Abstract
Objective: To study the self-reported level of physical activity (PA) and quality of life (QOL) in patients receiving physical activity on prescription (PAP) for up to 24 months.
Design: Observational study conducted in a regular healthcare setting.
Setting: A primary care population in Sweden receiving physical activity on prescription as part of regular care was studied alongside a reference group.
Subjects: The group comprised 146 patients receiving PAP at two different primary care locations (n = 96 and 50, respectively). The reference group comprised 58 patients recruited from two different primary care centres in the same region.
Main outcome measurements: We used two self-report questionnaires – the four-level Saltin-Grimby Physical Activity Level Scale (SGPALS) to assess physical activity, and SF-36 to assess QOL.
Results: A significant increase in the PA level was found at six and 12 months following PAP, with an ongoing non-significant trend at 24 months (p = .09). A clear improvement in QOL was seen during the period. At 24 months, significant and clinically relevant improvements in QOL persisted in four out of eight sub-scale scores (Physical Role Limitation, Bodily Pain, General Health,Vitality) and in one out of two summary scores (Physical Component Summary).
Conclusion: Patients receiving PAP showed an increased level of self-reported PA at six and 12 months and improved QOL for up to 24 months in several domains. The Swedish PAP method seems to be a feasible method for bringing about changes in physical activity in different patient populations in regular primary healthcare.
While increased physical activity (PA) is shown to improve health, the implementation of methods designed to increase activity is still being developed.
The present study confirms that the Swedish physical activity on prescription (PAP) method increases the self-reported level of PA in the primary care setting at six and 12 months.
Furthermore, this study shows that PAP recipients report a clinically relevant long-term improvement in quality of life, persisting for two years post-prescription, thus extending earlier findings.
These findings have clinical implications for the implementation of PAP in healthcare.
Key points
Introduction
The burden of non-communicable disease (NCD) is increasing [Citation1,Citation2] and the World Health Organisation (WHO) currently ranks physical inactivity as the fourth most important risk factor for premature death worldwide after hypertension, tobacco use and high blood glucose.[Citation1] Physical inactivity alone is estimated to account for five million disability-adjusted life years (DALY), i.e. years lost by people living in high-income countries.[Citation1]
It is universally considered to be important to promote physical activity (PA) in general and within the healthcare system in particular. The Swedish National Board of Health and Welfare published national guidelines in 2011 that summarised which methods should preferably be used to change patients' unhealthy lifestyle habits.[Citation3] One method to improve PA behaviour is 'Physical activity on prescription' (PAP).[Citation4] The Swedish model of this method has over the past 10 years been introduced throughout the primary healthcare sector in Sweden and has been adjusted locally.
The Swedish PAP method comprises a written, individualised prescription of PA by a physician or another authorised healthcare professional. It is used primarily for routine care of patients with a condition that can be affected by PA. The method includes five main parts, varying somewhat between areas due to environmental and demographic conditions. The most central element of the method is the patient-centred perspective. This includes factors such as the patient’s health status, previous experience, limitations and strengths, interest and self-efficacy. This is an important part as it has been shown that the physician’s preventive consultations can be challenging and applying patient-centred perspective is central in order to increase the possibility of changing behaviour.[Citation5] The second part is the written prescription, which is based on the patient-centred perspective mentioned above. The third part is the guidebook “Physical activity in the prevention and treatment of disease” (FYSS in Swedish), which summarises the current scientific knowledge of how PA can be used for prevention and treatment, including recommendations for the type and level of activity for each condition.[Citation4] The prescribed physical activity should be individually adapted to each patient and can be either self-monitored or arranged by a public PA organisation. Hence, the fourth part of the PAP model includes cooperation with different activity centres and other PA providers outside the healthcare system. The fifth part of the PAP method is the follow-up, which could vary considerably between areas and healthcare providers. Typically, but not always, the patient is referred to a so-called PAP coordinator, who is usually based at a local training facility and/or primary care site. In most cases the PAP coordinator is responsible for the patient-centred interview, setting goals, motivation, guidance, follow-up and feedback to the prescriber.[Citation6]
The PAP method has previously been evaluated in different Swedish settings [Citation7–10] and has been shown to increase the level of self-reported PA at six and 12 months.[Citation7,Citation11] Improved quality of life (QOL) [Citation7,Citation10] and of the cardio-metabolic risk-marker profile following PAP have also been shown.[Citation12] The Swedish PAP method thus appears to be promising in terms of bringing about behavioural change, resulting in improvement in different health outcomes, at least over a limited period of time. However, many aspects of using the method still remain a challenge, including maintaining behavioural change over a longer period of time. To date, no study has examined the effects of the Swedish PAP system for more than 12 months.
In other countries, including the UK, Spain and Denmark, different exercise referral schemes or exercise interventions have shown some promising but mostly divergent short-term effects on PA level.[Citation13,Citation14] The exercise referral schemes in other countries are not identical to the Swedish PAP system and this makes it difficult to compare the results and particularly draw conclusions with regard to the efficacy of different methods.
In addition to the actual self-reported PA level, the use of a range of surrogate outcome variables is warranted when assessing the efficacy of different methods such as PAP. Thus, quality of life (QOL) is frequently used to evaluate the effects of PA interventions.[Citation15] In the Swedish Björknäs study, several lifestyle behaviours included in PA were targeted. At the 36-month follow-up, it was shown that the intervention was superior to regular care with regard to QOL and cost-effectiveness.[Citation16] In another study conducted in a Swedish primary care setting, a low-cost PAP intervention compared to a more high-cost PAP regime showed promising results for QOL (8) at 12 months. The long-term effects of PAP on QOL need to be studied further.
The primary aim of the present study was to study the self-reported level of physical activity (PA) and quality of life (QOL) in patients receiving physical activity on prescription (PAP) for up to 24 months. Patients receiving PAP regardless of the reason for the prescription were included. A further aim was to explore the effect of PAP on quality of life.
Methods
Overall study design
This is an observational study that includes patients receiving PAP as part of regular primary healthcare. The study was conducted between March 2007 and February 2008 and was performed in the Västra Götaland Region in Sweden, which has a population of ∼1.6 million. Two primary care sites in the region were selected for inclusion (one in a medium-sized town with some rural areas (Site I) and one in a large city (Site II)). An unmatched group of patients seeking primary healthcare was included as a reference group.
The PAP method
The PAP method used by the regular care providers included in this study resembles the general PAP model described in the “Introduction” section. Following the written prescription, the patients are referred to a PAP coordinator, typically a physiotherapist, who conducted a patient-centred interview covering goal setting, level of motivation, regular support and follow-up. The prescribed activity was either performed in the same building as the coordinator (Site II), as part of regular activities outside the coordinator's location or by the patients themselves (Site I).
Study population
The study population comprised patients who were received PAP and attended the subsequent visit to the local PAP coordinator (inclusion criteria). The reasons for PAP referral can be compiled into four groups: (1) musculoskeletal pain, (2) metabolic disorders (which includes diabetes, cardiovascular disease, overweight), (3) psychiatric disorders and 4) other (which included osteoporosis, asthma, chronic obstructive pulmonary disease, vertigo and cancer).
The exclusion criteria were (1) prior PAP, (2) low BMI (<18.5), (3) age >75 years, (4) severe disease that could constitute a possible barrier to physical activity, i.e. major stroke or advanced disability, and (5) impaired vision or language difficulties.
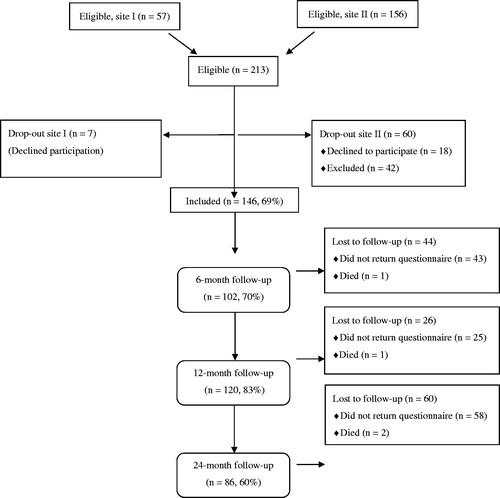
At Site I, 75 prescriptions were written out during the study period. Eighteen of those receiving PAP did not attend the scheduled meeting with the coordinator (two because of their medical condition). The remaining 57 patients were asked if they were willing to participate in the study and if that was the case they gave their informed consent. Seven eventually declined participation and the final study group from the rural site comprised 50 patients or 67% of all patients receiving PAP in the area.
At Site II, the number of prescriptions in the area was not known. A total of 156 patients met the PAP coordinator during the study inclusion period. Forty-two of these met different exclusion criteria: language difficulties (n = 10), low BMI (<18.5) (n = 11), prior PAP (n = 8), severe disease (n = 6), age >75 (n = 5) and impaired vision (n = 2). Of the 114 remaining patients, 18 declined participation and 96 patients gave their informed consent.
The final study population thus consisted of 146 patients, of whom 75% (n = 109) were women. The baseline characteristics of the study population are outlined in . Information regarding inclusion/exclusion is provided in the flow chart ().
Table 1. Baseline characteristics of the patients (n = 146) included in the study who have received PAP.
For patients from Site I, 34 prescriptions were written out by a physician (68%), 13 by nurses and one by a dietician. Information about the prescriber was lacking in two cases. For Site II, 68 prescriptions were made by physicians (71%), 12 by physiotherapists, 13 by nurses and three by dieticians.
Dropout analysis
The proportion of women among those not included for different reasons (74%), or those who did not complete the 24-month follow-up (80%), did not differ from the study group as whole (75%). The mean age did not differ between the group that was included (55 years (SD 11)) in the study and the group that was not included (54 years (SD 13)). The completers at 24 months were significantly older than the group that was included but who did not complete the study (mean age 58 (SD 10) and 51 (SD 12) respectively) (p < .05). The BMI did not differ between the groups (data not shown).
Of the 85 patients who were not included, 28 (33%) had a metabolic disorder-related diagnosis (cardiovascular disease, diabetes and overweight) and 29 (34%) patients were reported to have musculoskeletal pain, 10 had a psychiatric diagnosis, five had other reasons and for the remaining five patients information was lacking. This pattern is slightly different from the patients who were included in the study in that a higher percentage of the patients with a metabolic disorder-related diagnosis entered the study (45%) compared to those who were not included (33%) (p = 0.08).
The pattern with regard to which profession prescribed the PAP was similar for those who were included and those who were not included. The majority (around 70%) of the prescriptions for all groups were written out by a physician, regardless of whether they were included or not and regardless of whether they had completed the study or not (data not shown).
Reference group
A reference group was included, consisting of randomly selected patients who sought medical care at two primary care centres in the same geographical area as the units from where the study participants were recruited. No intention was made to change the PA level in this group. The primary aim of the reference group was to follow the main outcome measures (self-reported PA level and QOL) during the study period in order to detect any general change over time that might have occurred regardless of PAP in a similar primary care population. The questionnaires and the information letter at inclusion were the only diversions from the usual care process for these patients.
The reference group was included on three separate occasions, on two different days in the large city area and on one day in the medium-sized town area. Almost two-thirds of all patients below the age of 75 who visited the primary care centres on these days were asked to participate in the study. In the large city area, 57 out of 71 who were asked (80%) agreed to participate and in the other area, 14 out of a possible 20 agreed to participate (70%). Thirteen patients were then excluded as they met one of the exclusion criteria: Prior PAP (n = 7), low BMI <18.5 (n = 2) and age >75 (n = 4). The reference group thus finally consisted of 58 patients (30 women and 28 men) with a mean BMI of 27 (SD 5.0) (n = 54) and a mean age of 49 years (SD 17). They had a significantly lower BMI and were significantly younger compared to the PAP group. The reasons for their health visits were musculoskeletal pain, a psychiatric condition, cardiovascular disease, infections, blood tests and prophylactic interventions, such as vaccinations. During the study period, four patients received PAP and these patients were excluded from the analysis.
Data collection and questionnaire
The baseline questionnaire was filled in at the inclusion site prior to PAP intervention at the coordinator (study group) or at the primary care centre (reference group). For follow-ups at six, 12 and 24 months post-inclusion, postal questionnaires were sent out to all participants. One reminder was sent out at six months and after two reminders at 12 and 24 months followed by a phone call if necessary. The questionnaire included items about the self-reported level of PA [Citation17,Citation18] and the quality of life assessment described below.[Citation19] Baseline details of age, weight and height were also collected. At the 24-month follow-up, the questions also included marital status and education.
Assessment of the physical activity level
To evaluate the self-reported PA level, we used the validated and extensively used Saltin-Grimby Physical Activity Level Scale (SGPALS). This scale was first developed in the late 1960s [Citation17,Citation20] and it has up till now been used by more than 600,000 subjects, especially in different population studies in the Nordic countries.[Citation21] It has been validated against cardiovascular risk factors, cardiorespiratory fitness and long-term morbidity and it is concluded that both the concurrent and predictive validity of the scale has been shown to be good.[Citation21]
The SGPALS consists of a single question/statement: “How much do you move around and exert yourself physically during your leisure time? If your activity varies greatly between, for example, summer and winter, try to estimate an average. The question refers to the past year”. Four options were given as possible answers, making up the four self-assessed physical activity level groups: (1) Sedentary/Physically inactive (S): Almost completely inactive and only reading, watching television, watching movies, using computers or doing other sedentary activities during leisure time; (2) Some light physical activity (LPA): Physically active for at least four hours per week, such as riding a bicycle or walking to work, walking or skiing with the family, gardening, fishing, playing table tennis, bowling, etc.; (3) Regular physical activity and training (moderate PA): Spending time doing heavy gardening, running, swimming, playing tennis, badminton, calisthenics and similar activities for at least 2–3 hours per week; (4). Regular, strenuous physical training for competitive sports (vigorous PA): Spending time running, orienteering, skiing, swimming, playing soccer, handball, etc., several times per week.
Similar to earlier studies,[Citation18,Citation22] few patients reported the highest level of PA, i.e. “Regular, strenuous physical training for competitive sports” (vigorous PA), and this group was merged with the third group in the present study, forming the previously described moderate-to-vigorous PA group (MVPA).[Citation18]
At 24 months, all the subjects were asked, using a simple question, whether they had increased their PA level irrespective of any change revealed by SGPALS. Four possible answers to the question were available: (a) Yes, very much, (b) Yes, some, (c) No, (d) Don’t know. The participants were also asked to estimate how important the prescription was for their changed PA behaviour. Four different answers were given: (a) Very important, (b) Fairly important, (c) Not so important and (d) Unimportant. These questions were included as a complement to the validated SGPALS and have not been validated.
Quality of life assessment
We used the widely used SF-36 questionnaire to assess quality of life (QOL).[Citation19] This questionnaire has also been used previously to assess the clinical effect of PAP in primary care.[Citation7,Citation10]
SF-36 was developed in English and was translated and validated in Sweden in 1995 [20]. It consists of 36 items. Using the results of the patient’s self-assessment of each item, eight different modalities are calculated: Physical Functioning (PF), Role-Physical (RP), Bodily Pain (BP), General Health (GH), Vitality (VT), Social Functioning (SF), Role-Emotional (RE) and Mental Health (MH). These were presented as a scale score between 0 (worst) and 100 (best). The different modalities of SF-36 are also grouped together to form a Physical Component Summary (PCS) score (consisting of Physical Functioning, Role-Physical, Bodily Pain and General Health), and a Mental Component Summary (MCS) score (consisting of Vitality, Social Functioning, Role-Emotional and Mental Health).
An advocated minimal important difference (MID) of three units in the MCS or PCS score is considered to be a clinical relevant change.[Citation23] Moreover, both component summary scores have a mean of 50, with an SD of 10 in an average US population, and are therefore more useful for comparing different interventions rather than individual subscale scores, which are subject to greater variation.
Statistical analysis
All analyses were performed using SPSS version 22.0 (IBM SPSS Inc., Chicago, IL).
We used McNemar’s test for paired proportions after a dichotomised outcome with regard to the self-reported physical activity level in the study group. All patients who increased their level of self-reported PA were defined as successful and all those who reported a lower level of PA were defined as unsuccessful. We also performed a marginal homogeneity test with all levels of PA included.
For an analysis of quality of life, we used a paired t-test to analyse the mean change from baseline. Only those who had complete data at baseline and follow-up were included in this analysis. To compare the baseline characteristics of the dropouts and completers, we used a Student t-test for numerical data and a chi-square test for categorical data, as we did for a comparison of the reference group versus the PAP group.
Ethical review board
This study was approved by the Regional Ethical Review Board, Gothenburg, Sweden (number 440-06) and all participants signed informed consent before entering the study.
Results
Self-reported physical activity level following PAP
At baseline, 142 patients answered the SGPALS question. Almost a third reported sedentary behaviour, as shown in . Four patients did not report their PA level. At six and 12 months there was a significant improvement in the self-reported PA level and a trend at 24 months (p = .09) ().
Table 2. Self-reported physical activity level according to the Saltin-Grimby Physical Activity Level Scale at baseline and at six, 12 and 24 months (M) after receiving physical activity on prescription.
Looking specifically at the 22 patients who reported sedentary behaviour at baseline, only 11 remained sedentary at 24 months. After 24 months, two of the initially sedentary patients reported MVPA and nine patients reported LPA. Seven patients reported a lower level of PA at 24 months compared to baseline.
When asked specifically at 24 months, 52 (62%) of the patients (n = 84) reported that they had an increased PA level. Of these 52 patients, 35 (67%) rated PAP as very important or important for their change in behaviour. Only one individual rated it as unimportant and 14 rated it as not so important. When analysing the whole group, regardless of whether they had an increased PA level or not, 45 (60%) rated PAP as important or very important (n = 75).
Quality of life at baseline and after intervention
As described in , there were significant changes in the majority of SF-36 subscales at all follow-ups. At 24 months the PCS score was significantly improved but not the MCS.
Table 3. Mean changes in quality of life, measured by using SF-36, from baseline to six, 12 and 24 months follow-up among patients prescribed physical activity.
Looking specifically at the 52 out of 84 study patients who reported an increased PA level during the study, the effect on QOL was greater (data not shown). In these patients, seven out of eight modalities were significantly increased at 24 months compared to baseline, the only exception being Mental Health. No decrease in QOL could be seen in any modality from baseline to 24 months post-intervention.
Self-reported physical activity and QOL in the reference group
The reference group was more physically active and had a higher QOL at baseline than the study group, with only 17% reporting sedentary levels on SGPALS, 66% reporting LPA and 17% reporting MVPA. During the 24-month follow-up period, four patients from the reference group received PAP for clinical reasons. The PA level did not change over the study period in the reference group. The percentage of sedentary patients who remained stable during the whole study period were 17%, 21%, 17% and 22% for the four time points. No significant general change in QOL was seen in this group during the study period ().
Table 4. Mean changes in quality of life from baseline to 24-month follow-up among the reference group.
Discussion
The main findings of the present study are that physical activity on prescription seems to have positive, long-term effects on unselected patients in the clinical primary care setting. Self-reported PA is significantly increased at six and 12 months in patients receiving PAP. However, an observed positive change did not reach significance at 24 months. Furthermore, the significant positive effects on quality of life persisted after 24 months.
These results are in accordance with previous results, showing that the Swedish PAP method results in increased self-reported PA level in patients after both six and 12 months.[Citation11,Citation24] The PAP method seems to be a feasible method of increasing the PA level in a clinical setting. However, all patients do not increase their level of activity and more in-depth analysis, using a larger patient population, should be performed to explore the character and the context of the patients who are not responding. It is known from previous studies that different individuals do need different kind of support to achieve a lifestyle changes and the PAP method might not be sufficient for i.e. patient with low sense of coherence who most probably need another approach to support lifestyle changes.[Citation24]
This study was conducted as part of regular clinical practice and the patients included received PAP for many different reasons. The PA level prescribed is individually adapted and can differ considerably depending on the reason for PAP, individual capacity and the goals that are set. The aim of this study was not to study different diagnoses or the level of PA, but to explore whether the use of the PAP model in regular clinical practice, regardless of the reason for prescription, could influence the self-reported PA level.
This study is one of the several studies that have shown the feasibility of using the Swedish PAP intervention to increase the self-reported level of PA in primary care populations. No increase in the self-reported level of PA was seen in the reference group that did not receive PAP during the same time period and in the same geographical region as the study population. This indicates that no general increase in PA has occurred among the population living in the same area. However, the reference group included was not comparable with the study group, and thus not suitable as a pure control group. Age, the reason for seeking care and above all the PA level differed between the groups at baseline.
Comparing the findings of this study to finding from other countries such as Denmark [Citation26] and the UK [Citation27] is difficult. As pointed out earlier, the Swedish PAP model differs from other methods, which often include referral to a centre for a specified period of training (exercise referral schemes). The Swedish model focuses more on integrating exercise into daily activity by means of patient-centred counselling with scheduled follow-ups in the regular healthcare system and at the PAP coordinator. The PAP model was studied as a whole in the present paper and we had no possibility to analyse the different components that make up the method.
Quality of life improved following PAP. This was already evident after six months and it was even more pronounced after 24 months. We cautiously conclude that this effect could be due to the increased level of PA, especially since the patients with increased PA, had a greater increase in QoL. Further support for this is that no significant change in any aspect of QOL was seen in the reference group during the study period.
In four of the scale scores (PF, RLP, BP, GH), greater effects on QOL were noted compared to those previously reported by Kallings et al. six months post-PAP.[Citation7] An observed increment of 3–5 points for each domain is considered clinically relevant.[Citation16] In this study, the positive changes in all domains except mental health exceeded three points, at the 24 months post-intervention. The improvement in the physical component score (+3.8), for example, is considerably greater than could be seen in the Björknäs lifestyle intervention study (+1.3) (using supervised exercise, diet counselling and group meetings) at 24 months.[Citation16]
Strengths and limitations
One of the strengths of the present study is that it was conducted within the regular healthcare system, thus making the results more externally valid in terms of applicability. A further strength is that by adopting a more conservative approach, using the last observations carried forward amongst dropouts, the results remained close to the improvements shown among the long-term completers. There was a high dropout rate during the study period, which affect generalisability of the results. The dropouts were younger and had a higher age-adjusted BMI at baseline. This group is perhaps in need of more intense intervention and PAP might not be a suitable method to increase the PA level in these patients. Furthermore, relatively few patients completed the study and thus relatively few patients reported increased PA levels at 24 months, making the statistical strength too low to reach any firm conclusions regarding 24 months.
The sample size was too small to conduct any sub-group analysis with regard to diagnosis or sex and with regard to the profession of the prescriber. Some selection bias may also be present in this study, including the awareness of both the patients and the PAP coordinators that they were a part of a study. Furthermore, some of the physicians could have received the information from the patients that they were a part of a study and thus the motivation and support towards the patients could have been affected by this.
Factors other than an increased self-reported PA level could account for the improvement in quality of life. Also, indirect effects could be present as it can be assumed that lifestyle changes, such as an increased PA level, results in several other positive changes in life that could directly or indirectly improve quality of life. However, analysis of the reference group did not reveal any general improvement in quality of life. Another limitation is the possibility of regression toward the mean, i.e. those starting at low QOL might to a greater extent improve over time by themselves, unrelated to the actual intervention. However, since no change in any QOL measure was seen in the reference group, we can cautiously conclude that the improvement seen is at least in some part due to PAP.
Ideally, a randomised, controlled trial should have been performed but this was not feasible. We cannot conclude that the PAP method is superior to other methods, as this was not tested. The intervention group and the reference group were contacted by the research group at the same time intervals and this hopefully rules out any influence the study procedures could have on the participants. Any change in PA or QOL that could have been due to interference with the study group would have been detected in both groups and this does not seem to be the case.
Objective measures with regard to PA would have added some useful information but the self-reported questionnaire used in this study has been well validated and been shown to measure the PA level in relation to both cardiorespiratory fitness and different health measures.[Citation21]
Clinical implications
It seem feasible to use the Swedish PAP method in clinical practice in order to improve PA behaviour in patients with unselected diagnoses. The sustainable effects on the self-reported PA level and quality of life after one and two years, respectively, is a important finding. However, not all patients increase their PA level after receiving PAP and more studies are needed to further explore which methods are suitable for different patients in order to successfully change behaviour and to maintain the change over a longer period of time.
In healthcare, in general, different methods to promote patients' lifestyles, including the PA level, as part of regular treatment are still underutilised. Since 2011, the Swedish National Board of Health and Welfare has recommended the use of individualised prescription, with adjuvants such as pedometers and written prescriptions and with a structured follow-up, in order to increase PA among patients who are insufficiently active.[Citation3] As some scepticism has been reported among physicians,[Citation28] the present findings may also be an important means of motivating healthcare personnel to follow existing recommendations by showing that the method is effective and feasible in clinical practice.
Conclusion
The present study concurs with previous studies that physical activity on prescription increases self-reported physical activity in patients in the clinical primary care setting at both six and 12 months. Moreover, PAP results in long-term improvements in quality of life persisting two years post-prescription for the physical dimension but not for the mental health dimension, thus extending earlier findings. PAP can be used for patients with different diagnoses but more studies are needed to explore why some patients are not responding. Relatively few patients (86/146) were still participating at two years follow-up, thus larger studies are needed to confirm the present findings. In addition, studies with other designs are also warranted, including having a true control group, which is fully comparable to the intervention group.
Acknowledgements
We would like to thank Marita Snällman for her logistical support during the study and Emina Hadzibajramovic for her statistical advice. We would also like to thank the healthcare units for allowing us to include the reference population and all the personnel at the sites responsible for including the patients receiving physical activity on prescription. We are sincerely grateful to the patients who completed the questionnaires.
Disclosure statement
The authors state no conflict of interest. The authors are solely responsible for the content and writing of the paper.
Funding
The project was funded by the Västra Götaland Region.
References
- World Health Organization. Global health risks: mortality and burden of disease attributable to selected major risks. Geneva: World Health Organization; 2009.
- Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260.
- Socialstyrelsen. Nationella riktlinjer för sjukdomsförebyggande metoder 2011. Stockholm: Socialstyrelsen; 2011. p. 002E.
- Swedish Professional Associations for Physical Activity. Physical activity in the prevention and treatment of disease. Stockholm, Sweden: Swedish National Institute of Public Health; 2010.
- Guassora AD, Nielsen SB, Reventlow S. Deciding if lifestyle is a problem: GP risk assessments or patient evaluations? A conversation analytic study of preventive consultations in general practice. Scand J Prim Health Care. 2015;33:191–198.
- Kallings LV, editor. FaR(R) Individanpassad skriftlig ordination av fysisk aktivitet. Östersund: Statens Folkhälsoinstitut; 2011.
- Kallings LV, Leijon M, Hellenius ML, et al. Physical activity on prescription in primary health care: a follow-up of physical activity level and quality of life. Scand J Med Sci Sports. 2008;18:154–161.
- Leijon M. Activating people-physical activity in general population and referral schemes among primary health care patients in a Swedish county [dissertation]. Linköping: Linköping University; 2009.
- Rome A, Persson U, Ekdahl C, et al. Physical activity on prescription (PAP): costs and consequences of a randomized, controlled trial in primary healthcare. Scand J Prim Health Care. 2009;27:216–222.
- Rome A. Prescribed physical activity. A health economic analysis [dissertation]. Lund: University of Lund; 2014.
- Leijon ME, Bendtsen P, Nilsen P, et al. Does a physical activity referral scheme improve the physical activity among routine primary health care patients? Scand J Med Sci Sports. 2009;19:627–636.
- Kallings LV, Sierra Johnson J, Fisher RM, et al. Beneficial effects of individualized physical activity on prescription on body composition and cardiometabolic risk factors: results from a randomized controlled trial. Eur J Cardiovasc Prev Rehabil. 2009;16:80–84.
- Pavey TG, Taylor AH, Fox KR, et al. Effect of exercise referral schemes in primary care on physical activity and improving health outcomes: systematic review and meta-analysis. BMJ. 2011;343:d6462.
- Liira H, Engberg E, Leppavuori J, et al. Exercise intervention and health checks for middle-aged men with elevated cardiovascular risk: a randomized controlled trial. Scand J Prim Health Care. 2014;32:156–162.
- Bize R, Johnson JA, Plotnikoff RC. Physical activity level and health-related quality of life in the general adult population: a systematic review. Prev Med. 2007;45:401–415.
- Eriksson MK, Hagberg L, Lindholm L, et al. Quality of life and cost-effectiveness of a 3-year trial of lifestyle intervention in primary health care. Adv Intern Med. 2010;170:1470–1479.
- Saltin B, Grimby G. Physiological analysis of middle-aged and old former athletes. Comparison with still active athletes of the same ages. Circulation. 1968;38:1104–1115.
- Rodjer L, Jonsdottir IH, Rosengren A, et al. Self-reported leisure time physical activity: a useful assessment tool in everyday health care. BMC Public Health. 2012;12:693.
- Sullivan M, Karlsson J, Ware JE Jr. The Swedish SF-36 health survey–I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Soc Sci Med. 1995;41:1349–1358.
- Rosengren A, Wilhelmsen L. Physical activity protects against coronary death and deaths from all causes in middle-aged men. Evidence from a 20-year follow-up of the primary prevention study in Goteborg. Ann Epidemiol. 1997;7:69–75.
- Grimby G, Borjesson M, Jonsdottir IH, et al. The “Saltin-Grimby Physical Activity Level Scale and its application to health research”. Scand J Med Sci Sports. 2015;25(Suppl 4):119–125.
- Jonsdottir IH, Rodjer L, Hadzibajramovic E, et al. A prospective study of leisure-time physical activity and mental health in Swedish health care workers and social insurance officers. Prev Med. 2010;51:373–377.
- Frendl DM, Ware JE Jr. Patient-reported functional health and well-being outcomes with drug therapy: a systematic review of randomized trials using the SF-36 health survey. Med Care. 2014;52:439–445.
- Kallings LV, Leijon ME, Kowalski J, et al. Self-reported adherence: a method for evaluating prescribed physical activity in primary health care patients. J Phys Act Health. 2009;6:483–492.
- Miettola J, Viljanen AM. A salutogenic approach to prevention of metabolic syndrome: a mixed methods population study. Scand J Prim Health Care. 2014;32:217–225.
- Sorensen JB, Kragstrup J, Skovgaard T, et al. Exercise on prescription: a randomized study on the effect of counseling vs counseling and supervised exercise. Scand J Med Sci Sports. 2008;18:288–297.
- Isaacs AJ, Critchley JA, Tai SS, et al. Exercise Evaluation Randomised Trial (EXERT): a randomised trial comparing GP referral for leisure centre-based exercise, community-based walking and advice only. Health Technol Assess. 2007;11:1–165.
- Persson G, Brorsson A, Ekvall Hansson E, et al. Physical activity on prescription (PAP) from the general practitioner's perspective: a qualitative study. BMC Fam Pract. 2013;14:128.