Abstract
Objective
To investigate if wearing surgical face mask by doctors and nurses during suturing of traumatic wounds has any impact on postoperative infection rate.
Design
Randomized controlled study with masked or unmasked health personnel groups.
Setting
A Norwegian Minor Injury Department.
Subjects
Adult patients with traumatic wounds sutured at the clinic between 7 October 2019 and 28 May 2020.
Main outcome measures
Postoperative infections of sutured wounds.
Results
One hundred and sixty-five patients with 176 wounds were included in the study. Nine out of 88 wounds (10.2%) in the masked group and 11 out of 88 wounds in the unmasked group (12.5%) had a wound infection.
Conclusions
Despite a higher percentage of postoperative infections in the unmasked than in the masked group (12.5% versus 10.2%), the difference was not statistically significant (p = .6). This might imply that the use of facemasks during suture of traumatic wounds in an outpatient setting does not significantly reduce the number of infections. However, due to the covid pandemic, the study had to be prematurely stopped before the planned number of participants had been recruited (n = 594). This increases the risk of type II error.
Key points
Few studies from hospital setting have found significant difference in postoperative wound infections if surgical face masks were worn or not during surgery.
High quality studies about face masks and wound infections from primary care are lacking.
This randomized study at a minor injury department outside hospital found no significant difference in frequency of postoperative wound infection if health personnel had worn surgical face masks or not while suturing traumatic wounds.
Introduction
Late in the nineteenth century, Mikulicz described the wearing of face mask to help control surgical sepsis [Citation1]. A study of more than 1000 photographs of surgeons in European and U.S. operating hospitals indicated that almost all of them wore masks by 1935 [Citation2]. The use of face masks is now standard procedure in operating theatres all over the world. They are also widely used during operative procedures in an outpatient setting. Presently, there are no national guidelines in Norway concerning the use of face masks while suturing traumatic lacerations in primary health care. An investigation done by the Norwegian Research Centre (NORCE) at Norwegian Accident and Emergency departments (A&Es) in 2015 showed that approximately 60% wore face masks after individual assessments of the traumatic laceration and patient characteristics. The use of face masks was part of the general routines in 38% of A&Es in the major Norwegian cities, while 5–6% used face masks routinely in medium and small A&Es [Citation3].
Few studies have investigated if the use of surgical face masks or not influence the postoperative infection rate [Citation4–8]. The aim of our study was to investigate if wearing of surgical face masks by health personnel during suturing of traumatic lacerations in an A&E outside hospital had any significant impact on the frequency of postoperative wound infection.
Materials and methods
Study design
This study is a single blinded randomized controlled clinical trial (RCT). The study personnel assessing postoperative infection at follow-up, did not know if the patient’s wound had been treated and sutured by masked or unmasked health personnel.
Our planned design was a non-inferiority study. Due to the SARS-Cov-2 pandemic, surgical face masks during all close contacts with patients were recommended and the study had to be prematurely stopped in May 2020.
However, we found that our available data material was sufficiently large, and the hypothesis important enough to undergo further analysis. Unfortunately, the preconditions for a non-inferiority analysis had substantially changed due to the number of participants in our study. We therefore changed the analysis to a 0-hypothesis testing, assuming no difference in postoperative wound infection frequency with or without face mask worn by health personnel during suture of traumatic wounds.
Calculation of statistical power
A previous study from Bergen A&E found the prevalence of clinically significant infections in sutured wounds was 4% [Citation9]. Based on this assumed frequency of infections in both study arms and non-inferiority limit of 4, we calculated we needed 297 patients in each of the two groups to reach a power of 80% and a significance level of .025.
However, we included only 165 patients before the study was stopped due to the covid pandemic.
Setting
The study was conducted at Bergen Minor Injury Department (MID), a major A&E department in Norway. This clinic treats almost all minor injuries in Bergen, not in need of hospitalization. The staff consists of primary health care physicians. Annually, 1000–1500 wounds are treated with suture at the MID. It is standard procedure to use face masks while suturing traumatic laceration. The department is closed during night-time (from 10.30 pm to 8.30 am). The study period was from 7 October 2019 to 28 May 2020.
Study population
All adult patients (16 years or older) with a traumatic wound sutured at the MID were eligible for inclusion. Exclusion criteria were: Patients unable to give informed consent, patients admitted to hospital, patients with an infectious disease that could transmit to health personnel, bite injuries, unable to understand Norwegian or English, address outside Norway, patients with diseases that increase wound infection risk (such as diabetes, active cancer or other immunodeficiencies), use of immunosuppressant drugs (such as corticosteroids, cytostatica or other immunosuppressive medication), use of oral antibiotic within the last seven days prior to the laceration and patients who received prophylactic antibiotics immediately after wound closure.
Intervention and randomization
Study participants decided to participate after written and oral information about the study. They filled out a semi-structured questionnaire about wound cause, time since injury, personalia, chronic diseases, regular medication or use of any antibiotics within the last week. Health personnel then filled out information about wound location, depth, length, underlying tissue affected, and number of cutaneous and subcutaneous sutures used. The study participants were block randomized in groups of 10 with the use of Sealed Envelope Ltd (London, UK) [Citation10] into two groups:
Masked group: Both doctor and nurse used primarily surgical face masks type IIR, and less frequent type II, during the procedure (type II surgical masks have a bacterial filtration efficiency of ≥98%, and the R signifies splash resistance).
Unmasked group: Neither doctor nor nurse used face masks during the procedure.
Closed envelopes, with the assigned intervention according to the randomization program (masked or unmasked), were used. All other procedures followed the established procedures at the MID and were identical for both groups, including use of operation caps, sterile gloves and fenestrated drapes. Wound management and preparation were done according to the MID’s own method-book, based on the Norwegian A&E manual [Citation11].
Infection criteria
To evaluate wound infection parameters, we used the same definition of wound infection as used by a former study at the MID (former Bergen A&E) that investigated infections in wounds sutured at the A&E in 2011 [Citation9]. This definition is based on an internationally applied and validated classification system to grade wound infection and have been used in other studies at emergency departments in the U.S. and U.K. [Citation12,Citation13]. This definition classifies postoperative infections as follows:
0 – no sign of infection;
1 – simple stitch abscesses;
2 – surrounding cellulitis >1 cm;
3 – accompanying lymphangitis or lymphadenitis;
4 – systemic symptoms.
We considered infection grade 2–4 as clinically relevant and defined these groups as an infection in our study. Patients who received systemic antibiotics after a doctor’s visit due to symptoms of infection after primary suture, were also included in the study as a clinically relevant infection.
Evaluation by health personnel at the minor injury department or by phone
If the stitches were removed at the MID, the health or study personnel filled out a standardized questionnaire about infection grade. If the stiches were removed elsewhere, the study personnel contacted the patients by phone 14–28 days after the primary contact and registered wound infection data on the same questionnaire. The use of local or systemic antibiotics or any visits to a medical doctor due to suspected infection was also registered.
Data analysis
All collected parameters from the questionnaire were registered on registration forms without any patient sensitive information. If any specific parameters were missing, we searched the Electronic Patient Journal (EPJ) for the missing information. The data were then transferred to and analyzed with SPSS statistical software version 26/27 (SPSS Inc., Chicago, IL).
Statistics
A significance level of 5% was chosen (p < .05). We used Pearson’s Chi-square test in the data analysis.
Ethics
The study was approved by the regional ethical committee (REK Nord, ref. nr: 2019/1145), the Data Protection Officer in Helse Bergen (ID: 1173, 2019) and the clinical director of the MID.
Results
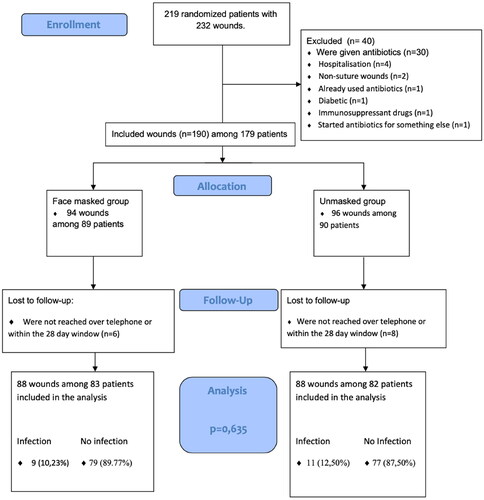
A flowchart of the study is shown in . We included a total of 165 patients with 176 wound injuries. Both allocation groups contained 88 wounds, distributed among 83 patients in the masked group and 82 patients in the unmasked group. Seventy-two percent were men and 28% women. The mean age for men was 38 (SD: 15) years and for women 38 (SD: 17) years. The differences between patient characteristics in the two randomized groups were small (). Apart from more patients using regular medication in the unmasked than the masked groups, the two patient groups were quite similar. Likewise, wound characteristics, including mechanism of injury, time from injury to suturing and number of sutures differed little between the two groups (). However, more patients had head wounds and deeper wounds, and less patients had hand and wrist wounds and squeeze wounds in the masked group compared to the unmasked group ().
Table 1. Patient characteristics of 165 patients in the masked (n = 83) and unmasked groups (n = 82).
Table 2. Wound characteristics, number of sutures, injury mechanism and time from injury to treatment among 176 wounds in the masked group (n = 88) and the unmasked group (n = 88).
Eleven of 88 wounds treated in the unmasked group (12.5%) and nine of 88 in the masked group (10.2%) fulfilled the study definition of a clinically relevant wound infection (). The difference between the groups was not statistically significant (p = .6).
Table 3. Postoperative infections (number and percentage) among 176 wounds in the masked group (n = 88) and the unmasked group (n = 88).
Postoperative infection was assessed by telephone for 95% of the patients (157 out of 165).
A total of 14 patients out of 179 patients (7.8%) were lost to follow up, because they were not reached by telephone at all or within the defined timeframe of 28 days from time of suturing.
Discussion
Main principal findings
To our knowledge, this is the first randomized Norwegian study to examine the postoperative wound infection rate in relation to health personnel’s use or no use of face masks while suturing traumatic lacerations in an outpatient setting. We found more infections in the unmasked group compared to the masked group (12.5% versus 10.2%), but this difference was not significant (p = .6).
Effect of differences between the allocation groups
In the unmasked group, we found substantially more patients on regular medication, fewer head lacerations and more squeezing injures, all with the potential to increase the risk of wound infection, but also fewer deep wounds, lowering the risk of wound infection. All these factors could, in theory, influence the infection rate and render comparison between the groups difficult.
Strengths and weaknesses of the study
Our study was a RCT with block randomization in groups of 10, which made it possible to ensure adequate group allocations despite the need to end the study earlier than primarily planned. It was blinded at follow up both for the health personnel at the MID assessing infection at stitch removal there, and also for the authors when they asked about patient self-assessment of infection by telephone when stiches had been removed elsewhere. Follow up by telephone were done by the same three authors (KSae, SH, KSt) using standardized questions. Loss to follow-up was quite limited (7.8%).
Most patients (95%) did not remove their stitches at the MID and were therefore not assessed for infection by the MID’s health personnel. They were asked standardized questions about objective signs of wound infection over telephone within 28 days, entirely relying on the patient’s self-assessment. By contacting them shortly after suture removal and using questions easy to answer, it is reasonable to assume that we avoided (some) recall biases.
The main weakness of the study was the small sample size, which reduces its power and increases the risk of type II error. The study had to be prematurely stopped due to the covid pandemic and thus by a cause completely unrelated to the study itself, rendering the risk of bias in the sampled groups unlikely.
Wound injuries treated at night in the A&E were not included in this study and may have caused a selection bias by not including many wounds acquired by violence or patients under the influence of drugs and alcohol.
We failed to initially exclude five patients that did not meet the inclusion criteria before they were randomized; respectively one patient with diabetes, one on immunosuppressive medication, one on antibiotics and two patients with wounds that did not need suturing. Later, they were fortunately excluded before the study personnel had been un-blinded. Patients who received prophylactic antibiotics or were hospitalized after inclusion and randomization were excluded at follow-up.
Findings in relation to other studies
Face masks and hospital
Few previous studies, nearly all of them done in hospitals, have examined if the use of face masks affects post-operative infection frequency [Citation4–8].
This is a prospective study from 1980 by Orr from a general surgical ward performing only planned surgery such as prostatectomies, gastrostomies, hernia operations, etc. Only operations involving skin incisions were included (n = 432). Face masks were discarded by all personnel in the operating theatre for 6 months. The previous years, when face masks were routinely used, were control groups. Orr found a 50% reduction in postoperative infection rate (1.8% versus 3.7 to 5.4%) when face masks were not used [Citation4].
A few years later, an attempt to reproduce these findings was done at a gynecological ward. The patients were operated alternatively by masked or unmasked personnel. This prospective study was stopped after the recruitment of 41 patients due to the high number of infections in large-scale operations such as hysterectomies in the unmasked group (three of five) versus the masked group (0 of 4). However, smaller operations such as curettage and laparoscopy, showed no significant differences between the masked and unmasked group [Citation5].
A large prospective study from a surgical department in Sweden (Danderyd Hospital) including 3088 patients, found no statistical difference in infection rates between masked (4.7%) and unmasked groups (3.5%) during operations. The use of face masks during operations was pseudo-randomized by whole weeks. Both emergency operations and elective operations were included, while outpatients, orthopedic patients and urological patients were excluded [Citation6].
Webster et al. did a randomized controlled study at a tertiary hospital in Australia to investigate if the use or not of face masks by un-scrubbed personnel at the operating theatre had any significant influence on postoperative infection rate. This study included patients undergoing both emergency and elective obstetric, gynecological, orthopedic, breast and urological surgery in 2007 and 2008. A total of 811 patients completed the study. The infection rate was 11.5% in the masked group and 9.0% in the unmasked group. The difference was not statistically significant [Citation7].
On the other hand, a prospective case–control study from the U.K. done over a 12-month period in 1999–2000, investigating possible risk factors for endophthalmitis after cataract surgery (cases: N = 214, controls: N = 445) found that not wearing a face mask in the operating theatre increased the risk of endophthalmitis significantly [Citation8].
The studies differ both in quality, design and results. Therefore, it is difficult to draw any firm conclusions on the effect of surgical face masks on the frequency of postoperative infections. We think it is not possible to give any recommendations.
Face masks, emergency room and primary care
The use of face masks was probably transferred from surgical procedures at hospitals to emergency rooms and primary care, without any assessment of necessity. We have found only two studies looking into this, both from emergency rooms in the U.S., and none from general practice. One study was small (n = 92), unblinded and done by the same physician using alternatively face masks or not when suturing traumatic lacerations. He found no significant difference in infection frequency between the two groups [Citation14].
Another pseudo-randomized study from a U.S. emergency department examined if the use of both face masks and caps (n = 239) or not (n = 203) by the physicians during suturing wounds at the emergency department influenced the frequency of postoperative infections. This study found no significant difference in postoperative infection rate between the patients operated with no face masks and caps (2.5%) versus facemasks and caps (3.9%) [Citation15].
Both studies are quite old and are of poor scientific quality, and probably cannot be used as a basis for recommendations on use of face masks or not in primary care.
Face masks, different indications and complications
Surgical face mask was originally developed to minimize transmission of oro- and nasopharyngeal microorganism from operating theatre staff to the patient to prevent surgical site infections (SSIs). Splash resistant surgical face masks type IIR has also been thought to protect the surgical staff from infected blood- and body fluids from the patient [Citation16]. Orr states that the effectiveness of a face mask depends on its material, how you wear it and its shape [Citation4]. The face mask’s ability to filter efficiently also involves the length of time it can maintain this efficiency. Earlier studies recognized that wet masks become ineffective, and should be changed after a given time [Citation1].
Schweizer showed that the use of a tightly molded face mask significantly resulted in more bacterial growth than the use of a filter mask (p = .011). This higher infection rate could be explained by the assumed higher risk of friction between the mask and a wearers skin, resulting in more skin cells falling and contaminating the wound [Citation4,Citation16].
Wound infections
Presently, there is no generally accepted definition of post-operative wound infection. Different criteria have been used to define wound infection in research studies, ranging from doctors’ prescription of antibiotics [Citation17] wound characteristics [Citation13] or positive bacterial culture [Citation4], to some studies using a combination of these methods [Citation7,Citation17]. We chose to use the same wound infection definition as used in former A&E studies from Norway, U.S.A. and U.K., that found infection rates varying from 3.4% to 15% [Citation9,Citation12,Citation13,Citation16]. We included grade two infections or higher in our study. Some studies also included grade one infections.
In a former study from Norway, grade two infections were seen in 4% of wounds, but 6% of the study participants had received prophylactic antibiotics [Citation9]. Our study had a higher infection rate (11.4%), which might be influenced by the low sample size, and exclusion of patients who received prophylactic antibiotics from the analysis. In the studies from U.K. and U.S.A., the participants were younger (17–23 years) and they had a higher use of topical antibiotics [Citation12,Citation13]. Other studies have found a significant relationship between wound infection and wound length or depth, wound location, patient age or time since injury. Multiple studies and a review study showed no significant findings on number of hours since trauma to suturing, and infection risk [Citation15–18]. Lammers et al. found that wound age and patient age were important risk factors for infection, and that wound infections were more frequent on the extremities than on other body locations [Citation19]. Quinn et al. concluded that a history of wound contamination, jagged or stellate edges, wound length > 5 cm, non-head/neck location and history of diabetes were important risk factors for wound infection [Citation17]. Hollander et al. found that bite injuries, wound length, depth and width, contamination with foreign material, patient age, diabetes, and a non-head or -neck location were important risk factors for wound infection in traumatic lacerations [Citation12,Citation16]. It is difficult to assess to what degree the difference between the patients and wound characteristics between the two groups affect the infection rates in the two study arms of our study.
Other hygienic measures
It is possible that the use of face masks in combination with other hygienic measures such as the use of sterile gloves, sterile fenestrated drapes and sterile dressings could influence postoperative infections. A recent Dutch study did not find that a combination of different sterile measures or not (gloves, drapes and dressings) had any effect on postoperative infection rate [Citation20]. It seems unlikely that adding face masks to other hygienic procedures would affect postoperative infection rates in a primary care setting.
Implications of the study
In this study, we did not find any significant difference in postoperative infection rate after suturing of traumatic wounds in an outpatient setting if surgical face masks were worn or not by health personnel. Our findings might imply that it is not necessary to wear surgical face masks as a routine procedure to reduce postoperative infections when suturing traumatic wounds in primary care. However, our study is too small to draw a firm conclusion.
Acknowledgements
We thank MD PhD Teresa Osland for participating in planning the study with us, Head Nurse Linda Eikefet Revheim and nurse Juliane Medås for their engagement in the conduction of the study, and all nurses and doctors at the MID for collecting data to the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Spooner JL. History of surgical face masks: the myths, the masks, and the men and women behind them. AORN J. 1967;5(1):76–80. doi: 10.1016/s0001-2092(08)71359-0.
- Strasser BJ, Schlich T. A history of the medical mask and the rise of throwaway culture. Lancet. 2020;396(10243):19–20. doi: 10.1016/S0140-6736(20)31207-1.
- Steen K, Morken T, Hunskår S. Steril prosedyre ved sårlukking i allmennpraksis og på legevakt [Sterile procedure while closing wounds in general practice and at accident and emergency departments]. Utposten. 2018;6:31–36.
- Orr NW. Is a mask necessary in the operating theatre? Ann R Coll Surg Engl. 1981;63(6):390–392.
- Chamberlain GV, Houang E. Trial of the use of masks in the gynaecological operating theatre. Ann R Coll Surg Engl. 1984;66(6):432–433.
- Tunevall TG. Postoperative wound infections and surgical face masks: a controlled study. World J Surg. 1991;15(3):383–387, discussion 387–388. doi: 10.1007/BF01658736.
- Webster J, Croger S, Lister C, et al. Use of face masks by non-scrubbed operating room staff: a randomized controlled trial. ANZ J Surg. 2010;80(3):169–173. doi: 10.1111/j.1445-2197.2009.05200.x].
- Kamalarajah S, Ling R, Silvestri G, et al. Presumed infectious endophthalmitis following cataract surgery in the UK: a case-control study of risk factors. Eye. 2001;21(5):580–586. doi: 10.1038/sj.eye.6702368.
- Brudvik C, Tariq H, Bernardshaw SV, et al. Infections in traumatic wounds sutured at a Norwegian Accident and Emergency Department. Tidsskr Nor Laegeforen. 2015;135(8):759–762. doi: 10.4045/tidsskr.13.1536.
- Sealed Envelope. Sealed envelope randomisation and online databases for clinical trials; 2001. Available from: https://www.sealedenvelope.com
- Legevakthåndboken. Gyldendal Akademiske; 2018 [Internet] [cited 2018 11]. Available from: https://www.lvh.no/skader/saarskader
- Hollander JE, Singer AJ, Valentine S, et al. Wound registry: development and validation. Ann Emerg Med. 1995;25(5):675–685. doi: 10.1016/s0196-0644(95)70183-4.
- Dire DJ, Coppola M, Dwyer DA, et al. Prospective evaluation of topical antibiotics for preventing infections in uncomplicated soft-tissue wounds repaired in the ED. Acad Emerg Med. 1995;2(1):4–10. doi: 10.1111/j.1553-2712.1995.tb03070.x.
- Caliendo JE. Letter: surgical masks during laceration repair. JACEP. 1976;5(4):278–279. doi: 10.1016/s0361-1124(76)80013-5.
- Ruthman JC, Hendricksen D, Miller RF, et al. Effect of cap and mask on infection rates in wounds sutured in the emergency department. IMJ Ill Med J. 1984;165(6):397–399.
- Hollander JE, Singer AJ, Valentine SM, et al. Risk factors for infection in patients with traumatic lacerations. Acad Emerg Med. 2001;8(7):716–720. doi: 10.1111/j.1553-2712.2001.tb00190.x.
- Quinn JV, Polevoi SK, Kohn MA. Traumatic lacerations: what are the risks for infection and has the ‘golden period’ of laceration care disappeared? Emerg Med J. 2014;31(2):96–100. doi: 10.1136/emermed-2012-202143.
- Steen K. Should traumatic wounds be closed within eight hours? Tidsskr Nor Laegeforen. 2014;134(17):1657–1660. doi: 10.4045/tidsskr.13.1551.
- Lammers RL, Hudson DL, Seaman ME. Prediction of traumatic wound infection with a neural network-derived decision model. Am J Emerg Med. 2003;21(1):1–7. doi: 10.1053/ajem.2003.50026.
- Zwaans JJM, Raven W, Rosendaal AV, et al. Non-sterile gloves and dressings versus sterile gloves, dressings and drapes for suturing of traumatic wounds in the emergency department: a non-inferiority multicentre randomised controlled study. Emerg Med J. 2022;39(9):650–654. doi: 10.1136/emermed-2021-211540.