Abstract
Objective. The efficacy of programmes to reduce long-term benzodiazepine use could be compromised by subsequent increases in contacts with the family practice. In this study the hypothesis was tested as to whether participation in a benzodiazepine discontinuation programme affects the frequency of contacts with the family practice. Design. A controlled stepped-care intervention programme to decrease long-term benzodiazepine use. Setting. Family practices in the Netherlands. Subjects. The experimental group consisted of 996 long-term benzodiazepine users and a control group of 883 long-term benzodiazepine users. Main outcome measures. Practice contacts before and up to 12 months after the start of the programme. Results. There was a general tendency visible for contacts to decrease during the follow-up time. The course of the number of contacts during the follow-up was not different for the experimental and control groups (p=0.45). The level of non-benzodiazepine prescriptions was generally not altered. The number of non-benzodiazepine prescriptions decreased in benzodiazepine quitters during the follow-up of the programme. Conclusion. No clinically important differences in practice contacts were observed when the course of the number of contacts and non-benzodiazepine prescriptions were compared between the experimental and control groups. Family practitioners do not have to anticipate an increased workload associated with participation in such a benzodiazepine discontinuation programme.
Long-term use of benzodiazepines remains widespread, irrespective of national and international guidelines advising short-term use Citation[1–4]. Patient- as well as physician-related factors may be responsible for the initiation and persistence of this prescribing practice Citation[5], Citation[6]. It appears that physicians and patients think differently about the therapeutic efficacy and risks of using benzodiazepines Citation[7]. Long-term users of benzodiazepines often mention high treatment satisfaction without being very concerned about the side effects Citation[8]. On the other hand, many physicians perceive that the chances for long-term benzodiazepine users to quit their use are small Citation[9]. Furthermore, family practitioners anticipate difficulties in persuading patients to withdraw and suppose that quitting benzodiazepine use will increase other patient demands as a substitution Citation[9], Citation[10]. These opinions may prevent family practitioners from taking an active role in addressing this subject with their patients. Within a study aimed at the reduction of long-term benzodiazepine use in family practice in the Netherlands, the Benzoredux study Citation[11], Citation[12], we had the opportunity to test the hypothesis as to whether participation in a benzodiazepine discontinuation programme affects the frequency of contacts with the family practice.
Whether intervening with long-term benzodiazepine use may lead to subsequent increases in other patient demands is not clear.
Participation in a benzodiazepine discontinuation programme did not increase the number of family practice contacts by long-term benzodiazepine users.
Family practitioners do not have to anticipate an increased workload as a spin-off of the reported benzodiazepine discontinuation programme.
This finding removes a possible obstacle for implementation of this successful benzodiazepine discontinuation strategy.
Material and methods
The Benzoredux study (source population)
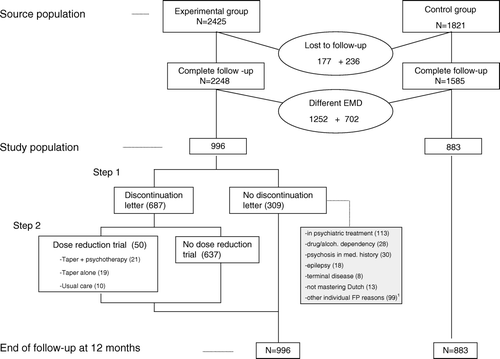
The Benzoredux study was a family practice based prospective controlled study. It used a stepped intervention with a benzodiazepine discontinuation letter as the first step, followed by the second step: a three-group randomized clinical trial with a guided taper programme, with or without additional group psychotherapy, or usual care Citation[11], Citation[12]. The long-term benzodiazepine users received benzodiazepine prescriptions for more than three months. In 30 family practices 2964 long-term benzodiazepine users were identified. After applying exclusion criteria, 2004 were included. Three months after the discontinuation letter subjects were invited to make an appointment with the family practitioner (FP) for an evaluation consultation. Of the subjects that still used benzodiazepines and had an evaluation consultation (n=1036), 180 gave informed consent and were randomized in step 2 Citation[12]. From the Dutch National Information Network of Family Practices (LINH), 19 practices were selected as control practices (long-term users, n=2061) Citation[13]. Six months after the discontinuation letter 24% of the long-term users in the participating practices had no more benzodiazepine prescriptions Citation[11]. In the second step, of the subjects receiving taper, 61% discontinued benzodiazepine use Citation[12].
Study population
From the participating practices of the Benzoredux study, six practices (three experimental and three control) had insufficient data follow-up. From the remaining practices (27 experimental and 16 control), those practices were selected that used PROMEDICO™ as electronic medical dossier (EMD) Citation[14]. Eleven experimental practices (n = 996) and nine control practices (n = 883) complied with this criterion ().
Variables
The following data were extracted anonymously from the EMD: (1) demographic data, (2) prescriptions, and (3) dates and administration codes of handlings, operations, financial declarations, consultations, diagnostic reports, referral, etc.
Practice contacts, defined as a notification in the EMD, were classified in six types. In hierarchical sequence: (1) home visit, (2) consultation, (3) telephone contact, (4) (practice) assistant contact (for instance, diagnostic operations like urine checks and treatment operations such as vaccination), (5) other contact (any other non-specified notification), and (6) a prescription line without any other notification in the EMD (“separate prescription line”).
We dichotomized contacts per day into “yes” or “no contact”. If more contact types were present on the same day, we recorded the contact with the highest ranking according to the hierarchical sequence mentioned above. In the experimental practices the intervention started at the moment when the discontinuation letter was sent. To rule out seasonal differences, a similar starting date was applied for the control practices (matched by date at the level of practice). Baseline was defined as the period of three months before the start of the intervention. The 12-month follow-up was divided into four subsequent periods of three months (periods 1–4).
Analysis
We compared the course of the number of contacts in the experimental group with the control group. The experimental group (n = 996 long-term benzodiazepine users) contained the subjects that were addressed by the discontinuation letter (n = 687) and those who were not (n = 309) (see ). A “quitter” was defined as a subject who did not receive any benzodiazepine prescription in the second follow-up period (months 4 up to and including 6) Citation[11]. We used a Poisson regression model with correction for over-dispersion (GENMOD procedure in SAS) Citation[15] with number of contacts (or prescriptions) as the dependent variable. There were five time points: baseline and four three-month periods. Apart from the variables “group” (experimental/control), “time” (five time points) and their interaction term, we entered the following co-variables in the analysis: “health insurance” (NHS or private), “gender” (M/F), “baseline benzodiazepine use” (number of prescribed daily standard dosages (PDD) in the baseline period), “number of baseline non-benzodiazepine prescriptions” (number of non-benzodiazepine prescriptions in the baseline period), and “age” (year). The statistical significance of the estimate of the interaction term between the group (experimental or control) and time provided the information on whether there was a difference in the course of the number of contacts (or prescriptions) during the follow-up between the experimental and control groups.
We analysed prescription separately from contacts in two different categories: benzodiazepine prescriptions and non-benzodiazepine prescriptions. Furthermore, we analysed practice contacts also without prescriptions (“prescription excluded contacts”). In order to establish whether benzodiazepine quitters had a different course of contacts compared with non-quitters, we entered the variables “quitter” (yes/no), “time” and their interaction term in a model with “health insurance”, “gender”, “baseline benzodiazepine use”, “number of baseline non-benzodiazepine prescriptions”, and “age” as covariates. Separate analysis was performed in the control group, the experimental group, and the pure intervention group (those subjects actually receiving the discontinuation letter).
In all analysis comparisons were made for subjects with a complete follow-up. Two sided p-values were used with a significance level of 0.05.
Results
Baseline characteristics
The distribution of the demographic variables compared favourably, as well between the study population and the source population as between the experimental and the control condition ().
Table I. Charateristics of study subjects.
Contacts
The average numbers of contacts per three-month period in the experimental and control groups are given in . The number of practice contacts (SD) per three months varied between 5.7 (4.3) and 6.3 (4.2) in the experimental group and 6.4 (4.8) and 6.9 (4.9) in the control group. There was a general tendency visible for contacts to decrease during follow-up. The course of the number of contacts during the follow-up was not different for the experimental and control groups (p = 0.45). Quitters had lower contacts and showed a larger decrease in contacts compared with non-quitters (p < 0.001). When prescriptions were excluded from the counting of contacts, the course of contacts was not different for the experimental and control groups (p = 0.06), but statistically different for quitters and non-quitters (p = 0.04). The course of the number of consultations was statistically different for the experimental group compared with the control group (p = 0.002). This was due to an increase in consultations in the experimental group relative to the control group in the first two three-month periods (comparison with baseline and period 1: p = 0.002; period 2: p < 0.001; period 3: p = 0.10; period 4: p = 0.22). The course of the number of consultations was statistically different for the quitters and non-quitters (p = 0.02), where quitters had fewer consultations compared with the non-quitters. The analysis of the course of the number of consultations in the control, experimental, and pure intervention groups separately showed no statistically significant difference between quitters and non-quitters (control group: p = 0.23; experimental group: p = 0.10; pure intervention group: p = 0.14).
Table II. Average number of contacts (SD) per three-month period in the experimental group (n = 996) and the control group (n = 883).
Prescriptions
The average numbers of prescriptions per three-month period in the experimental and control groups are given in . The number of non-benzodiazepine prescriptions (SD) per three months varied between 6.0 (5.7) and 5.6 (5.6) in the experimental group and 6.6 (6.8) and 6.1 (6.7) in the control group. The level of non-benzodiazepine prescriptions was generally not altered. The differences in course of number of non-benzodiazepine prescriptions between the experimental and control groups were not statistically significant (p = 0.96), but were statistically significant for benzodiazepines (p < 0.001).
Table III. Average number of prescriptions1 (SD) per three-month period in the experimental group (n = 996) and the control group (n = 883).
Although the level of non-benzodiazepine prescriptions was stable in non-quitters, quitters, in both the experimental and the control groups, showed a decrease in number of non-benzodiazepine prescriptions during the follow-up. The decrease in non-benzodiazepine prescriptions of quitters was statistically different compared with non-quitters in all subjects (p < 0.001), the experimental group subjects (p = 0.001) and in the pure intervention group (p = 0.01), but not in the control group subjects (p = 0.18).
Other contacts
At baseline, in the experimental group, the mean number of home visits was 0.27 (SD 0.99), the mean number of telephone consultations was 0.17 (SD 0.89), the mean number of practice assistant contacts was 0.07 (SD 0.26), and other 0.82 (SD 1.21). These numbers were statistically not different from the baseline values of the control group: home visits 0.46 (SD 1.38; p = 0.12), telephone contacts 0.14 (SD 0.63; p = 0.99), assistant contacts 0.17 (SD 0.46; p = 0.43) and other 0.84 (SD 1.36; p = 0.74). During follow-up these numbers did not change significantly.
Discussion
The most important finding of this study was that the course of the number of both non-benzodiazepine prescriptions and practice contacts excluding separate prescriptions was not relevantly influenced by participation in this benzodiazepine reduction programme. Moreover, benzodiazepine quitters showed a larger decrease in contacts and non-benzodiazepine prescriptions, compared with non-quitters. These observations clearly do not support the hypothesis that quitting benzodiazepine use will increase other patient demands as a substitution Citation[9], Citation[10].
In our data analysis, we generally made two comparisons. First, we compared the experimental group with the control group. This is methodologically the most valid comparison, thereby accepting a loss of specificity due to the fact that about one-third of the experimental group subjects were excluded from the intervention. The second comparison, between quitters and non-quitters, should be interpreted with caution as quitters probably constitute a selected group.
This study was not primarily set up for the measuring of practice contacts. We composed practice contacts afterwards using all information available in the EMD, including clinical, administration, and financial data, and all prescriptions. We are confident of our measurement as only on the rare occasion that, for a contact, nothing was entered in the EMD, we missed it. We had very large samples. A power estimation, made afterwards, suggested the study had power (beta 0.2) to detect a difference of 5% in overall contacts. This was in our opinion sufficient. Regression to the mean effects may be responsible for part of the decrease in the number of benzodiazepine prescriptions. However, these effects do not invalidate our comparison as they appear in both the experimental and the control group. Differences in timing of data acquisition might have invalidated the comparison of the lost to follow-up groups. Therefore, we decided to analyse study completers only. We do not consider that the intervention itself caused a substantial migration of patients, as the presentation of the intervention by the family practitioner was not compulsory.
We did observe a statistically significant increase in number of consultations in only the first six months in the experimental group. Quitters had fewer consultations than non-quitters. This suggests that it was a temporary effect related to the positioning of evaluation consultations for which the subjects were invited around three months after the discontinuation letter. As these consultations were used also for other encounter reasons, we could not filter them out in the counting of contacts.
Our analysis of family practice contacts is unique. Only one earlier, much smaller, study reported no change in the number of consultations in the first six months after a brief intervention to reduce chronic use of benzodiazepines Citation[17].
We conclude that this benzodiazepine discontinuation programme does not increase family practice contacts. This contradicts expectations of family practitioners that discourage them from starting benzodiazepine discontinuation interventions. These expectations may be based too often on experiences with individual patients but appear to be not valid for the whole group of long-term benzodiazepine users.
Acknowledgements
The Dutch Health Care Insurance Council funded the study. No author has competing interests. Ethical approval was obtained by the CMO Arnhem/Nijmegen.
References
- GIP/College voor zorgverzekeringen (Dutch Health Care Insurance Council). Available at: http://www.gipdatabank.nl (2006).
- Ashton H. Guidelines for the rational use of benzodiazepines. When and what to use. Drugs 1994; 48: 25–40
- Gorgels WJMJ, Oude Voshaar RC, Mol AJ, Breteler MH, Lisdonk EH van de, Zitman FG. [Long-term use of benzodiazepines]. Ned Tijdschr Geneeskd 2001; 145: 1342–6
- Lader MH. Limitations on the use of benzodiazepines in anxiety and insomnia: Are they justified?. Eur Neuropsychopharmacol 1999; 9(Suppl. 6)S399–S405
- Anthierens S, Habraken H, Petrovic M, Christiaens T. The lesser evil? Initiating a benzodiazepine prescription in general practice. Scand J Prim Health Care 2007; 25: 214–19
- Anthierens S, Habraken H, Petrovic M, Deveugele M, De Maeseneer J, Christiaens T. First benzodiazepine prescription. Can Fam Physician 2007; 53: 1200–1
- Mah L, Upshur REG. Long term benzodiazepine use for insomnia in patients over the age of 60: Discordance of patient and physician perceptions. BMC Family Practice 2002; 3: 9
- Vissers F, Grinten R van der, Horst F van der, Kester A, Knottnerus JA. Use of hypnotic and tranquillising drugs in general practice – Determinants, satisfaction and motivation to stop: The patients’ view. Scand J Prim Health Care 2003; 21: 159–61
- Cook JM, Marshall R, Masci C, Coyne JC. Physicians’ perspectives on prescribing benzodiazepines for older adults: A qualitative study. J Gen Intern Med 2007; 22: 303–7
- Iliffe S, Curran HV, Collins R, Kee SCY, Fletcher S, Woods B. Attitudes to long-term use of benzodiazepine hypnotics by older people in general practice: Findings from interviews with service users and providers. Aging & Mental Health 2004; 8: 242–8
- Gorgels WJ, Oude Voshaar RC, Mol AJ, Lisdonk EH van de, Balkom AJ van, Hoogen HJ van den et al Discontinuation of long-term benzodiazepine use by sending a letter to users in family practice: A prospective controlled intervention study. Drug Alcohol Depend 2005;78:49–56.
- Oude Voshaar RC, Gorgels WJMJ, Mol AJJ, van Balkom AJLM, van de Lisdonk EH, Breteler MH, et al. Tapering-off long-term benzodiazepine use with or without simultaneous group cognitive behavioural therapy: A three-condition randomised controlled trial. Br J Psychiatry 2003; 182: 498–504
- Verheij RA, te Brake JHM, Abrahamse H, van den Hoogen H, Braspenning J, van Althuis T. Landelijk Informatienetwerk Huisartsenzorg. Feiten en cijfers over huisartsenzorg in Nederland [National Information Network of Family Practices. Facts and figures of the care of family practitioners in the Netherlands]. Utrecht/Nijmegen: NIVEL/WOK. Available at: http://www.LINH.nl (2006).
- PROMEDICO-VDF. Available at: http://www.promedico.nl (2005).
- SAS Online Docs, Version 8. Cary, NC: SAS Institute; 1999.
- Zitman FG, Couvee JE. Chronic benzodiazepine use in general practice patients with depression: An evaluation of controlled treatment and taper-off: report on behalf of the Dutch Chronic Benzodiazepine Working Group. Br J Psychiatry 2001; 178: 317–24
- Bashir K, King M, Ashworth M. Controlled evaluation of brief intervention by general practitioners to reduce chronic use of benzodiazepines. Br J Gen Pract 1994; 44: 408–12