Abstract
Objective
This Swedish study aimed to assess the prevalence, associated clinical factors, and mortality rates of heart failure patients diagnosed without echocardiograms in both hospital and primary care settings.
Design
We conducted a retrospective population-based study using data from the Region Halland healthcare database in Sweden covering 330,000 residents.
Subjects
From 2013–2019, 3,903 patients received an incidental heart failure diagnosis without an echocardiogram and they were followed for one year.
Main outcome measures
Using logistic and Cox regression analyses, we evaluated the prevalence, clinical characteristics, and all-cause mortality at intervals of 30, 100, and 365 days post-diagnosis.
Results
In this Swedish cohort, the one-year all-cause mortality rate was markedly higher for patients diagnosed in hospitals (42%) compared to those in primary care (20%, p < 0.001). Patients diagnosed in primary care were older and had fewer comorbidities and lower NT-proBNP levels. Hospital-diagnosed patients faced a significantly higher mortality rate in the initial 30 days but saw similar rates to primary care patients thereafter.
Conclusion
In a Swedish region, heart failure diagnoses without echocardiograms were more common in hospitals, and these patients initially faced worse prognoses. After the first month, however, the prognosis of hospital-diagnosed patients mirrored that of those diagnosed in primary care. These findings emphasize the need for improved diagnostic and treatment approaches in both care settings to enhance outcomes.
KEY POINTS
In a Swedish study, 58% of heart failure patients diagnosed without an echocardiogram were identified in a hospital setting. Patients diagnosed in primary care were generally older with fewer comorbidities and lower NT-proBNP levels. The first-year post-diagnosis mortality rate was higher for patients diagnosed in hospitals (42%) compared to those diagnosed in primary care (20%).
Despite a higher initial mortality for hospital-diagnosed patients, the rates became comparable with primary care diagnoses after the first month.
Introduction
Heart failure is a common condition in the western world with a prevalence of approximately 2%, corresponding to 200,000 individuals in Sweden [Citation1,Citation2]. The prevalence of heart failure increases with age, from 1% among 40-year-olds to 10% in individuals aged >75 years [Citation3]. Heart failure has a poor prognosis and entails an increased risk of mortality and need for hospitalization [Citation3–5]. Furthermore, it negatively impacts patient quality of life and is costly for healthcare systems, including primary care, where these patients are often seen [Citation1, Citation4].
To establish a correct diagnosis, it is necessary to determine the extent of left ventricular dysfunction, which is preferably investigated by echocardiography. Based on the ejection fraction (EF), heart failure patients are further classified as one of three phenotypes. Heart failure with reduced EF (HFrEF) and heart failure with mildly reduced EF (HFmrEF) have been shown to benefit from pharmacotherapy with beta blockers (BB), renin-angiotensin-aldosterone-system-inhibitors (RAASi), and mineralocorticoid-receptor-antagonists (MRA) [Citation6]. The recommendation for treating HFmrEF with BB, RAAS inhibitors, and MRA is categorized as a class IIb recommendation, in contrast to HFrEF, where it holds a class 1a recommendation. The European guidelines from 2021 advocate the use of SGLT2 inhibitors for all subtypes of HF. However, these treatments do not show the same positive effect in heart failure with preserved EF (HFpEF) and, since treatment plans are based on heart failure phenotype, determining the patients’ ventricular function is of the utmost importance [Citation6].
Typical symptoms and clinical signs suggestive of heart failure are regarded as non-specific and diagnosing heart failure solely based on these findings is perceived as diagnostically insufficient. A previous study showed that clinical criteria together with the patient’s medical history alone had a limited value and half of the patients with suspected heart failure were incorrectly diagnosed [Citation7]. According to a Spanish study, only half of the patients with heart failure in primary care medical healthcare records have a confirmed diagnosis [Citation8]. Another study has reported that ejection fraction in patients with heart failure is poorly documented in primary care medical records and is associated with poorer prognosis [Citation9]. The importance of echocardiography increases when the patient’s condition is more severe [Citation7]. Although the guidelines regarding heart failure diagnostics are well established, the majority of patients are not properly examined and instead, the diagnosis of heart failure is based on clinical criteria. A previous study has shown that 43% of patients who received heart failure diagnosis never had a diagnostic echocardiography and it was unclear whether those patients were diagnosed in primary care on in hospital [Citation10].
The diagnostic process often begins with a clinical suspicion based on signs and symptoms. Further investigation through clinical examination, ECG and analysis of N-terminal pro brain natriuretic peptide (NT-proBNP) can lead to a diagnosis based on clinical criteria [Citation11–13]. An abnormal NT-proBNP supports the suspicion of heart failure and is often indication enough to proceed with echocardiographic examination. However, elevated NT-proBNP may in some cases be considered equivalent to HF, and clinicians could refrain from performing echocardiography with a subsequent risk of incorrect diagnosis and treatment. There are other conditions and factors which can elevate NT-proBNP even in light of normal cardiac function [Citation14–17].
A previous study based on the Swedish heart failure registry showed that heart failure patients in primary care were older, more commonly women, had a higher occurrence of hypertension and pulmonary disease, and less frequent ischemic heart disease compared with patients registered in conjunction with hospitalization [Citation18]. Echocardiography is generally not available in primary care which could cause a higher number of heart failure patients to be diagnosed based on clinical criteria.
It has not been shown which clinical characteristics are associated with an inadequate diagnosis. The objective of this study was to investigate the prevalence of heart failure patients diagnosed without a diagnostic echocardiogram in hospital care compared to those primary care, the association to clinical factors and mortality.
Materials and methods
This is a retrospective, population-based study of patients in Region Halland who were newly diagnosed with heart failure without a diagnostic echocardiography. Region Halland is located in southwestern Sweden and has an estimated population of 320,000 residents. Within Region Halland, there are three acute care hospitals, 40 inpatient wards, two emergency departments, 30 outpatient specialized clinics and 48 primary care facilities.
Patient data was collected via the Regional Healthcare Information Platform (RHIP), a platform containing complete and comprehensive data from electronic healthcare records from residents of Region Halland. This regards both hospital care and public/private primary care [Citation19]. RHIP contains all clinical investigation results (i.e. laboratory assessments, radiological examinations) and care delivery resources.
Study population
All patients ≥18 years of age within Region Halland, where a heart failure diagnosis according to ICD-10 () was documented in the electronic medical record for the first time during the period 2013–2019. A lookback period from 2008 to 2012 was implemented to ensure a cohort of incidental and newly diagnosed patients, devoid of any previous history of HF upon entry into the study. The study process is displayed in .
Figure 1. A flow chart illustrating the inclusion of heart failure patients without echocardiogram from the onset heart failure and the follow-up period of one year during the period 2013–2019.
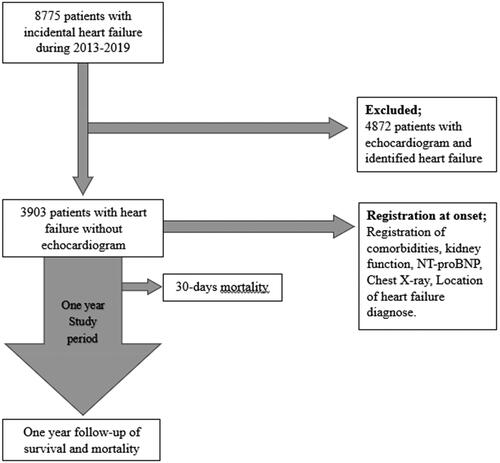
Table 1. Baseline characteristics and distribution of patients first diagnosed with HF in hospital care and primary health care.
The main inclusion criterion was a first-time diagnosis of heart failure according to the ICD-10 code () without an echocardiogram to determine the ejection fraction at the time of diagnosis. Echocardiography performed greater than one year from the date of diagnosis was considered clinically irrelevant and as such these patients were included as heart failure without diagnostic echocardiography. In clinical practice, patients presenting unexplained deterioration as a result of suspected heart failure and a current echocardiogram i missing, a new echocardiogram should be should be considered. During the study period, the waiting time for echocardiography in Region Halland was one to three months.
The patients must have received their care within Region Halland. There were 115 patients who had no contact with regional healthcare during 2013 and beyond, and these patients were subsequently excluded as they were considered to have received care outside of Region Halland or incorrectly diagnosed. In total, 8775 patients were diagnosed with heart failure during 2013-2019 and of these, 3903 (44%) had not undergone an echocardiography. All data were collected within the RHIP database, starting from the initial diagnosis and extending up to one year.
Study process
The variables collected were sex, age, and comorbidities according to ICD-10 (). The level of healthcare in which the first heart failure diagnosis appeared was recorded and further divided into primary care and hospital care. Hospital care is presented in total and further divided into emergency department, hospital inpatient care and hospital outpatient care. NT-proBNP and eGFR were documented.
NT-proBNP levels were assessed based on new onset of heart failure symptoms and the values registered were those closest to the initial heart failure diagnosis date but not older than three months. The NT-proBNP values were divided into three groups to determine the likeliness that they were associated with heart failure. Those with normal NT-proBNP levels were defined as “heart failure unlikely” and an elevated value was defined either as “grey zone” or “heart failure likely” based on age [Citation16,Citation17, Citation20]. Patients with NT-proBNP <300 pg/mL are unlikely to have heart failure, regardless of age. The "grey zone" is defined as NT-proBNP 300-450 pg/mL for patients aged <50 years, 300-900 pg/mL for patients aged 50-75 years and 300-1800 for patients >75 years. An NT-proBNP >450 pg/mL for patients <50 years, >900 pg/mL in patients aged 50-75 years and >1800 pg/mL in patients aged >75 years is considered to likely be associated with heart failure. This is shown in detail in .
Table 2. Illustrates a Cox regression for 30-days, 100-days and 365-days all-cause mortality adjusted for age groups, diagnosed in primary care versus hospital care, eGFR, NT-proBNP levels and comorbidities.
Kidney function was assessed with the available eGFR (ml/min/1.73 m2) closest to index [Citation21]. Based on eGFR, kidney function was defined as either normal with eGFR ≥60 ml/min, lowered with eGFR 30-59 ml/min or impaired with eGFR <30 ml/min.
Statistics
Descriptive statistics were performed to describe the population. Categorical data were analysed using Chi-2 tests and Student t-tests were used when comparing continuous data. Kruskal-Wallis tests were applied to compare mean values between multiple groups.
The patients were categorized based on where they first received the heart failure diagnosis, distributed into primary or hospital care. Hospital care was further divided into ED, hospital inpatient care and hospital outpatient care.
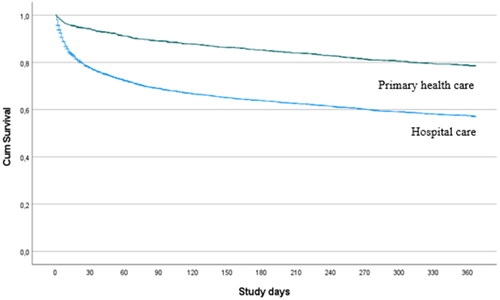
One-year all-cause mortality is presented in a Kaplan-Meier curve separated for hospital and primary care.
NT-proBNP was divided into normal and elevated values at the time of diagnosis [Citation17, Citation20]. This division of NT-proBNP is age-controlled according to a validated model. Kidney function was divided into normal with an eGFR ≥60 ml/min, lowered with eGFR 30-59 ml/min or impaired with eGFR <30 ml/min [Citation21]. Since kidney dysfunction is known to elevate NT-proBNP, a correlation analysis was performed in order to determine if kidney dysfunction was a potential cause of falsely elevated NT-proBNP levels. A logistic regression analysis was performed to determine the odds ratio that patients were diagnosed in hospital- or primary care. Analyses were performed to examine the clinical characteristics of the two groups and adjusted for age, renal function, NT-proBNP and presence of ischemic heart disease, cerebrovascular disease, diabetes and COPD. Survival was analysed using unadjusted and adjusted Cox regression models at intervals of 30-, 100-, and 365-days after diagnosis. The models were adjusted for age, primary care diagnosis, eGFR, NT-proBNP, ischemic heart disease, cerebrovascular stroke, atrial fibrillation, diabetes, and chronic obstructive pulmonary disease (COPD). A p-value <0.05 is considered significant. Data was processed using IBM SPSS Statistics 27, Armonk, New York, USA.
Ethical considerations
The study was approved by the Swedish Ethical Review Board, Stockholm Department 2 Medicine, and registration number 2020-00455. Since this was a retrospective study, an informed consent was waived, and the study procedure was granted approval by the Swedish Ethical Review Board. All methods and procedures in this study were conducted in accordance with relevant guidelines and regulations.
Results
A total of 3903 patients were included in the study. Of these, 2272 (58%) were first diagnosed with HF in hospital with hospital inpatient care accounting for 82%, followed by emergency department (10%) and hospital outpatient care (8%). The remaining 1631 (42%) patients were first diagnosed in primary care. The distribution and baseline characteristics are illustrated in . A total of 373 patients, ultimately diagnosed with HF, had previously undergone a normal echocardiogram more than 12 months prior, but did not receive a HF diagnosis in conjunction with the examination. Of these, 202 (54%) where later diagnosed in primary care and 171 (46%) where diagnosed in hospital.
A Spearman correlation analysis between NT-proBNP and eGFR resulted in a correlation of 36% (p < 0.001).
A total of 1273 (33%) patients died within a year of receiving a heart failure diagnosis, 42% of which were diagnosed in hospital care and 20% in primary care. Mortality over time for patients diagnosed in hospital care and primary care is presented in . In the study cohort, 598 individuals (15%) died within 30 days of the initial diagnosis of heart failure. Among the deceased patients diagnosed, 534 were diagnosed with heart failure in hospital care, corresponding to a 30-day mortality rate of 22%. The remaining 112 patients had been diagnosed in primary care resulting in a 30-day mortality rate of 6% (p < 0.001).
Figure 2. Cumulative survival from index for patients first diagnosed in hospital and in primary health care.
A logistic regression analysis was performed to assess the clinical characteristics of patients diagnosed without echocardiography, using those diagnosed in primary care as the reference group. Patients diagnosed in primary care were more commonly of age >75 years, had lower NT-proBNP levels, less kidney dysfunction and fewer comorbidities compared to patients diagnosed in hospital. The results are shown in .
A Cox-regression analysis for mortality adjusted for age, level of healthcare, eGFR, NT-proBNP levels, and comorbidities is shown in . Advanced age, impaired renal function and high levels of NT-proBNP were associated with higher mortality.
Table 3. Illustrates a logistic regression for diagnosed in primary care adjusted for age groups, eGFR, NT-proBNP levels and comorbidities.
Discussion
In the present study, 58% of heart failure patients diagnosed without echocardiography were diagnosed in hospital care, of which the majority were diagnosed while admitted to hospital. Patients diagnosed in primary care were more likely to be older than 75 years while those diagnosed in hospital care were more likely to have elevated NT-proBNP values and more commonly occurring chronic comorbidities including ischemic heart disease, cerebrovascular disease, diabetes and COPD. Patients diagnosed in hospital care had a one-year all-cause mortality of 42% compared to only 20% for those diagnosed in primary care. The all-cause mortality was highest within 30-days of the initial diagnosis and was particularly evident amongst patients diagnosed in hospital care. After the first month, the mortality rate stabilized and was similar regardless of where the patient was diagnosed.
In primary care, accessibility to echocardiography is limited and could potentially explain a diagnosis of HF without echocardiography. Surprisingly, however, the majority of patients diagnosed with HF without echocardiography in this study were diagnosed in hospital care, despite a relatively high accessibility. Furthermore, in cases where heart failure patients are referred from hospital care to primary care for follow-up, the diagnosis has been set and a pharmacotherapy plan has often already been initiated, thus limiting the primary care physician’s ability to commence an investigation that would include diagnostic echocardiography.
In the present study, 43% of patients were diagnosed without echocardiography, which is substantial when compared to a previous study that showed between 26-30% with echocardiogram [Citation18]. The high portion of patients diagnosed without echocardiography in the previous study may reflect the comprehensiveness of this cohort, which includes individuals from an unselected pool from the total population. In contrast, heart failure registry studies primarily focus on selecting patients specifically diagnosed with heart failure. Patients diagnosed in primary care were generally older, had fewer comorbidities and were more likely to receive recommended heart failure pharmacotherapy compared to those diagnosed in hospital care according to the Swedish Heart Failure Registry study [Citation18]. A previous study also showed that primary care-based patients were older and had a decreased incidence of ischemic heart disease, but a higher incidence of hypertension [Citation2]. In the present study, the presence of age-related comorbidities such as ischemic heart disease, CVI, diabetes and COPD was lower in primary care patients compared to those diagnosed in hospital care [Citation2].
The results of this study show that patients diagnosed with heart failure in primary care have fewer comorbidities compared to those diagnosed in hospital care. A previous study based on the Swedish heart failure registry showed that patients in primary care had a higher degree of comorbidity compared to hospital care [Citation22]. However, the study was aimed at patients diagnosed with HFpEF, while patients in the current study were diagnosed with heart failure without echocardiography and thus have no defined phenotype, potentially giving rise to greater heterogeneity within the study population.
Mortality within 30 days of diagnosis was 22% amongst patients diagnosed in hospital care compared to just 6% for those diagnosed in primary care. This discrepancy is consistent with other studies, which also show a lower all-cause mortality in primary care-based populations [Citation2, Citation23]. Patients diagnosed in hospital had a higher incidence of comorbidity, which may reflect the severity of illness and thus the higher 30-day mortality rate. In such instances, it may be justifiable to refrain from further diagnostic procedures, including echocardiography. However, beyond 30 days, the mortality rate stabilizes and essentially mirrors that of patients diagnosed in primary care, making the decision to refrain from diagnostic echocardiography, recommended by current guidelines, more difficult to explain. Most likely, several of these patients have been diagnosed with heart failure based on clinical criteria, NT-proBNP or chest X-ray, and there is a risk that physicians settle for this. It is possible that the decision to not perform echocardiography is due to increased frailty, which is not investigated in this study. However, even elderly and frail patients benefit from pharmacotherapy and the choice of pharmacotherapy is based on determining the heart failure phenotype through correct diagnostic procedure.
Limitations
In this study, comorbidity is present to a lesser extent among patients diagnosed with heart failure in primary care. Incidentally, the use of ICD diagnoses is also lower in primary care compared to hospital care. The difference is possibly the result of the reimbursement system being based on diagnoses in hospital care but not in primary care. At the same time, the ICD diagnoses for the comorbidities are obtained during the period from 2013 to 2019 and can be registered both in hospital care and primary care, independently. Therefore, this difference should have limited impact on the result.
In the results, it can be noted that NT-proBNP and chest X-rays occur to a greater extent in the hospital care cohort. NT-proBNP is more quickly analysed and chest X-ray more readily available in hospital care, which arguably increases the likelihood that these diagnostic steps be performed. It could be argued that increased NT-proBNP levels may be affected by kidney dysfunction, but the impact is assessed as limited since the Spearman correlation was 36%.
Patients with heart failure and chronic obstructive pulmonary disease have similar symptoms and functional level [Citation24]. Undeniably, there is an obvious risk that patients with these symptoms who have not undergone an echocardiogram may be misdiagnosed. The actual reason why echocardiography has not been performed cannot be determined and the explanation is likely to be multifactorial. The results shown are part of an observational study, meaning they cannot be considered conclusive or causal, rather are limited to illustrating associations.
Conclusion
Heart failure patients diagnosed without echocardiography were more commonly seen in hospital care andwas associated with a poorer prognosis compared to patients diagnosed in primary care. Patients with a clinical heart failure diagnosis in primary care were associated with older age but with fewer comorbidities including COPD and cardiovascular disease burden. Mortality among patients diagnosed in hospital care was highest within 30 days of diagnosis and thereafter compared similarly to patients diagnosed in primary care. In patients with an expected survival of more than one month, a diagnostic echocardiography should be considered to determine the heart failure phenotype and enable administration of appropriate pharmacotherapy.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Yasin ZM, Anderson PD, Lingman M, et al. Receiving care according to national heart failure guidelines is associated with lower total costs - an observational study in region Halland, Sweden. Eur Heart J Qual Care Clin Outcomes. 2021;7(3):280–286. doi: 10.1093/ehjqcco/qcaa020.
- Jonsson Å, Edner M, Alehagen U, et al. Heart failure registry: a valuable tool for improving the management of patients with heart failure. Eur J Heart Fail. 2010;12(1):25–31. doi: 10.1093/eurjhf/hfp175.
- Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics–2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113(6):e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600.
- Agvall B, Paulsson T, Foldevi M, et al. Resource use and cost implications of implementing a heart failure program for patients with systolic heart failure in Swedish primary health care. Int J Cardiol. 2014;176(3):731–738. doi: 10.1016/j.ijcard.2014.07.105.
- Zarrinkoub R, Wettermark B, Wändell P, et al. The epidemiology of heart failure, based on data for 2.1 million inhabitants in Sweden. Eur J Heart Fail. 2013;15(9):995–1002. doi: 10.1093/eurjhf/hft064.
- McDonagh T, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur Heart J. 2021;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368.
- Fonseca C, Morais H, Mota T, EPICA Investigators, et al. The diagnosis of heart failure in primary care: value of symptoms and signs. Eur J Heart Fail. 2004;6(6):795–800. doi: 10.1016/j.ejheart.2004.08.002.
- Verdú-Rotellar JM, Frigola-Capell E, Alvarez-Pérez R, et al. Validation of heart failure diagnosis registered in primary care records in two primary care centres in Barcelona (Spain) and factors related. A cross-sectional study. Eur J Gen Pract. 2017;23(1):107–113. doi: 10.1080/13814788.2017.1305104.
- Muñoz MA, Mundet-Tuduri X, Real J, et al. Heart failure labelled patients with missing ejection fraction in primary care: prognosis and determinants. BMC Fam Pract. 2017;18(1):38. doi: 10.1186/s12875-017-0612-6.
- Davidge J, Ashfaq A, Ødegaard KM, et al. Clinical characteristics and mortality of patients with heart failure in Southern Sweden from 2013 to 2019: a population-based cohort study. BMJ Open. 2022;12(12):e064997. doi: 10.1136/bmjopen-2022-064997.
- Stoupakis G, Klapholz M. Natriuretic peptides: biochemistry, physiology, and therapeutic role in heart failure. Heart Dis. 2003;5(3):215–223. doi: 10.1097/01.HDX.0000074517.30102.64.
- Ewald B, Ewald D, Thakkinstian A, et al. Meta-analysis of B type natriuretic peptide and N-terminal pro B natriuretic peptide in the diagnosis of clinical heart failure and population screening for left ventricular systolic dysfunction. Intern Med J. 2008;38(2):101–113. doi: 10.1111/j.1445-5994.2007.01454.x.
- Costello-Boerrigter LC, Boerrigter G, Redfield MM, et al. Aminoterminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2006;47(2):345–353. doi: 10.1016/j.jacc.2005.09.025.
- Jernberg T, Stridsberg M, Lindahl B. Usefulness of plasma N-terminal proatrial natriuretic peptide (proANP) as an early predictor of outcome in unstable angina pectoris or non-ST-elevation acute myocardial infarction. Am J Cardiol. 2002;89(1):64–66. doi: 10.1016/s0002-9149(01)02166-x.
- Redfield MM, Rodeheffer RJ, Jacobsen SJ, et al. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002;40(5):976–982. doi: 10.1016/s0735-1097(02)02059-4.
- Anwaruddin S, Lloyd-Jones DM, Baggish A, et al. Renal function, congestive heart failure, and amino-terminal pro-brain natriuretic peptide measurement: results from the ProBNP investigation of dyspnea in the emergency department (PRIDE) study. J Am Coll Cardiol. 2006;47(1):91–97. doi: 10.1016/j.jacc.2005.08.051.
- Januzzi JL, Chen-Tournoux AA, Christenson RH, et al. N-terminal pro-B-type natriuretic peptide in the emergency department: the ICON-RELOADED study. J Am Coll Cardiol. 2018;71(11):1191–1200. doi: 10.1016/j.jacc.2018.01.021.
- Dahlström U, Håkansson J, Swedberg K, et al. Adequacy of diagnosis and treatment of chronic heart failure in primary health care in Sweden. Eur J Heart Fail. 2009;11(1):92–98. doi: 10.1093/eurjhf/hfn006.
- Ashfaq A, Lönn S, Nilsson H, et al. Data resource profile: regional healthcare information platform in Halland, Sweden. Int J Epidemiol. 2020;49(3):738–739f. doi: 10.1093/ije/dyz262.
- Mueller C, McDonald K, de Boer RA, Heart Failure Association of the European Society of Cardiology, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019;21(6):715–731. doi: 10.1002/ejhf.1494.
- Nyman U, Grubb A, Larsson A, et al. The revised Lund-Malmö GFR estimating equation outperforms MDRD and CKD-EPI across GFR, age and BMI intervals in a large Swedish population. Clin Chem Lab Med. 2014;52:815–824.
- Verdu-Rotellar JM, Vaillant-Roussel H, Abellana R, et al. Precipitating factors of heart failure decompensation, short-term morbidity and mortality in patients attended in primary care. Scand J Prim Health Care. 2020;38(4):473–480. doi: 10.1080/02813432.2020.1844387.
- Agvall B, Alehagen U, Dahlström U. The benefits of using a heart failure management programme in Swedish primary healthcare. Eur J Heart Fail. 2013;15(2):228–236. doi: 10.1093/eurjhf/hfs159.
- Giezeman M, Theander K, Zakrisson AB, et al. Exploration of the feasibility to combine patients with chronic obstructive pulmonary disease and chronic heart failure in self-management groups with focus on exercise self-efficacy. Scand J Prim Health Care. 2022;40(2):208–216. doi: 10.1080/02813432.2022.2073961.

