (B) Schematic diagram of the microfluidic cell trap array for real‐time monitoring of nanoparticles uptake. (a) A sequential injection of cells and nanoparticles into the microfluidic cell trap array. (b, c, d) Temporal aspects of the protocol: cell trapping, nanoparticles delivery, and antigen processing. Reproduced from Cunha-Matos et al. (Citation2016) with permission from The Royal Society of Chemistry.
In addition to the abovementioned microfluidic techniques for monitoring single-cell ROS formation and nanoparticles uptake, we believe many other microfluidic-based single-cell systems also have great potential in evaluating PM toxicity at both cellular and molecular levels, although they have not been used in such applications so far. Some representative techniques are single-cell “omics” and single-cell immunoassay. Different microfluidic techniques (e.g., droplet-based RNA sequence) have been developed for single-cell “omics” studies (Macosko et al. Citation2015; Prakadan, Shalek, and Weitz Citation2017), providing the possibility for large-scale single-cell “omics” analysis or simultaneous studies of “multi-omics” including genomics, transcriptomics, and proteomics. Single-cell immunoassay can be a complementary approach of single-cell “omics”, which detects secreted proteins from single cells. Microwell-based array method is particularly appealing in single-cell immunoassay, in which single cells are isolated within microchambers containing or capped with an antibody barcode array (Lu et al. Citation2015; Xue et al. Citation2015). The secreted proteins can then be captured by antibodies and quantified using fluoroimmunoassay. These in-depth characterizations are highly informative and would greatly help us understand the cellular and molecular mechanisms of how cells respond to PM perturbation at the single-cell level.
3.3. 3D-coculture and lung-on-a-chip model to mimic physiologically relevant culture conditions
To date, most in vitro cellular assays are performed using two-dimensional (2D) monolayer cell culture method. However, 2D cell culture does not adequately reconstruct the natural three-dimensional (3D) cellular microenvironments in vivo, excluding the interactions with neighboring cells and extracellular matrix (ECM). It has been suggested in diverse research studies that 3D cell culture systems are better models than 2D cell culture systems due to improved simulation of in vivo situations such as cell morphology, cell population, cell-cell and cell-ECM interactions (Edmondson et al. Citation2014; Kasurinen et al. Citation2018; Ravi et al. Citation2015). Microfluidics has become a valuable technology to further increase the physiological relevance of 3D cell culture by enabling spatiotemporal control over fluids in micrometer-sized channels (Van Duinen et al. Citation2015). Some microfluidic 3D cell culture systems have been established for PM toxicity evaluation (Huh et al. Citation2010; Xu et al. Citation2020; Zhang et al. Citation2020; Zheng et al. Citation2019). In general, these systems consist of two tissue layers separated by a thin, porous, ECM-coated membrane, which mimics the alveolar-capillary interface. Human alveolar epithelial cells are cultured on the top side of the membrane, and human vascular endothelial cells are cultured on the opposite side of the membrane (a and b). Zhang et al. (Citation2020) developed a 3D coculture-based pulmonary alveolus microsystem and compared a variety of cellular responses between either microfluidic coculture and monoculture or on-chip and off-chip culture. Two highly toxic pollutants (i.e., nicotine and benzo[a]pyrene) that may exist in ambient PM, were exposed to cells and used for the comparative assessment. Two important conclusions emerged from the results: first, cellular responses from on-chip culture are more sensitive than off-chip culture; second, the coculture of the epithelium with the endothelium layer strengthens the chemical resistance of the pulmonary alveolus system to the exogenous pollutants (). These results point to the importance of creating complex in vitro tissue microenvironments to explore pollution-induced human pathology.
Figure 5. Representatives of microfluidic techniques for 3D cell culture and lung‐on‐a‐chip model. (A): (a) Diagram of coculture cell lines. (b) Overview of the microfluidic 3D coculture device. (c) ROS production upon exposure to benzo[a]pyrene and nicotine based on microfluidic coculture, microfluidic monoculture, and off-chip monoculture methods. Adapted with permission from Zhang et al. (Citation2020), Copyright (2020) American Chemical Society. (B) Schematic diagram of the lung‐on‐a‐chip model for PM exposure. Reprinted with permission from Xu et al. (Citation2020). Copyright (2020) American Chemical Society.
![Figure 5. Representatives of microfluidic techniques for 3D cell culture and lung‐on‐a‐chip model. (A): (a) Diagram of coculture cell lines. (b) Overview of the microfluidic 3D coculture device. (c) ROS production upon exposure to benzo[a]pyrene and nicotine based on microfluidic coculture, microfluidic monoculture, and off-chip monoculture methods. Adapted with permission from Zhang et al. (Citation2020), Copyright (2020) American Chemical Society. (B) Schematic diagram of the lung‐on‐a‐chip model for PM exposure. Reprinted with permission from Xu et al. (Citation2020). Copyright (2020) American Chemical Society.](/cms/asset/fc7c0353-0c8e-42da-8737-7313bea1165e/uast_a_1879373_f0005_c.jpg)
The developments of microengineering approaches for 3D cell culture have been further improved to reconstitute 3D organ-level structures. Various organ-on-a-chip models have been constructed on microfluidic platforms, which recapitulate biochemical and mechanical microenvironments, in addition to having a 3D matrix for cell growth (Huh, Hamilton, and Ingber Citation2011). For example, in lung-on-a-chip models, air is introduced into the epithelial channel to mimic the air-liquid interface within the lung. The respiratory tract is a major route for airborne PM to enter human body. There have been a few studies using lung-on-a-chip models to evaluate the pulmonary risk of PM exposure in an organotypic manner (Huh et al. Citation2010; Xu et al. Citation2020; Zheng et al. Citation2019). In the model developed by Huh et al. (Citation2010), in addition to 3D cell culture, two lateral microchambers were incorporated into the device to simulate dynamic mechanical distortion of the alveolar-capillary interface caused by breathing movements. This lung-on-a-chip model was used to study the toxicology of silica nanoparticles. Results indicate that cyclic mechanical strain contributes to the activation of pro-inflammatory activities of silica nanoparticles. Mechanical strain also enhances epithelial and endothelial uptake of nanoparticles and stimulates their transport into the underlying microvascular channel. Similarly, Xu et al. (Citation2020) employed a lung-on-a-chip model to probe the adverse effects of ambient PM in the respiratory system (). The model enables investigating multiple cellular responses (e.g., ROS generation, apoptosis, and inflammatory cytokines expression) and cell-cell interactions (e.g., changes of adherens junctions and permeability of the alveolar-capillary barrier) induced by PM. Further, immunocytes could be introduced to the vessel channel forming a triple coculture system to investigate the interaction of injured tissue and immunocytes. Interestingly, an increase in permeability of the alveolar‐capillary barrier was observed in both the studies of Xu et al. (Citation2020) and Huh et al. (Citation2010), although different microfluidic designs and PM samples were tested in the two studies. These complexities in the tissue model could potentially push the relevance of the in vitro system toward better mimicking the physiology in vivo.
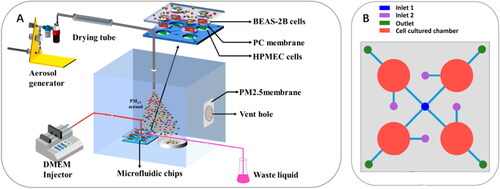
It is worth noting that the abovementioned studies use submerged methods (i.e., extracted PM in culture media) for exposing PM to cells. The submerged methods are simpler and easier to perform compared to air-liquid interface exposure systems, and therefore are widely used in assessing the chemical toxicology of PM, especially for the water-soluble fraction of PM. There are also attempts in incorporating a direct deposition of aerosolized PM to epithelial cells at an air-liquid interface (Dong et al. Citation2019; Leslie et al. Citation2011; Zheng et al. Citation2019). This exposure method preserves the physicochemical properties (e.g., chemical composition, size, and surface area, etc.) of PM, and thus may be more relevant to the real situation in the respiratory tract compared to the submerged method (Lichtveld et al. Citation2012; Ward et al. Citation2020; Zheng et al. Citation2019). Leslie et al. (Citation2011) reported a method enabling the delivery of aerosolized PM to the lung-on-a-chip model developed by Huh et al. (Citation2010), where they coupled an aerosol delivery system to the microdevice through a fused-silica capillary. Zheng et al. (Citation2019) also developed an organ-level lung microfluidic platform for air-liquid interface exposure of PM to cells (). The platform consists of four microchambers with the top layers open to the air. A vertical aerosol flow is introduced through the microchambers and hence PM can be directly delivered onto cells. This design is similar to transwell-based air-liquid interface exposure (Jeannet et al. Citation2015; Kooter et al. Citation2019) but in microscale and allows a perfusion flow to supply cell culture media instead of a static approach.
Figure 6. An organ‐level lung microfluidic platform that allows for exposing PM to cells at an air‐liquid interface. (A) Overview of the entire system for aerosolized PM generation, PM exposure, and the microfluidic platform. (B) Schematic diagram of the microfluidic platform. Reprinted with permission from Zheng et al. (Citation2019). Copyright (2019) American Chemical Society.
Collectively, these studies demonstrate the great potential of 3D cell culture and lung-on-a-chip models to investigate complex cellular responses upon PM exposure in a physiologically relevant condition, which is not available in 2D monoculture, nor can be easily quantified in animal studies. The lung-on-a-chip models are promising to serve as low-cost alternatives to animal studies for PM toxicity assessment.
4. Future outlook
Microfluidic techniques have grown rapidly in the past decades (Whitesides Citation2006), and there have been substantial fabrications and applications of microfluidic systems in diverse research. Their applications in aerosol toxicology, however, is still at an early stage. In our opinion, one important reason is that fabrication and usage of microfluidic systems demand expert operators, which may have hindered non-experts from using microfluidic systems. For example, the mold fabrication of microfluidic devices must be done in a cleanroom environment involving a large set of equipment. The expertise and experience in the optimization of device design and fabrication of microfluidic devices could be another challenge. Thus, more collaborations between microfluidic researchers and aerosol scientists are needed to facilitate employing microfluidic techniques for PM toxicity evaluation.
Even so, from the small number of studies that we have highlighted in this article, microfluidic techniques have demonstrated their versatility to overcome/circumvent many of the limitations experienced with traditional acellular and cellular assays. lists the applications and advantages of microfluidic techniques that have been employed for in vitro acellular and cellular assays to study PM toxicity. We believe the benefits and contributions from microfluidic techniques in aerosol toxicology can be far more than the current stage, which should be extensively explored in future studies. For instance, one future direction might be to further integrate microfluidic systems with aerosol sampling systems for both acellular and cellular assays, since there are only sparse demonstrations as reviewed in this article. One particular area would be the integration of aerosol sampling systems with various microfluidic platforms in cellular assays, which would allow real‐time monitoring of high‐throughput cellular response upon PM exposure. For example, there are microfluidic electrochemical sensors that have been developed for selective measurements of different types of ROS and reactive nitrogen species (RNS) produced by cells (Amatore et al. Citation2007; Li et al. Citation2018). Similar to applying microfluidic electrochemical sensors for online OP measurements, coupling such microfluidic platforms with aerosol sampling systems (e.g., PILS) could provide high‐throughput, real‐time measurement of different types of cellular ROS and RNS production induced by PM. However, for cellular assays, the aerosol flow rate (from mesoscale in aerosol sampling system to microscale in microfluidic cell culture system) must be optimized to prevent disruption of the cells cultured in microfluidic platforms.
Table 1. A summary of microfluidic techniques and their applications and advantages in PM toxicity studies.
For microfluidic techniques in acellular OP assays, µPAD systems are promising to serve as a complementary approach to the current instrumental techniques. The current approaches are able to perform daily OP measurements for PM samples collected at fixed sampling sites. The OP results can be associated with the health outcomes from epidemiologic studies at the population level (Abrams et al. Citation2017). The µPAD systems, which are developed to measure OP in the context of personal exposure assessment, can be complementary to conduct the personal-level analysis of the health effects of ambient OP. Nevertheless, the capability of µPAD for large-scale studies is yet to be demonstrated. The current µPAD systems for OP measurements are based on colorimetric detection, which can suffer from low sensitivity. Further developments to improve sensitivity are needed. In addition, the µPAD systems fabricated by wax printing techniques are only amenable to aqueous flow. The contribution of water‐insoluble PM components to OP may not be fully accounted for in the current µPAD systems. Future designs perhaps should incorporate alternatives that are compatible with organic solvents.
For microfluidic techniques in cellular assays, there are a variety of microfluidic platforms that have been developed for diverse research purposes. Many of them can be easily employed/adjusted to study PM toxicity. For example, the microfluidic-based systems that have been applied to drug screening and nanomaterial toxicity evaluation, are promising in extending their application to PM toxicity (He, Ranganathan, and Li Citation2018; Mahto et al. Citation2015). Despite the rapid developments of microfluidic systems in cellular assays, one major issue is that diverse experimental conditions employed in different microfluidic systems, indeed, make the comparison among systems rather difficult. The complexity of experimental conditions could come from different flow conditions, designs of microfluidic platforms, cell types, and the interaction between cells and stimulus under different conditions (Bhatia and Ingber Citation2014; He, Ranganathan, and Li Citation2018; Mitxelena-Iribarren et al. Citation2017; Teh et al. Citation2008). Transport and kinetic processes should be carefully designed and controlled, perhaps standardizing requirements of dimensionless groups such as Re, Sh, and De. To resolve this issue, standardized platforms and protocols should be established. National and international workshops gathering experts from industry, academia, and government could be a good initiative for the standardization in the microfluidics arena. The first workshop for standards of microfluidics held in 2017 by National Institute of Standards and Technology (NIST) could serve as a good example (Reyes and van Heeren Citation2019). In terms of microfluidic systems in PM toxicity studies, the assay systems should also be robust and easy to perform in average atmospheric science and biology/toxicology research labs.
While PDMS is the material that is mostly used for the fabrication of microfluidic devices because of its gas permeability, optical transparency, and biocompatibility, PDMS can absorb small hydrophobic organic compounds (Regehr et al. Citation2009; Toepke and Beebe Citation2006). This can be an issue for PM toxicity evaluation, since there are considerable amounts of hydrophobic organic components in PM and some of them (such as polycyclic aromatic hydrocarbons) are highly toxic (Jiang, Xu et al. Citation2019). Further research may be needed on biocompatible, optically transparent, economical, and easy-to-fabricate materials that are also resistant to absorption of small organic molecules (Berthier et al. Citation2019; Bhatia and Ingber Citation2014; Domansky et al. Citation2013; Sackmann, Fulton, and Beebe Citation2014).
Further, exposure to PM has been linked to diverse systemic effects as well as organ-specific effects, indicating the health effects caused by PM are beyond the lung tissue. For example, ultrafine particles have been observed in organs such as brain, liver, and kidney (Li et al. Citation2019; Maher et al. Citation2016). Thus, other cell types, organs-on-a-chip, human brain organoid-on-a-chip, and model-organism-on-chip platforms should be considered to understand the health effects from PM exposure. Further development of human body-on-a-chip will facilitate in vitro studies of systemic effects caused by PM (Novak et al. Citation2020). Although much effort has been made, there is still a large gap between state-of-the-art organ/organoid/organism-on-a-chip models and the reality of in vivo function. Given the complexities of how organs function, organ-on-a-chip models are not likely to replace animal-based assays and studies involving human populations; rather, they could lead to a decrease of animal studies by recreating more physiologically relevant conditions.
Acknowledgments
We would like to thank Gongchen Sun and Emily L. Jackson‐Holmes for helpful discussions.
References
- Abrams, J. Y., R. J. Weber, M. Klein, S. E. Sarnat, H. H. Chang, M. J. Strickland, V. Verma, T. Fang, J. T. Bates, J. A. Mulholland, et al. 2017. Associations between ambient fine particulate oxidative potential and cardiorespiratory emergency department visits. Environ. Health Perspect. 125 (10):107008. doi:https://doi.org/10.1289/EHP1545.
- Ahmed, D., C. Y. Chan, S.-C S. Lin, H. S. Muddana, N. Nama, S. J. Benkovic, and T. J. Huang. 2013. Tunable, pulsatile chemical gradient generation via acoustically driven oscillating bubbles. Lab Chip. 13 (3):328–31. doi:https://doi.org/10.1039/C2LC40923B.
- Alfaro-Moreno, E., C. García-Cuellar, A. De-Vizcaya-Ruiz, L. Rojas-Bracho, and A. R. Osornio-Vargas. 2010. Cellular mechanisms behind particulate matter air pollution related health effects.In Air Pollution: Health & Environmental Impacts, ed. R. Gurjar, L. T. Molina, and CSP Ojha, 249–74. Taylor & Francis
- Altschuler, S. J., and L. F. Wu. 2010. Cellular heterogeneity: Do differences make a difference? Cell 141 (4):559–63. doi:https://doi.org/10.1016/j.cell.2010.04.033.
- Amatore, C., S. Arbault, Y. Chen, C. Crozatier, and I. Tapsoba. 2007. Electrochemical detection in a microfluidic device of oxidative stress generated by macrophage cells. Lab Chip. 7 (2):233–8. doi:https://doi.org/10.1039/B611569A.
- Ardon-Dryer, K., C. Mock, J. Reyes, and G. Lahav. 2020. The effect of dust storm particles on single human lung cancer cells. Environ. Res. 181:108891. doi:https://doi.org/10.1016/j.envres.2019.108891.
- Atkinson, R. W., E. Samoli, A. Analitis, G. W. Fuller, D. C. Green, H. R. Anderson, E. Purdie, C. Dunster, L. Aitlhadj, F. J. Kelly, et al. 2016. Short-term associations between particle oxidative potential and daily mortality and hospital admissions in London. Int. J. Hyg. Environ. Health. 219 (6):566–72.,doi:https://doi.org/10.1016/j.ijheh.2016.06.004.
- Bates, J. T., T. Fang, V. Verma, L. Zeng, R. J. Weber, P. E. Tolbert, J. Y. Abrams, S. E. Sarnat, M. Klein, J. A. Mulholland, et al. 2019. Review of acellular assays of ambient particulate matter oxidative potential: Methods and relationships with composition, sources, and health effects. Environ. Sci. Technol. 53 (8):4003–19. doi:https://doi.org/10.1021/acs.est.8b03430.
- Bates, J. T., R. J. Weber, J. Abrams, V. Verma, T. Fang, M. Klein, M. J. Strickland, S. E. Sarnat, H. H. Chang, J. A. Mulholland, et al. 2015. Reactive oxygen species generation linked to sources of atmospheric particulate matter and cardiorespiratory effects. Environ. Sci. Technol. 49 (22):13605–12.,doi:https://doi.org/10.1021/acs.est.5b02967.
- Berg, K. E., L. R. Turner, M. L. Benka-Coker, S. Rajkumar, B. N. Young, J. L. Peel, M. L. Clark, J. Volckens, and C. S. Henry. 2019. Electrochemical dithiothreitol assay for large-scale particulate matter studies. Aerosol Sci Technol 53 (3):268–75. doi:https://doi.org/10.1080/02786826.2018.1560391.
- Berthier, E., A. M. Dostie, U. N. Lee, J. Berthier, and A. B. Theberge. 2019. Open microfluidic capillary systems. Anal. Chem. 91 (14):8739–50. doi:https://doi.org/10.1021/acs.analchem.9b01429.
- Bhatia, S. N., and D. E. Ingber. 2014. Microfluidic organs-on-chips. Nat. Biotechnol. 32 (8):760–72. doi:https://doi.org/10.1038/nbt.2989.
- Blay, V., B. Tolani, S. P. Ho, and M. R. Arkin. 2020. High-throughput screening: Today’s biochemical and cell-based approaches. Drug Discov. Today. 25: 1807–21 doi:https://doi.org/10.1016/j.drudis.2020.07.024.
- Burnett, R., H. Chen, M. Szyszkowicz, N. Fann, B. Hubbell, C. A. Pope, J. S. Apte, M. Brauer, A. Cohen, S. Weichenthal, et al. 2018. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc. Natl. Acad. Sci. USA. 115 (38):9592–7. doi:https://doi.org/10.1073/pnas.1803222115.
- Cate, D. M., J. A. Adkins, J. Mettakoonpitak, and C. S. Henry. 2015. Recent developments in paper-based microfluidic devices. Anal. Chem. 87 (1):19–41. doi:https://doi.org/10.1021/ac503968p.
- Chen, S., D. Li, H. Zhang, D. Yu, R. Chen, B. Zhang, Y. Tan, Y. Niu, H. Duan, B. Mai, et al. 2019. The development of a cell-based model for the assessment of carcinogenic potential upon long-term PM2.5 exposure. Environ. Int. 131:104943. doi:https://doi.org/10.1016/j.envint.2019.104943.
- Chingozha, L., M. Zhan, C. Zhu, and H. Lu. 2014. A generalizable, tunable microfluidic platform for delivering fast temporally varying chemical signals to probe single-cell response dynamics. Anal. Chem. 86 (20):10138–47. doi:https://doi.org/10.1021/ac5019843.
- Cho, A. K., C. Sioutas, A. H. Miguel, Y. Kumagai, D. A. Schmitz, M. Singh, A. Eiguren-Fernandez, and J. R. Froines. 2005. Redox activity of airborne particulate matter at different sites in the Los Angeles basin. Environ. Res. 99 (1):40–7. doi:https://doi.org/10.1016/j.envres.2005.01.003.
- Cho, C.-C., W.-Y. Hsieh, C.-H. Tsai, C.-Y. Chen, H.-F. Chang, and C.-S. Lin. 2018. In vitro and in vivo experimental studies of PM2.5 on disease progression. IJERPH 15 (7):1380. doi:https://doi.org/10.3390/ijerph15071380.
- Chowdhury, P. H., Q. He, R. Carmieli, C. Li, Y. Rudich, and M. Pardo. 2019. Connecting the oxidative potential of secondary organic aerosols with reactive oxygen species in exposed lung cells. Environ. Sci. Technol. 53 (23):13949–58. doi:https://doi.org/10.1021/acs.est.9b04449.
- Chowdhury, P. H., Q. He, T. Lasitza Male, W. H. Brune, Y. Rudich, and M. Pardo. 2018. Exposure of lung epithelial cells to photochemically aged secondary organic aerosol shows increased toxic effects. Environ. Sci. Technol. Lett. 5 (7):424–30. doi:https://doi.org/10.1021/acs.estlett.8b00256.
- Chung, K., C. A. Rivet, M. L. Kemp, and H. Lu. 2011. Imaging single-cell signaling dynamics with a deterministic high-density single-cell trap array. Anal. Chem. 83 (18):7044–52. doi:https://doi.org/10.1021/ac2011153.
- Cui, S., Z-z He, Z-w Zhu, Z. Sun, Y-t Xu, J-l Wang, Y-y Bao, D-y Ji, S. Liu, J-t Liu, et al. 2015. Microfluidic analysis of PM2.5-induced epithelial–mesenchymal transition in human bronchial epithelial 16HBE cells. Microfluid. Nanofluid. 19 (2):263–72. doi:https://doi.org/10.1007/s10404-014-1499-3.
- Cunha-Matos, C. A., O. R. Millington, A. W. Wark, and M. Zagnoni. 2016. Real-time assessment of nanoparticle-mediated antigen delivery and cell response. Lab Chip. 16 (17):3374–81. doi:https://doi.org/10.1039/C6LC00599C.
- Dedoussi, I. C., S. D. Eastham, E. Monier, and S. R. Barrett. 2020. Premature mortality related to united states cross-state air pollution. Nature 578 (7794):261–5. doi:https://doi.org/10.1038/s41586-020-1983-8.
- Demello, A. J. 2006. Control and detection of chemical reactions in microfluidic systems. Nature 442 (7101):394–402. doi:https://doi.org/10.1038/nature05062.
- Deng, Y., A. Finck, and R. Fan. 2019. Single-cell omics analyses enabled by microchip technologies. Annu. Rev. Biomed. Eng. 21:365–93. doi:https://doi.org/10.1146/annurev-bioeng-060418-052538.
- Di Carlo, D., N. Aghdam, and L. P. Lee. 2006. Single-cell enzyme concentrations, kinetics, and inhibition analysis using high-density hydrodynamic cell isolation arrays. Anal. Chem. 78 (14):4925–30. doi:https://doi.org/10.1021/ac060541s.
- Dittrich, P. S., and A. Manz. 2006. Lab-on-a-chip: Microfluidics in drug discovery. Nat. Rev. Drug Discov. 5 (3):210–8. doi:https://doi.org/10.1038/nrd1985.
- Domansky, K., D. C. Leslie, J. McKinney, J. P. Fraser, J. D. Sliz, T. Hamkins-Indik, G. A. Hamilton, A. Bahinski, and D. E. Ingber. 2013. Clear castable polyurethane elastomer for fabrication of microfluidic devices. Lab Chip. 13 (19):3956–64. doi:https://doi.org/10.1039/C3LC50558H.
- Donaldson, K., V. Stone, A. Seaton, and W. MacNee. 2001. Ambient particle inhalation and the cardiovascular system: Potential mechanisms. Environ. Health Perspect. 109:523–7. doi:https://doi.org/10.1289/ehp.01109s4523.
- Dong, H., L. Zheng, X. Duan, W. Zhao, J. Chen, S. Liu, and G. Sui. 2019. Cytotoxicity analysis of ambient fine particle in BEAS-2B cells on an air-liquid interface (ALI) microfluidics system. Sci. Total Environ. 677:108–19. doi:https://doi.org/10.1016/j.scitotenv.2019.04.203.
- Du, G., Q. Fang, and J. M. den Toonder. 2016. Microfluidics for cell-based high throughput screening platforms—A review. Anal. Chim. Acta. 903:36–50. doi:https://doi.org/10.1016/j.aca.2015.11.023.
- Dungchai, W., Y. Sameenoi, O. Chailapakul, J. Volckens, and C. S. Henry. 2013. Determination of aerosol oxidative activity using silver nanoparticle aggregation on paper-based analytical devices. Analyst 138 (22):6766–73. doi:https://doi.org/10.1039/c3an01235b.
- Edmondson, R., J. J. Broglie, A. F. Adcock, and L. Yang. 2014. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol 12 (4):207–18. doi:https://doi.org/10.1089/adt.2014.573.
- Eiguren-Fernandez, A., N. Kreisberg, and S. Hering. 2017. An online monitor of the oxidative capacity of aerosols (o-MOCA). Atmos. Meas. Tech. 10 (2):633–644. doi:https://doi.org/10.5194/amt-10-633.2017.
- El-Ali, J., P. K. Sorger, and K. F. Jensen. 2006. Cells on chips. Nature 442 (7101):403–11. doi:https://doi.org/10.1038/nature05063.
- Elowitz, M. B., A. J. Levine, E. D. Siggia, and P. S. Swain. 2002. Stochastic gene expression in a single cell. Science 297 (5584):1183–6. doi:https://doi.org/10.1126/science.1070919.
- Fang, T., V. Verma, J. T. Bates, J. Abrams, M. Klein, M. J. Strickland, S. E. Sarnat, H. H. Chang, J. A. Mulholland, P. E. Tolbert, et al. 2016. Oxidative potential of ambient water-soluble PM2.5 in the southeastern United States: Contrasts in sources and health associations between ascorbic acid (AA) and dithiothreitol (DTT) assays. Atmos. Chem. Phys. 16 (6):3865–79. doi:https://doi.org/10.5194/acp-16-3865-2016.
- Fang, T., V. Verma, H. Guo, L. King, E. Edgerton, and R. Weber. 2015. A semi-automated system for quantifying the oxidative potential of ambient particles in aqueous extracts using the dithiothreitol (DTT) assay: Results from the southeastern center for air pollution and epidemiology (scape). Atmos. Meas. Tech. 8 (1):471–482. doi:https://doi.org/10.5194/amt-8-471-2015.
- Forouzanfar, M. H., A. Afshin, L. T. Alexander, H. R. Anderson, Z. A. Bhutta, S. Biryukov, M. Brauer, R. Burnett, K. Cercy, F. J. Charlson, et al. 2016. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the global burden of disease study 2015. The Lancet 388 (10053):1659–724. doi:https://doi.org/10.1016/S0140-6736(16)31679-8.
- Gao, D., S. Ripley, S. Weichenthal, and K. J. G. Pollitt. 2020. Ambient particulate matter oxidative potential: Chemical determinants, associated health effects, and strategies for risk management. Free Radic. Biol. Med. 151: 7–25. doi:https://doi.org/10.1016/j.freeradbiomed.2020.04.028.
- Halldorsson, S., E. Lucumi, R. Gómez-Sjöberg, and R. M. Fleming. 2015. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens. Bioelectron. 63:218–31. doi:https://doi.org/10.1016/j.bios.2014.07.029.
- He, L., A. D. Raddatz, F. Zhou, H. Hwang, M. L. Kemp, and H. Lu. 2019. Dynamic mitochondrial migratory features associated with calcium responses during t cell antigen recognition. J. Immunol. 203 (3):760–8. doi:https://doi.org/10.4049/jimmunol.1800299.
- He, Z., N. Ranganathan, and P. Li. 2018. Evaluating nanomedicine with microfluidics. Nanotechnology 29 (49):492001. doi:https://doi.org/10.1088/1361-6528/aae18a.
- Hosokawa, M., T. Hayashi, T. Mori, T. Yoshino, S. Nakasono, and T. Matsunaga. 2011. Microfluidic device with chemical gradient for single-cell cytotoxicity assays. Anal. Chem. 83 (10):3648–54. doi:https://doi.org/10.1021/ac2000225.
- Huh, D., G. A. Hamilton, and D. E. Ingber. 2011. From 3D cell culture to organs-on-chips. Trends Cell Biol. 21 (12):745–54. doi:https://doi.org/10.1016/j.tcb.2011.09.005.
- Huh, D., B. D. Matthews, A. Mammoto, M. Montoya-Zavala, H. Y. Hsin, and D. E. Ingber. 2010. Reconstituting organ-level lung functions on a chip. Science 328 (5986):1662–8. doi:https://doi.org/10.1126/science.1188302.
- Jeannet, N., M. Fierz, M. Kalberer, H. Burtscher, and M. Geiser. 2015. Nano aerosol chamber for in-vitro toxicity (NACIVT) studies. Nanotoxicology 9 (1):34–42. doi:https://doi.org/10.3109/17435390.2014.886739.
- Jiang, H., C. Ahmed, A. Canchola, J. Y. Chen, and Y.-H. Lin. 2019. Use of dithiothreitol assay to evaluate the oxidative potential of atmospheric aerosols. Atmosphere 10:571. doi:https://doi.org/10.3390/atmos10100571.
- Jiang, X., F. Xu, X. Qiu, X. Shi, M. Pardo, Y. Shang, J. Wang, Y. Rudich, and T. Zhu. 2019. Hydrophobic organic components of ambient fine particulate matter (PM2.5) associated with inflammatory cellular response. Environ. Sci. Technol. 53 (17):10479–86. doi:https://doi.org/10.1021/acs.est.9b02902.
- Kasurinen, S., M. S. Happo, T. J. Rönkkö, J. Orasche, J. Jokiniemi, M. Kortelainen, J. Tissari, R. Zimmermann, M.-R. Hirvonen, and P. I. Jalava. 2018. Differences between co-cultures and monocultures in testing the toxicity of particulate matter derived from log wood and pellet combustion. PLoS One. 13 (2):e0192453. doi:https://doi.org/10.1371/journal.pone.0192453.
- Kniss-James, A. S., C. A. Rivet, L. Chingozha, H. Lu, and M. L. Kemp. 2017. Single-cell resolution of intracellular T cell Ca2+ dynamics in response to frequency-based H2O2 stimulation. Integr. Biol. (Camb.) 9 (3):238–47. doi:https://doi.org/10.1039/C6IB00186F.
- Koehler, K. A., J. Shapiro, Y. Sameenoi, C. Henry, and J. Volckens. 2014. Laboratory evaluation of a microfluidic electrochemical sensor for aerosol oxidative load. Aerosol Sci. Technol. 48 (5):489–97. doi:https://doi.org/10.1080/02786826.2014.891722.
- Kooter, I., M. Ilves, M. Gröllers-Mulderij, E. Duistermaat, P. C. Tromp, F. Kuper, P. Kinaret, K. Savolainen, D. Greco, P. Karisola, et al. 2019. Molecular signature of asthma-enhanced sensitivity to CuO nanoparticle aerosols from 3D cell model. ACS Nano. 13 (6):6932–46. doi:https://doi.org/10.1021/acsnano.9b01823.
- Kumagai, Y., S. Koide, K. Taguchi, A. Endo, Y. Nakai, T. Yoshikawa, and N. Shimojo. 2002. Oxidation of proximal protein sulfhydryls by phenanthraquinone, a component of diesel exhaust particles. Chem. Res. Toxicol. 15 (4):483–9. doi:https://doi.org/10.1021/tx0100993.
- Landreman, A. P., M. M. Shafer, J. C. Hemming, M. P. Hannigan, and J. J. Schauer. 2008. A macrophage-based method for the assessment of the reactive oxygen species (ROS) activity of atmospheric particulate matter (PM) and application to routine (daily-24 h) aerosol monitoring studies. Aerosol. Sci. Tech. 42 (11):946–57. doi:https://doi.org/10.1080/02786820802363819.
- Lelieveld, J., J. S. Evans, M. Fnais, D. Giannadaki, and A. Pozzer. 2015. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 525 (7569):367–71. doi:https://doi.org/10.1038/nature15371.
- Lelieveld, J., K. Klingmüller, A. Pozzer, U. Pöschl, M. Fnais, A. Daiber, and T. Münzel. 2019. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur. Heart J. 40 (20):1590–6. doi: https://doi.org/10.1093/eurheartj/ehz200.
- Leslie, D. C., K. Domansky, G. A. Hamilton, A. Bahinski, and D. E. Ingber. 2011. Aerosol drug delivery for lung on a chip. In 15th International Conference on Miniaturized Systems for Chemistry and Life Sciences.
- Li, D., Y. Li, G. Li, Y. Zhang, J. Li, and H. Chen. 2019. Fluorescent reconstitution on deposition of PM2.5 in lung and extrapulmonary organs. Proc. Natl. Acad. Sci. USA. 116 (7):2488–93. doi:https://doi.org/10.1073/pnas.1818134116.
- Li, J., J. Li, G. Wang, K. F. Ho, W. Dai, T. Zhang, Q. Wang, C. Wu, L. Li, L. Li, et al. 2020. Effects of atmospheric aging processes on in vitro induced oxidative stress and chemical composition of biomass burning aerosols. J. Hazard. Mater. 401:123750. doi:https://doi.org/10.1016/j.jhazmat.2020.123750.
- Li, N., C. Sioutas, A. Cho, D. Schmitz, C. Misra, J. Sempf, M. Wang, T. Oberley, J. Froines, and A. Nel. 2003. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 111 (4):455–60. doi:https://doi.org/10.1289/ehp.6000.
- Li, Q., A. Wyatt, and R. M. Kamens. 2009. Oxidant generation and toxicity enhancement of aged-diesel exhaust. Atmos. Environ 43 (5):1037–42. doi:https://doi.org/10.1016/j.atmosenv.2008.11.018.
- Li, Y., J. D. Motschman, S. T. Kelly, and B. B. Yellen. 2020. Injection molded microfluidics for establishing high-density single cell arrays in an open hydrogel format. Anal. Chem. 92 (3):2794–801. doi:https://doi.org/10.1021/acs.analchem.9b05099.
- Li, Y., C. Sella, F. Lemaître, M. Guille-Collignon, C. Amatore, and L. Thouin. 2018. Downstream simultaneous electrochemical detection of primary reactive oxygen and nitrogen species released by cell populations in an integrated microfluidic device. Anal. Chem. 90 (15):9386–94. doi:https://doi.org/10.1021/acs.analchem.8b02039.
- Lichtveld, K. M., S. M. Ebersviller, K. G. Sexton, W. Vizuete, I. Jaspers, and H. E. Jeffries. 2012. In vitro exposures in diesel exhaust atmospheres: Resuspension of PM from filters versus direct deposition of PM from air. Environ. Sci. Technol. 46 (16):9062–70. doi:https://doi.org/10.1021/es301431s.
- Liu, F., M. G. Saavedra, J. A. Champion, K. K. Griendling, and N. L. Ng. 2020. Prominent contribution of hydrogen peroxide to intracellular reactive oxygen species generated upon exposure to naphthalene secondary organic aerosols. Environ. Sci. Technol. Lett. 7 (3):171–7. doi:https://doi.org/10.1021/acs.estlett.9b00773.
- Liu, F., J. Whitley, N. L. S. Ng, and H. Lu. 2020. Time-resolved single-cell assay for measuring intracellular reactive oxygen species upon exposure to ambient particulate matter. Environ. Sci. Technol. 54 (20):13121–30. doi:https://doi.org/10.1021/acs.est.0c02889.
- Lu, Y., Q. Xue, M. R. Eisele, E. S. Sulistijo, K. Brower, L. Han, E.-A D. Amir, D. Pe'er, K. Miller-Jensen, and R. Fan. 2015. Highly multiplexed profiling of single-cell effector functions reveals deep functional heterogeneity in response to pathogenic ligands. Proc. Natl. Acad. Sci. USA. 112 (7):E607–E615. doi:https://doi.org/10.1073/pnas.1416756112.
- Macosko, E. Z., A. Basu, R. Satija, J. Nemesh, K. Shekhar, M. Goldman, I. Tirosh, A. R. Bialas, N. Kamitaki, E. M. Martersteck, et al. 2015. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161 (5):1202–14. doi:https://doi.org/10.1016/j.cell.2015.05.002.
- Maher, B. A., I. A. Ahmed, V. Karloukovski, D. A. MacLaren, P. G. Foulds, D. Allsop, D. M. Mann, R. Torres-Jardón, and L. Calderon-Garciduenas. 2016. Magnetite pollution nanoparticles in the human brain. Proc. Natl. Acad. Sci. USA. 113 (39):10797–801. doi:https://doi.org/10.1073/pnas.1605941113.
- Mahto, S. K., V. Charwat, P. Ertl, B. Rothen-Rutishauser, S. W. Rhee, and J. Sznitman. 2015. Microfluidic platforms for advanced risk assessments of nanomaterials. Nanotoxicology 9 (3):381–95. doi:https://doi.org/10.3109/17435390.2014.940402.
- Martinez, A. W., S. T. Phillips, G. M. Whitesides, and E. Carrilho. 2010. Diagnostics for the developing world: Microfluidic paper-based analytical devices. Anal. Chem. 82 (1):3–10. doi:https://doi.org/10.1021/ac9013989.
- Mentele, M. M., J. Cunningham, K. Koehler, J. Volckens, and C. S. Henry. 2012. Microfluidic paper-based analytical device for particulate metals. Anal. Chem. 84 (10):4474–80. doi:https://doi.org/10.1021/ac300309c.
- Meredith, N. A., C. Quinn, D. M. Cate, T. H. Reilly, J. Volckens, and C. S. Henry. 2016. Paper-based analytical devices for environmental analysis. Analyst 141 (6):1874–87. doi:https://doi.org/10.1039/C5AN02572A.
- Metcalf, A. R., S. Narayan, and C. S. Dutcher. 2018. A review of microfluidic concepts and applications for atmospheric aerosol science. Aerosol. Sci. Tech. 52 (3):310–29. doi:https://doi.org/10.1080/02786826.2017.1408952.
- Mitxelena-Iribarren, O., C. Hisey, M. Errazquin-Irigoyen, Y. González-Fernández, E. Imbuluzqueta, M. Mujika, M. Blanco-Prieto, and S. Arana. 2017. Effectiveness of nanoencapsulated methotrexate against osteosarcoma cells: In vitro cytotoxicity under dynamic conditions. Biomed. Microdev. 19 (2):35. doi:https://doi.org/10.1007/s10544-017-0177-0.
- Novak, R., M. Ingram, S. Marquez, D. Das, A. Delahanty, A. Herland, B. M. Maoz, S. S. Jeanty, M. R. Somayaji, and M. Burt. 2020. Robotic fluidic coupling and interrogation of multiple vascularized organ chips. Nat. Biomed. Eng. 4: 407–420. doi:https://doi.org/10.1038/s41551-019-0497-x.
- Ozer, T., C. McMahon, and C. S. Henry. 2020. Advances in paper-based analytical devices. Annu. Rev. Anal. Chem. 13: 85–109 doi:https://doi.org/10.1146/annurev-anchem-061318-114845.
- Pardo, M., X. Qiu, R. Zimmermann, and Y. Rudich. 2020. Particulate matter toxicity is nrf2 and mitochondria dependent: The roles of metals and polycyclic aromatic hydrocarbons. Chem. Res. Toxicol. 33 (5):1110–20. doi:https://doi.org/10.1021/acs.chemrestox.0c00007.
- Pardo, M., F. Xu, M. Shemesh, X. Qiu, Y. Barak, T. Zhu, and Y. Rudich. 2019. NRF2 protects against diverse PM2.5 components-induced mitochondrial oxidative damage in lung cells. Sci. Total Environ. 669:303–13. doi:https://doi.org/10.1016/j.scitotenv.2019.01.436.
- Pietrogrande, M. C., M. Russo, and E. Zagatti. 2019. Review of PM oxidative potential measured with acellular assays in urban and rural sites across Italy. Atmosphere 10:626. doi:https://doi.org/10.3390/atmos10100626.
- Pope, C. A., III, Ezzati, M., and D. W. Dockery. 2009. Fine-particulate air pollution and life expectancy in the United States. N. Engl. J. Med. 360 (4):376–86. doi:https://doi.org/10.1056/NEJMsa0805646.
- Prakadan, S. M., A. K. Shalek, and D. A. Weitz. 2017. Scaling by shrinking: Empowering single-cell 'omics' with microfluidic devices. Nat. Rev. Genet. 18 (6):345–61. doi:https://doi.org/10.1038/nrg.2017.15.
- Puthussery, J. V., C. Zhang, and V. Verma. 2018. Development and field testing of an online instrument for measuring the real-time oxidative potential of ambient particulate matter based on dithiothreitol assay. Atmos. Meas. Tech. 11 (10):5767–5780. doi:https://doi.org/10.5194/amt-11-5767-2018.
- Ravi, M., V. Paramesh, S. Kaviya, E. Anuradha, and F. P. Solomon. 2015. 3D cell culture systems: Advantages and applications. J. Cell. Physiol. 230 (1):16–26. doi: https://doi.org/10.1089/adt.2014.573.
- Regehr, K. J., M. Domenech, J. T. Koepsel, K. C. Carver, S. J. Ellison-Zelski, W. L. Murphy, L. A. Schuler, E. T. Alarid, and D. J. Beebe. 2009. Biological implications of polydimethylsiloxane-based microfluidic cell culture. Lab Chip. 9 (15):2132–9. doi:https://doi.org/10.1039/b903043c.
- Reyes, D. R., and H. van Heeren. 2019. Proceedings of the first workshop on standards for microfluidics. J. Res. Natl. Inst. Stand. Technol. 124:1–22. doi:https://doi.org/10.6028/jres.124.001.
- Sackmann, E. K., A. L. Fulton, and D. J. Beebe. 2014. The present and future role of microfluidics in biomedical research. Nature 507 (7491):181–9. doi:https://doi.org/10.1038/nature13118.
- Sameenoi, Y., K. Koehler, J. Shapiro, K. Boonsong, Y. Sun, J. Collett, Jr, J. Volckens, and C. S. Henry. 2012. Microfluidic electrochemical sensor for on-line monitoring of aerosol oxidative activity. J. Am. Chem. Soc. 134 (25):10562–8. doi:https://doi.org/10.1021/ja3031104.
- Sameenoi, Y., M. M. Mensack, K. Boonsong, R. Ewing, W. Dungchai, O. Chailapakul, D. M. Cropek, and C. S. Henry. 2011. Poly(dimethylsiloxane) cross-linked carbon paste electrodes for microfluidic electrochemical sensing. Analyst 136 (15):3177–84. doi:https://doi.org/10.1039/C1AN15335H.
- Sameenoi, Y., P. Panymeesamer, N. Supalakorn, K. Koehler, O. Chailapakul, C. S. Henry, and J. Volckens. 2013. Microfluidic paper-based analytical device for aerosol oxidative activity. Environ. Sci. Technol. 47 (2):932–40. doi:https://doi.org/10.1021/es304662w.
- Shah, P., X. Zhu, X. Zhang, J. He, and C-z Li. 2016. Microelectromechanical system-based sensing arrays for comparative in vitro nanotoxicity assessment at single cell and small cell-population using electrochemical impedance spectroscopy. ACS Appl. Mater. Interfaces 8 (9):5804–12. doi:https://doi.org/10.1021/acsami.5b11409.
- Shalek, A. K., R. Satija, X. Adiconis, R. S. Gertner, J. T. Gaublomme, R. Raychowdhury, S. Schwartz, N. Yosef, C. Malboeuf, D. Lu, et al. 2013. Single-cell transcriptomics reveals bimodality in expression and splicing in immune cells. Nature 498 (7453):236–40. doi:https://doi.org/10.1038/nature12172.
- Shembekar, N., C. Chaipan, R. Utharala, and C. A. Merten. 2016. Droplet-based microfluidics in drug discovery, transcriptomics and high-throughput molecular genetics. Lab Chip. 16 (8):1314–31. doi:https://doi.org/10.1039/C6LC00249H.
- Shiraiwa, M., K. Ueda, A. Pozzer, G. Lammel, C. J. Kampf, A. Fushimi, S. Enami, A. M. Arangio, J. Fröhlich-Nowoisky, Y. Fujitani, et al. 2017. Aerosol health effects from molecular to global scales. Environ. Sci. Technol. 51 (23):13545–67. doi:https://doi.org/10.1021/acs.est.7b04417.
- Sugiura, S., K. Hattori, and T. Kanamori. 2010. Microfluidic serial dilution cell-based assay for analyzing drug dose response over a wide concentration range. Anal. Chem. 82 (19):8278–82. doi:https://doi.org/10.1021/ac1017666.
- Sun, G., and H. Lu. 2019. Recent advances in microfluidic techniques for systems biology. Anal. Chem. 91 (1):315–29. doi:https://doi.org/10.1021/acs.analchem.8b04757.
- Švancara, I., K. VytřAs, K. Kalcher, A. Walcarius, and J. Wang, 2009. Carbon paste electrodes in facts, numbers, and notes: A review on the occasion of the 50‐years jubilee of carbon paste in electrochemistry and electroanalysis. Electroanalysis 21 (1):7–28. doi:https://doi.org/10.1002/elan.200804340.
- Teh, S.-Y., R. Lin, L.-H. Hung, and A. P. Lee. 2008. Droplet microfluidics. Lab Chip. 8 (2):198–220. doi:https://doi.org/10.1039/B715524G.
- Theberge, A. B., F. Courtois, Y. Schaerli, M. Fischlechner, C. Abell, F. Hollfelder, and W. T. Huck. 2010. Microdroplets in microfluidics: An evolving platform for discoveries in chemistry and biology. Angew. Chem. Int. Ed. Engl. 49 (34):5846–68. doi:https://doi.org/10.1002/anie.200906653.
- Toepke, M. W., and D. J. Beebe. 2006. Pdms absorption of small molecules and consequences in microfluidic applications. Lab Chip. 6 (12):1484–6. doi:https://doi.org/10.1039/B612140C.
- Toriello, N. M., E. S. Douglas, N. Thaitrong, S. C. Hsiao, M. B. Francis, C. R. Bertozzi, and R. A. Mathies. 2008. Integrated microfluidic bioprocessor for single-cell gene expression analysis. Proc. Natl. Acad. Sci. USA. 105 (51):20173–8. doi:https://doi.org/10.1073/pnas.0806355106.
- Tuet, W. Y., Y. Chen, S. Fok, J. A. Champion, and N. L. Ng. 2017. Inflammatory responses to secondary organic aerosols (SOA) generated from biogenic and anthropogenic precursors. Atmos. Chem. Phys. 17 (18):11423–40. doi:https://doi.org/10.5194/acp2017.
- Tuet, W. Y., Y. Chen, S. Fok, D. Gao, R. J. Weber, J. A. Champion, and N. L. Ng. 2017. Chemical and cellular oxidant production induced by naphthalene secondary organic aerosol (SOA): Effect of redox-active metals and photochemical aging. Sci. Rep. 7:1–10. doi:https://doi.org/10.1038/s41598-017-15071-8.
- Tuet, W. Y., S. Fok, V. Verma, M. S. T. Rodriguez, A. Grosberg, J. A. Champion, and N. L. Ng. 2016. Dose-dependent intracellular reactive oxygen and nitrogen species (ROS/RNS) production from particulate matter exposure: Comparison to oxidative potential and chemical composition. Atmos. Environ. 144:335–44. doi:https://doi.org/10.1016/j.atmosenv.2016.09.005.
- Tuet, W. Y., F. Liu, N. de Oliveira Alves, S. Fok, P. Artaxo, P. r Vasconcellos, J. A. Champion, and N. L. Ng. 2019. Chemical oxidative potential and cellular oxidative stress from open biomass burning aerosol. Environ. Sci. Technol. Lett. 6 (3):126–32. doi:https://doi.org/10.1021/acs.estlett.9b00060.
- Van Duinen, V., S. J. Trietsch, J. Joore, P. Vulto, and T. Hankemeier. 2015. Microfluidic 3D cell culture: From tools to tissue models. Curr. Opin. Biotechnol. 35:118–26. doi:https://doi.org/10.1016/j.copbio.2015.05.002.
- Verma, V., T. Fang, L. Xu, R. E. Peltier, A. G. Russell, N. L. Ng, and R. J. Weber. 2015. Organic aerosols associated with the generation of reactive oxygen species (ROS) by water-soluble PM2.5. Environ. Sci. Technol. 49 (7):4646–56. doi:https://doi.org/10.1021/es505577w.
- Verma, V., Z. Ning, A. K. Cho, J. J. Schauer, M. M. Shafer, and C. Sioutas. 2009. Redox activity of urban quasi-ultrafine particles from primary and secondary sources. Atmos. Environ. 43 (40):6360–8. doi:https://doi.org/10.1016/j.atmosenv.2009.09.019.
- Vlassakis, J., and A. E. Herr. 2017. Joule heating-induced dispersion in open microfluidic electrophoretic cytometry. Anal. Chem. 89 (23):12787–96. doi:https://doi.org/10.1021/acs.analchem.7b03096.
- Vyawahare, S., A. D. Griffiths, and C. A. Merten. 2010. Miniaturization and parallelization of biological and chemical assays in microfluidic devices. Chem. Biol. 17 (10):1052–65. doi:https://doi.org/10.1016/j.chembiol.2010.09.007.
- Wang, G., X. Zhang, X. Liu, J. Zheng, R. Chen, and H. Kan. 2019. Ambient fine particulate matter induce toxicity in lung epithelial-endothelial co-culture models. Toxicol. Lett. 301:133–45. doi:https://doi.org/10.1016/j.toxlet.2018.11.010.
- Ward, R. X., T. B. Tilly, S. I. Mazhar, S. E. Robinson, A. Eiguren-Fernandez, J. Wang, T. Sabo-Attwood, and C.-Y. Wu. 2020. Mimicking the human respiratory system: Online in vitro cell exposure for toxicity assessment of welding fume aerosol. J. Hazard. Mater. 395:122687. doi:https://doi.org/10.1016/j.jhazmat.2020.122687.
- Watson, C., J. Ge, J. Cohen, G. Pyrgiotakis, B. P. Engelward, and P. Demokritou. 2014. High-throughput screening platform for engineered nanoparticle-mediated genotoxicity using cometchip technology. ACS Nano. 8 (3):2118–33. doi:https://doi.org/10.1021/nn404871p.
- Weber, R., D. Orsini, Y. Daun, Y.-N. Lee, P. Klotz, and F. Brechtel. 2001. A particle-into-liquid collector for rapid measurement of aerosol bulk chemical composition. Aerosol. Sci. Tech. 35 (3):718–27. doi:https://doi.org/10.1080/02786820152546761.
- Weichenthal, S., D. L. Crouse, L. Pinault, K. Godri-Pollitt, E. Lavigne, G. Evans, A. van Donkelaar, R. V. Martin, and R. T. Burnett. 2016. Oxidative burden of fine particulate air pollution and risk of cause-specific mortality in the Canadian Census Health and Environment Cohort (CANCHEC). Environ. Res. 146:92–9. doi:https://doi.org/10.1016/j.envres.2015.12.013.
- Whitesides, G. M. 2006. The origins and the future of microfluidics. Nature 442 (7101):368–73. doi:https://doi.org/10.1038/nature05058.
- Williams, R., J. Suggs, C. Rodes, P. Lawless, R. Zweidinger, R. Kwok, J. Creason, and L. Sheldon. 2000. Comparison of PM2.5 and PM10 monitors. J. Expo. Anal. Environ. Epidemiol. 10 (5):497–505. doi:https://doi.org/10.1038/sj.jea.7500138.
- Xu, C., M. Zhang, W. Chen, L. Jiang, C. Chen, and J. Qin. 2020. Assessment of air pollutant PM2.5 pulmonary exposure using a 3D lung-on-chip model. ACS Biomater. Sci. Eng. 6 (5):3081–90. doi:https://doi.org/10.1021/acsbiomaterials.0c00221.
- Xue, M., W. Wei, Y. Su, J. Kim, Y. S. Shin, W. X. Mai, D. A. Nathanson, and J. R. Heath. 2015. Chemical methods for the simultaneous quantitation of metabolites and proteins from single cells. J. Am. Chem. Soc. 137 (12):4066–9. doi:https://doi.org/10.1021/jacs.5b00944.
- Yamada, K., T. G. Henares, K. Suzuki, and D. Citterio. 2015. Paper-based inkjet-printed microfluidic analytical devices . Angew. Chem. Int. Ed. Engl. 54 (18):5294–310. doi:https://doi.org/10.1002/anie.201411508.
- Ye, N., J. Qin, W. Shi, X. Liu, and B. Lin. 2007. Cell-based high content screening using an integrated microfluidic device. Lab Chip. 7 (12):1696–704. doi:https://doi.org/10.1039/b711513j.
- Zhang, F., W. Liu, S. Zhou, L. Jiang, K. Wang, Y. Wei, A. Liu, W. Wei, and S. Liu. 2020. Investigation of environmental pollutant-induced lung inflammation and injury in a 3D coculture-based microfluidic pulmonary alveolus system. Anal. Chem. 92 (10):7200–8. doi:https://doi.org/10.1021/acs.analchem.0c00759.
- Zhang, Y., L. Zheng, J. Tuo, Q. Liu, X. Zhang, Z. Xu, S. Liu, and G. Sui. 2017. Analysis of PM2.5-induced cytotoxicity in human hacat cells based on a microfluidic system. Toxicol. In Vitro. 43:1–8. doi:https://doi.org/10.1016/j.tiv.2017.04.018.
- Zheng, L., H. Dong, W. Zhao, X. Zhang, X. Duan, H. Zhang, S. Liu, and G. Sui. 2019. An air-liquid interface organ-level lung microfluidics platform for analysis on molecular mechanisms of cytotoxicity induced by cancer-causing fine particles. ACS Sens. 4 (4):907–17. doi:https://doi.org/10.1021/acssensors.8b01672.
- Zheng, L., S. Liu, G. Zhuang, J. Xu, Q. Liu, X. Zhang, C. Deng, Z. Guo, W. Zhao, T. Liu, et al. 2017. Signal transductions of BEAS-2B cells in response to carcinogenic PM2.5 exposure based on a microfluidic system. Anal. Chem. 89 (10):5413–21. doi:https://doi.org/10.1021/acs.analchem.7b00218.

