Abstract
Background
Advance care planning (ACP) allows patients to define their goals and preferences. Spending more time at home and less time in the hospital, along with avoiding death in the hospital, are often considered desirable outcomes of palliative care (PC). In 2015, 36% of cancer patients died in the hospital and 13% died at home in Norway.
Method
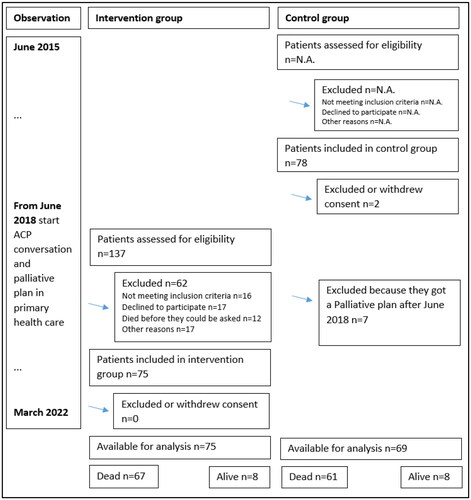
From 2015 to 2022, this prospective controlled non-randomized intervention trial observed 144 cancer patients with or without an organized ACP conversation in primary health care and a summarizing palliative plan (ClinicalTrials.gov Identifier: NCT02170168, 23 June 2014). The patients were identified through contact with the local cancer outpatient clinic or hospital-based PC team.
Results
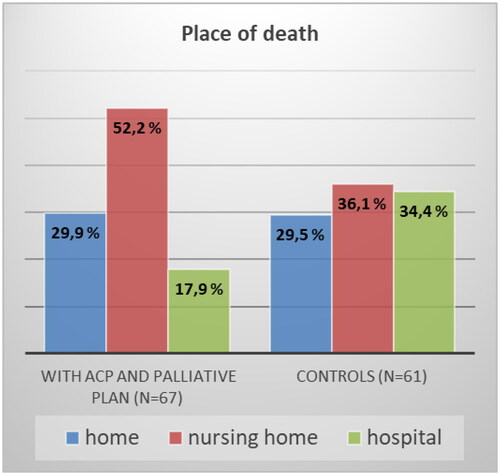
A total of 128 patients died during the observation period. Of these, 67 patients had an organized ACP conversation and summarizing palliative plan (intervention (I) group) and 61 had not (control (C) group). Dying in the hospital was significantly less common for patients in the I group compared to the C group (17.9% vs. 34.4%; X2 (1, n = 128) = 4.55, p = 0.033). There were no differences between the groups in terms of where they spent their time in the last 90 days of life (home, nursing home, or hospital). Most patients (62%) preferred to die at home. The observed differences between the groups regarding preferred and actual places of death did not reach statistical significance.
Conclusion
With organized ACP conversations in primary health care and a summarizing palliative plan, cancer patients died less often in the hospital in our observational study. A structured ACP approach integrating palliative care for cancer patients into primary health care can support patients´ preferences at the end of life.
Introduction
Advance care planning (ACP) is a continual process in which a patient’s current medical condition and prognosis are reviewed, their preferences for information regarding their illness are elicited, and likely medical dilemmas are presented and options discussed [Citation1]. The answer to the question, ‘Which medical treatment is right for me?’ depends on the patients’ goals, values, and preferences, and cannot be accurately predicted by the clinician, family, or other decision-makers [Citation2,Citation3]. ACP enables patients to discuss and define goals, set preferences for future medical treatment and care, and record and review these preferences [Citation4]. The ACP process is iterative and builds on existing and ongoing medical relationship [Citation5]. Using ACP in daily routine is often challenging, regarding when, where, and how to start, where to obtain all necessary information, how to document, and how to involve all future health care providers both in primary and specialist health care [Citation6]. The ACP definition and guidelines help implement ACP at a system level [Citation7]. ACP is a complex intervention at an individual level [Citation8]. Separating day-to-day care planning and ACP may be challenging, and ACP documentation may be scattered and difficult to find and use [Citation9]. There is always a right time and place for any conversation, and there is no exception for ACP conversation, which has resulted in an ongoing discussion between specialist and primary health care [Citation10,Citation11].
An ACP model within oncology treatment is feasible for community palliative care (PC) services [Citation12] and hospital-based services [Citation13]. Specialist PC teams can successfully contribute to the integration of oncology and PC [Citation14]. However, hospital-based specialist PC resources are limited, and palliative cancer patients live longer with modern oncology treatment [Citation15]. The gap between the availability of specialized palliative care resources and the number of cancer patients in need of palliative treatment is a significant challenge in many health care systems around the world [Citation16].
In 2018, the World Health Organization affirmed that providing palliative care to patients, families, and communities is at the core of the role and identity of primary care clinicians [Citation17]. It is important to build capacity in providing a palliative approach to care in the primary care setting to keep general practitioners (GPs) engaged in end-of-life (EoL) care [Citation18]. When GPs were actively involved with home visits, ACP conversations, and shared documentation of conclusions in a palliative plan, there was a higher likelihood of home death for cancer patients [Citation19].
Nurses often play leading roles in initiating and conducting ACP conversations [Citation20,Citation21]. They are well positioned to educate individuals and facilitate the ACP process in both primary and specialist health care [Citation22–24]. An international consensus supported by the European Association for Palliative Care provided guidance for clinical practice regarding the use of ACP and pointed out the role of trained non-physician facilitators in supporting the ACP process [Citation4].
Time spent at home is recognized as a valid outcome to assess the quality of EoL care and is in line with patients’ preferences [Citation25,Citation26]. Pinto et al. [Citation27] concluded from a literature review that home care is the most common preference for place of care and death for both patients and family members. In a study covering all deaths in Norway in 2012 and 2013 (n = 83.434), only 15% of deaths happened at home, being most frequent among patients with ‘Circulatory diseases’ and ‘Cancer’ [Citation28]. In Norway, most cancer patients die in nursing homes and hospitals. Historically, Norway had a low percentage of home deaths compared to other European countries, and although there has been an increase, the number of cancer deaths at home remains relatively low, at 16% in 2021 [Citation29–31].
In 2018, we started offering ACP conversations in primary health care and summarizing palliative plans in our region [Citation32]. The aim of the current study was to analyze the place of care prior to death and the place of death for individual patients based on whether they have had ACP conversations in a primary health care setting and a summarizing palliative plan, and to assess incurable cancer patients’ preferred place of death. We hypothesized that patients in the intervention group spent more time at home at the EoL and more often died at home.
Methods
Study design
This study was designed as a prospective, controlled, and non-randomized intervention trial. Participants were recruited between 2015 and 2020 from nine municipalities, with a total of 67.000 inhabitants in Møre og Romsdal County, North-Western Norway.
Setting
The municipalities in the catchment area have between 3.000 and 25.000 inhabitants and collaborate with the local hospital with an oncology outpatient clinic and a hospital-based PC team. Community cancer nurses offered support to patients and family caregivers in addition to the support from GPs and home-care nurses that the patient normally receives during the entire study period. Based on needs and symptom burden, cancer patients have access to the hospital-based PC team on referral from the hospital or primary health care. The PC team performed home visits and provided education and support to the patients and relatives. The local PC team and cancer outpatient clinic collaborate with nurses from all municipalities within a PC network and undertake educational conferences at least once a year. PC physicians from the hospital also collaborate with GPs through visits at the GP offices, common patient visits at home or nursing homes, and through bi-annual training courses within the general PC. Since 2015, home care nurses, nurses at nursing homes, and GPs in the municipalities have been able to contact a hospital PC physician by phone 24/7. Before 2015, three municipalities had implemented ‘Last days of life’ (former Liverpool care pathway [Citation33]), a structured guidance in Norway for PC in the last days and hours of life [Citation34]. These three municipalities cover 20.000 inhabitants. No hospice care was provided in the region during the study period.
Participants
The current study included cancer patients who could read and write Norwegian and (1) had advanced locoregional cancer and/or metastatic disease, (2) were > 18 years of age residing in one of the participating municipalities, and (3) were able to comply with the study procedures. Patients treated with curative intent were excluded. The patients were identified through contact with the local cancer outpatient clinic or hospital-based PC team.
Patients in the control group were included from June 2015 to December 2017, before the participating municipalities started to offer organized ACP conversations in primary health care and a summarizing palliative plan in 2018. The patients in the intervention group were included between September 2018 and March 2020. Community cancer nurses informed the local PC team about all cancer patients who had an organized ACP conversation in primary health care, and they asked eligible patients if a study contact (BD) from the hospital could contact them to explain the current study.
The observation period was from June 2015 to March 2022 for all the patients ().
Organized ACP conversations in primary health care and a summarizing palliative plan
From 2015 to 2017, a resource group consisting of health care professionals, including doctors, nurses, and specialists in palliative care, as well as patient representatives, developed the necessary requirements to offer organized ACP conversations in primary health care and summarizing palliative plans to all palliative patients in our county. They developed supporting tools such as information flyers for health care providers and patients, a template for summarizing palliative plans in the electronic patient journal (EPJ), and information videos published on the related website [Citation35]. Guidelines described how to document the palliative plan in the EPJ, including practical advice on how to send the plan electronically to possible future health care providers in our region, with permission from the patient [Citation32]. From January to June 2018, community cancer nurses shared the requirements and supporting tools to offer ACP and a summarizing plan within their respective municipalities, including the GPs. The hospital-based PC team supported the initiative in primary health care through training courses within general PC for GPs and one-day conferences for the established PC network of nurses in the region. These nurses shared information with the home care nurses in their municipality.
In this context, organized ACP conversations refer to structured and purposeful discussions between health care providers and patients about their values, goals, and preferences, building on an already achieved trusting relationship between health care personnel in primary health care and the patient. The summarizing palliative plan referred to a concise document that captured the essential elements and aimed to ensure that all health care providers involved had a clear understanding of patients’ preferences regarding future medical care.
One goal of this initiative was for primary health care providers to offer an ACP conversation and a summarizing palliative plan for all patients with non-curable cancer. The local hospital-based PC team supported primary health care with necessary information, such as medical status and prognosis, and recommended ACP conversations and a palliative plan in every discharge or outpatient note.
Intervention (I) group
Patients in the intervention group had an organized ACP conversation in primary health care and a summarizing palliative plan. Follow-up ACP conversation was offered, and the palliative plan was reassessed on demand when the patient’s medical condition changed.
Primary health care providers, mostly community cancer nurses, as well as home care nurses and GPs, decided if and when the patient should be offered an ACP conversation, and they were responsible for organizing and conducting it. They organized the conversation at the patients’ preferred place and proposed the possibility of having it at home [Citation32].
Control (C) group
Patients in the control group did not have an organized ACP conversation in primary health care with a summarizing palliative plan.
Data collection
Longitudinal data were collected through paper-based case report forms (patients’ self-report and health care providers’ report) as part of the data collected in the Orkdal Model Study (ClinicalTrials.gov Identifier: NCT02170168, 23 June 2014) in collaboration with nine community nurses and The Trial Office, Trondheim University Hospital. Additional data were extracted from the hospital and municipality EPJ.
Information on patient demographics, cancer diagnosis and prognosis, place of care, quality of life, and performance status was registered at inclusion and collected every four weeks during observation. The use of hospital services, admissions, use of community health care services, and date and place of death were recorded and verified, especially for the last 90 days of life for those patients who died within the observation period. Number of days the patient was not admitted to hospital or nursing home was counted as ‘days at home’. Whole-day stays in outpatient clinics, such as oncology units, were not included as hospital stays. Contact with the hospital-based PC team was verified by documentation in the hospital EPJ.
The patients responded to questionnaires at inclusion, every four weeks for two years, and thereafter every six months or until death. Data on quality of life (QoL) were collected (EORTC QLQ C15-PAL last question rated from 1 = very poor to 7 = excellent) [Citation36]. Furthermore, every 12 weeks, the patients stated their preferred place of death (PPOD). The question was formulated as follows: ‘We know from experience that you might change your mind over time. We would like to get your opinion about the next questions again, independent of what you have answered earlier’ 1)’ Many people, both healthy and ill, think about where they in time would like to die. When that time comes, and you yourself could choose, where would you prefer to die?’ The last responses before death were used to analyze whether PPOD was the actual place of death (APOD).
Data on the place of death of cancer patients were obtained from the Norwegian Cause of Death Registry (DÅR 18-0503) to obtain information on the pre-study proportion of cancer deaths at home in the catchment area of the study.
Outcomes
The primary outcome was the number of days spent at home in the last 90 days of life.
Secondary outcomes were the proportion of place of death, PPOD, and fulfilment of PPOD for those who died during the observation period, number of days at nursing homes or in hospitals, and number of hospital admissions during the last 90 days of life.
Data analyses and statistics
Descriptive statistics were used to summarize sex, age, education, living together with partner (yes/no), physician-reported estimated time of survival at inclusion (between 1 and 6 months, between 6 and 12 months, more than 1 year, between 1 and 5 years), Karnofsky index, QoL 1–7, number of days at home, in nursing homes or hospitals, PPOD and APOD, hospital admissions, ACP conversations in primary health care, GP home visits, and contact with the hospital-based PC team.
An independent two-sided t-test was used to examine differences in age, Karnofsky index at inclusion and follow-up, and number of days at home, in nursing homes, or in hospitals between the two groups. Comparison analyses between the groups according to gender, education, living together with partner, estimated survival time at inclusion, QoL 1–7 at inclusion and follow-up, GP home visits, contact with the hospital-based PC team, PPOD, and APOD were assessed using Pearson’s chi-square test.
For continuous variables, such as days at home in the last 90 days of life, 120 deaths (60 in each group) would allow the independent sample t-test to detect an effect size of 0,2 with a power of 80% and a two-sided significance level of 5%. Similarly, the same sample size allows a power of 80% for the detection of at least 22% difference in the proportions between the groups, in the hypothesis of a pre-intervention proportion of 13% (cancer death at home), and with a two-sided significance level of 5%. We assumed that 150 patients (75 in each group) would result in at least 120 deaths (60 in each group) during the observation period. In all cases, two-sided p values <0.05 were considered statistically significant. All statistical analyses were performed using the IBM SPSS Statistics version 28 (IBM Statistical Product and Service Solutions, Armonk, USA).
Ethics
The study was performed in accordance with the relevant guidelines and regulations of the Declaration of Helsinki and approved by the Regional Committee for Medical and Health Research Ethics (ID 2014/212) and the Cancer Department at Møre and Romsdal Hospital Trust. All participants provided written informed consent before participating in the study.
Results
One hundred and forty-four cancer patients were included in the current study: 75 in the intervention (I) group and 69 in the control (C) group (). Patients in the I group were included mean 24 days (SD, 37) after the first version of their summarizing palliative plan was saved in the EPJ.
Most of the 75 ACP conversations took place at patients’ homes (87%), in addition to at the GPs office (9%) or in nursing homes (4%). Participants in ACP conversations were patients (100%), relatives (90%), community cancer nurses (81%), GPs (55%), home-care nurses (33%), and hospital-based PC team members (28%). All palliative plans were verified and confirmed by patients’ GP. The 67 patients who died in group I received their first palliative plan mean 256 days before they died (SD 230).
Patients in group I were included later in their disease course, and the number of days from inclusion to death was significantly lower (). At inclusion, patients in the I group had shorter expected survival time, reported lower QoL, and had a lower Karnofsky index score than patients in the C group. During follow-up, the QoL and Karnofsky index scores did not differ between the groups (). There were no statistically significant differences between the two groups regarding gender, educational level, or living together with a partner, but patients in the I group were slightly older ().
Table 1. Patient characteristics.
During the observation period, 128 patients died. Of these 128, 67 patients had an ACP conversation and a summarizing palliative plan in primary health care (group I) and 61 had not (group C) ().
Primary outcomes
During the last 90 days of life, there were no statistically significant differences between the groups with or without intervention regarding days at home ().
Table 2. Place of care last 90 days of life and place of death.
Secondary outcomes
Data from the Norwegian Cause of Death Registry showed a pre-study average proportion of 12.4% deaths at home for cancer patients in the nine participating municipalities (DÅR 18-0503, years 2011–2015).
and summarize the differences between groups I and C in place of care, number of hospital admissions in the last 90 days of life, and place of death. Dying in primary health care (home/nursing home) was significantly more common in patients who received the intervention (82.2% vs. 65.6%, p = 0.033, ). Patients without intervention were more likely to die in the hospital. The probability of dying in the hospital for a patient without the intervention was more than 1.5 times higher than that for a patient with the intervention; N = 128, RR = 1.592 (95% CI: 0.98 − 2.58). However, there were no differences between groups in terms of home as place of death.
Figure 2. Place of death with and without ACP conversation in primary health care and a summarizing palliative plan, p = 0.033 for death in hospital.
Patients who underwent the intervention were significantly more likely to have home visits from their GP (p < 0.001; ). Patients in group I had more frequent contact with the hospital-based PC team than those in group C (). Contact with the hospital-based PC team was not associated with dying in the hospital or primary health care (X2 (1, n = 128) = 0.704; p = 0.401), but patients who had contact with the hospital-based PC team were significantly more likely to have an ACP conversation in primary health care and a summarizing palliative plan (X2 (1, n = 144) = 15.15; p < 0.001). Sixty-two patients (83%) in the I group and 69 patients (100%) in the C group underwent one or more oncology treatments at the local cancer outpatient clinic.
Of the 115 patients who stated a PPOD (last statement during observation), 71 (61.7%) wanted to die at home, 17 (14.8%) in nursing homes, 18 (15.7%) in hospitals, and 9 (7.8%) in other places. There were no statistically significant differences between the two groups (I and C) (X2 (3, n = 115) = 4.40, p = 0.222). We achieved a stated PPOD and APOD in 102 patients and observed differences between the groups in the fulfilment of PPOD (last), 40.4% in the I group, and 26.7% in the C group, but this did not reach statistical significance (X2 (1, n = 102) = 2.01; p = 0.148). Among the 102 patients who died and reported PPOD, 12 (12%) changed their preference during follow-up (first and last PPOD).
Discussion
In this prospective controlled non-randomized intervention trial, we evaluated the time at home and place of death of cancer patients with or without ACP conversations in primary health care settings and a summarizing palliative plan. In the current study, cancer patients spent 69,3% (group I) and 71,7% (group C) of their last 90 days of life at home, and 12,9% (I group) and 16,0% (C group) in the hospital. In contrast to our hypothesis, patients with ACP intervention in primary health care did not spend significantly more time at home in the last 90 days of life compared to the control group. However, having an ACP conversation in primary health care and a summarizing palliative plan was associated with more frequent deaths in primary health care (home/nursing home) and less frequent deaths in the hospital. In the current study, the proportion of home deaths among cancer patients was similar (29,9% in the I group and 29,5% in the C group) and overall high compared to the pre-study period (12,4%) and Norway in general (16% in 2021) [Citation30]. We also assessed cancer patients’ attitudes towards the preferred place of death (PPOD) and compared it with the actual place of death (APOD). There was a tendency towards more frequent fulfilment of PPOD for patients in the intervention group, but the overall proportion of fulfilment of the last PPOD and APOD was only 33% (40.4% in the I group and 26.7% in the C group). Contact with the hospital-based PC team did not influence the place of death; however, patients who had contact with the PC team were more likely to have a palliative plan in primary health care.
Almost the same number of days at home in the last 90 days of life and the overall high number of home deaths in groups I and C are most likely due to an established network within PC in primary health care developed over the last 12 years in our region. All patients in group C and the majority of patients in group I received oncology treatment at the local cancer outpatient clinic. Nurses at the cancer outpatient clinic knew and collaborated with all community cancer nurses in our region, which may have contributed to the early access to community cancer care for patients in both groups. Community cancer nurses knew about the principles of ACP and home as the preferred place of care for many cancer patients before the first patient was included in this study. The main difference between the I and C groups in this study was that from 2018, organized ACP conversations and summarizing palliative plans were offered systematically in primary health care, with shared definitions and supporting tools. This did not seem to have an impact on the number of days patients spent at home or the number of cancer deaths that occurred at home in our study population.
In the literature, the chosen timeframe for analyses of hospital utilization or time at home at EoL ranges between 180 and 30 days before death. The regional variation in the amount of time that dying cancer and non-cancer patients in the United States spent at home during the last 180 days of life was between 120 days (67%) and 146 days (81%) [Citation26]. Costantini et al. [Citation37] found that differences between groups with or without palliative home care teams were most marked in the last month of life, where patients spent between 30% and 19% of their last days in the hospital. In a previous retrospective analysis, we found that palliative cancer patients with an ACP conversation in primary health care spent approximately 10 days more at home in the last 90 days of life [Citation32].
Cohen et al. [Citation29] compared six developed countries and found that the percentage of all cancer deaths in 2003 occurring at home was 12.8 in Norway, 22.1 in England, 22.7 in Wales, 27.9 in Belgium, 35.8 in Italy, and 45.4 in the Netherlands. In Norway, EoL cancer care seemed to be hospital-centric with high expenditures and more hospital days in the last 180 days of life [Citation38]. Assareh et al. [Citation39] found that the majority of individuals aged 50 years or older who received palliative care in the hospital within 3 months to death stayed in the hospital until death. By establishing a home-based program, palliative care can successfully expand outside hospital walls to serve a high-need patient population with lower costs in the last three months of life [Citation40]. A shift in care delivery from inpatient to outpatient and at home care and improved community care support may help reduce hospitalizations in EoL care [Citation41,Citation42]. A well-integrated ACP and palliative care plan approach can be very effective in reducing the percentage of cancer patients spending many days or dying in the hospital [Citation43,Citation44]. In the current study, an ACP conversation in primary health care and a summarizing palliative plan were associated with a significantly reduced likelihood of dying in hospital, and patients with the intervention had significantly more frequent GP home visits. We believe that the affiliation of ACP conversation and a summarizing palliative plan to primary health care with the involvement of GPs is a key factor for fewer cancer deaths in hospitals in this study. The presence and involvement of a hospital-based PC team can contribute to a decrease in hospital deaths by actively supporting the structured transition of care from hospitals to communities [Citation45,Citation46].
Most patients express that they want to receive information on the course of the disease and that they want to talk about what is important at the EoL when they are asked [Citation47]. Meeting patient preferences is an important palliative care outcome [Citation48]. Most cancer patients prefer to die at home, which is more or less dependent on cultural influence [Citation49–51]. APOD is often not at home, especially in Norway [Citation28]. ACP improved the fulfilment of PPOD in a randomized study of elderly patients with various diagnoses [Citation52]. In an RCT, Skorstengaard et al. [Citation53] found no significant differences in the fulfilment of PPOD (52% vs. 35%) between cancer patients with or without ACP, but they found a significant difference in APOD favoring home death in the ACP group (40% vs. 17%). The degree of congruence between preferred and actual places of death is often unsatisfactory [Citation54]. We assume that decision-making about the most suitable place of care and death is easier with clarification of patients’ preferences and appropriate and available documentation. Affiliation of the ACP process to primary health care helps establish medical relationships in accordance with patient preferences. Recommendations and supporting tools for practical use helped offer ACP to as many palliative cancer patients and their families as possible in our region [Citation32]. There might be the potential to raise the fulfilment of PPOD for cancer patients by focusing even more on the possibility of home as place of death, if desired by the patient.
Strengths and limitations
As this was a single-center study with a limited number of patients and a non-randomized design, the analysis of results allowed us to look at associations rather than a causative relationship. Most of the patients were included in the local cancer outpatient clinic, indicating that they were eligible for cancer treatment. We selected patients who met the inclusion criteria until the desired sample size was achieved and did not consider selecting a sample that represented the entire cancer population in our region. Patients were included independently of their cancer diagnosis. Generalizability to other populations or communities is limited.
Some variables from the intervention group, such as estimated time of survival, QoL, or Karnofsky score, did not match the control group at inclusion. Patients in the intervention group were included later in their disease course and the number of days from inclusion to death was significantly lower. However, for all outcomes, the last assessment before death was used for comparison, and we analyzed the place of care in the last 90 days of life and place of death. Our assessments and analyses focused on EoL outcomes and not on the patient’s situation at inclusion. This study aimed to evaluate EoL outcomes based on the real-world clinical application of ACP intervention, where patients are often identified and treated later in their disease course [Citation5]. Offering a process of ACP, conducting the ACP conversation, and finishing and confirming the summarizing palliative plan takes time, and the inclusion of patients in the intervention group required that they have gone through the whole ACP process.
The control group was recruited before the municipalities started offering ACP conversations and a summarizing palliative plans to their patients. A long observation period was important to be able to follow as many patients as possible throughout the disease course, implying that we needed to reach a certain number of cancer deaths. However, comparisons with historical controls may be difficult, and a long study period makes temporal trends more likely to occur [Citation55]. In the intervention group, primary health care providers worked with common definitions, tools, and practice guidelines for organizing and conducting ACP conversations and documenting these within an electronically available summarizing palliative plan. In the control group, patients and relatives might also have had conversations with the community cancer nurse and their GP regarding preference for care and PPOD without a structured approach and summarizing documentation.
An RCT or a cluster-randomized trial was not feasible because of small-scale conditions and the already established and ongoing medical relationship between patients, GPs, and community cancer nurses. From 2018, primary health care providers decided whether and when a patient was offered an ACP conversation and summarizing palliative plan. Selection bias, especially from community cancer nurses, might have influenced the observed findings.
Implications for future work
In the current study, we analyzed how an individual patient-centered ACP intervention in primary health care influenced time at home, nursing home, or hospital, and PPOD and APOD for cancer patients undergoing palliative treatment. Many patients with cancer express a strong desire to remain at home for as long as possible, even during the last stage of their illness. GPs and community nurses play important roles in exploring, facilitating, and supporting patients’ values and preferences Citation22. In addition to the general recommendation to offer ACP to all advanced cancer patients, primary health care providers need practical guidance to overcome barriers in initiating the necessary communication and to use a routine approach with a flexible structure [Citation21].
Future research should be designed to establish a causal relationship between the defined ACP intervention and the observed EoL outcomes and to explore and measure the impact of ACP by describing and analyzing what has been done in real-life ACP.
Early integrated palliative care in oncology treatment should address patient preferences. Health care providers have the responsibility to help patients formulate their preferences based on their own values to improve care consistency with care preferences. ACP may be a possible approach to achieving better fulfilment in terms of, for example, preferred and actual place of death for cancer patients. Larger-scale studies and research focusing on which components are important to meet cancer patients’ preferences are needed.
Conclusion
This prospective controlled non-randomized intervention trial shows that integrated palliative and oncology care can be incorporated into the framework of primary health care disease management with the help of ACP conversations and summarizing palliative plans. A well-integrated ACP and palliative care plan approach in primary health care was associated with fewer cancer deaths in hospitals. The hospital-based PC team supported primary health care providers in initiating communication about ACP. Patients with ACP intervention did not spend more time at home at the EoL compared to the control group.
Ethics approval and consent to participate
The study was performed in accordance with the relevant guidelines and regulations of the Declaration of Helsinki and approved by the Regional Committee for Medical and Health Research Ethics (ID 2014/212) and the Cancer Department at Møre and Romsdal Hospital Trust. All participants provided written informed consent before participating in the study.
Authors’ contributions
BD was responsible for the conception, design, and procedures of the study, analysis, and interpretation of the data and was responsible for drafting and editing the manuscript. ATB was responsible for the conception and design of the study, interpretation of data, and took part in drafting and editing of the manuscript. BTP participated in the conception and design of the study and revised the manuscript accordingly. TH participated in the analysis and interpretation of the data and revised the manuscript. KES conceived the study and revised the manuscript accordingly. All authors have read and approved the final manuscript.
Availability of data and material
The dataset supporting the conclusions of this article, detailed information about these data, and the patient questionnaire can be received by the corresponding author, [email protected].
Acknowledgements
We would like to express our special thanks to the statistician from Møre og Romsdal Hospital trust, Tor Åge Myklebust, for his expertise and assistance during data analysis. We are grateful to the following study nurses at Molde Hospital and in the communities for their help in collecting data on ACP conversations and palliative plans: Randi Sunde, Liv Skjørsæther, Camilla Bugge Skåren, Camilla Søvde, Rita Skjegstad, Bente Winsjansen, Inger Anne Øyen, Marit Tangen Nautnes, Anita Støve and Ragnhild Eriksson. This project would not have been possible without the engagement of primary health care, including GPs, in implementing ACP in our region.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Sudore RL, Fried TR. Redefining the "planning" in advance care planning: preparing for end-of-life decision making. Ann Intern Med. 2010;153(4):256–261. doi:10.7326/0003-4819-153-4-201008170-00008.
- Bakitas M, Dionne-Odom JN, Jackson L, et al. There were more decisions and more options than just yes or no": evaluating a decision aid for advanced cancer patients and their family caregivers. Palliat Support Care. 2017;15(1):44–56. doi:10.1017/S1478951516000596.
- Chen YC, Huang HP, Tung TH, et al. The decisional balance, attitudes, and practice behaviors, its predicting factors, and related experiences of advance care planning in taiwanese patients with advanced cancer. BMC Palliat Care. 2022;21(1):189. doi:10.1186/s12904-022-01073-5.
- Rietjens JAC, Sudore RL, Connolly M, et al. Definition and recommendations for advance care planning: an international consensus supported by the European association for palliative care. Lancet Oncol. 2017;18(9):e543–e51. doi:10.1016/S1470-2045(17)30582-X.
- Lovell A, Yates P. Advance care planning in palliative care: a systematic literature review of the contextual factors influencing its uptake 2008–2012. Palliat Med. 2014;28(8):1026–1035. doi:10.1177/0269216314531313.
- Jones CA, Acevedo J, Bull J, et al. Top 10 tips for using advance care planning codes in palliative medicine and beyond. J Palliat Med. 2016;19(12):1249–1253. doi:10.1089/jpm.2016.0202.
- Sellars M, Silvester W, Masso M, et al. Advance care planning in palliative care: a national survey of health professionals and service managers. Aust Health Rev. 2015;39(2):146–153. doi:10.1071/AH14118.
- Ranganathan A, Gunnarsson O, Casarett D. Palliative care and advance care planning for patients with advanced malignancies. Ann Palliat Med. 2014;3(3):144–149. doi:10.3978/j.issn.2224-5820.2014.07.04.
- Kuusisto A, Santavirta J, Saranto K, et al. Advance care planning for patients with cancer in palliative care: a scoping review from a professional perspective. J Clin Nurs. 2020;29(13–14):2069–2082. doi:10.1111/jocn.15216.
- Mohan D, Sacks OA, O'Malley J, et al. A new standard for advance care planning (ACP) conversations in the hospital: results from a delphi panel. J Gen Intern Med. 2021;36(1):69–76. doi:10.1007/s11606-020-06150-0.
- Snyder S, Hazelett S, Allen K, et al. Physician knowledge, attitude, and experience with advance care planning, palliative care, and hospice: results of a primary care survey. Am J Hosp Palliat Care. 2013;30(5):419–424. doi:10.1177/1049909112452467.
- Blackford J, Street A. Is an advance care planning model feasible in community palliative care? A multi-site action research approach. J Adv Nurs. 2012;68(9):2021–2033. doi:10.1111/j.1365-2648.2011.05892.x.
- Hjorth NE, Schaufel MA, Sigurdardottir KR, et al. Feasibility and acceptability of introducing advance care planning on a thoracic medicine inpatient ward: an exploratory mixed method study. BMJ Open Respir Res. 2020;7(1). doi:10.1136/bmjresp-2019-000485
- Kaasa S, Loge JH, Aapro M, et al. Integration of oncology and palliative care: a lancet oncology commission. Lancet Oncol. 2018;19(11):e588–e653. doi:10.1016/S1470-2045(18)30415-7.
- Bajwah S, Oluyase AO, Yi D, et al. The effectiveness and cost-effectiveness of hospital-based specialist palliative care for adults with advanced illness and their caregivers. Cochrane Database Syst Rev. 2020;9:cd012780.
- Kebudi R, Cakir FB, Silbermann M. Palliative care in high and low resource countries. Curr Pediatr Rev. 2021;17(3):220–224. doi:10.2174/1573396317666210405143649.
- WHO, editor Declaration of Astana. Global Conference on Primary Health Care; 2018; Astana, Kazakhstan: https://www.who.int/docs/default-source/primary-health/declaration/gcphc-declaration.pdf.
- Mitchell S, Tan A, Moine S, et al. Primary palliative care needs urgent attention. BMJ. 2019;365:l1827. doi:10.1136/bmj.l1827.
- Neergaard MA, Vedsted P, Olesen F, et al. Associations between home death and GP involvement in palliative cancer care. Br J Gen Pract. 2009;59(566):671–677. doi:10.3399/bjgp09X454133.
- Dyar S, Lesperance M, Shannon R, et al. A nurse practitioner directed intervention improves the quality of life of patients with metastatic cancer: results of a randomized pilot study. J Palliat Med. 2012;15(8):890–895. doi:10.1089/jpm.2012.0014.
- Ólafsdóttir KL, Jónsdóttir H, Fridriksdóttir N, et al. Integrating nurse-facilitated advance care planning for patients newly diagnosed with advanced lung cancer. Int J Palliat Nurs. 2018;24(4):170–177. doi:10.12968/ijpn.2018.24.4.170.
- Head BA, Song MK, Wiencek C, et al. Palliative nursing summit: nurses leading change and transforming care: the nurse’s role in communication and advance care planning. J Hosp Palliat Nurs. 2018;20(1):23–29. doi:10.1097/NJH.0000000000000406.
- Johansen H, Helgesen AK. Palliative care in the community – the role of the resource nurse, a qualitative study. BMC Palliat Care. 2021;20(1):157. doi:10.1186/s12904-021-00860-w.
- Splendore E, Grant C. A nurse practitioner-led community workshop: increasing adult participation in advance care planning. J Am Assoc Nurse Pract. 2017;29(9):535–542. doi:10.1002/2327-6924.12467.
- Andersen SK, Croxford R, Earle CC, et al. Days at home in the last 6 months of life: a patient-Determined quality indicator for cancer care. J Oncol Pract. 2019;15(4):e308–e15. doi:10.1200/JOP.18.00338.
- Groff AC, Colla CH, Lee TH. Days spent at home – a patient-centered goal and outcome. N Engl J Med. 2016;375(17):1610–1612. doi:10.1056/NEJMp1607206.
- Pinto S, Lopes S, de Sousa AB, et al. Patient and family preferences about place of end-of-life care and death: an umbrella review. J Pain Symptom Manage. 2024;67(5):e439–e452. doi:10.1016/j.jpainsymman.2024.01.014.
- Kjellstadli C, Husebø BS, Sandvik H, et al. Comparing unplanned and potentially planned home deaths: a population-based cross-sectional study. BMC Palliat Care. 2018;17(1):69. doi:10.1186/s12904-018-0323-3.
- Cohen J, Houttekier D, Onwuteaka-Philipsen B, et al. Which patients with cancer die at home? A study of six european countries using death certificate data. J Clin Oncol. 2010;28(13):2267–2273. doi:10.1200/JCO.2009.23.2850.
- Health NIoP. Norwegian Cause of Death Registry. https://statistikkbank.fhi.no/dar/index.jsp?headers=DODSSTED&stubs=EU_SHORT_CODE&measure=common&virtualslice=Freq_value&layers=DAAR&layers=virtual&study=http%3A//10.0.3.47%3A80/obj/fStudy/d3b.eu-shortlist.dodssted&DAARsubset=2020&DAARslice=2020&mode=cube&DODSSTEDslice=Total&virtualsubset=Freq_value&v=2&EU_SHORT_CODEslice=Total&DODSSTEDsubset=Total%2C1+-+9&measuretype=4&cube=http%3A//10.0.3.47%3A80/obj/fCube/d3b.eu-shortlist.dodssted_C1&EU_SHORT_CODEsubset=Total%2C02_1&top=yes20212021.
- Kalseth J, Theisen OM. Trends in place of death: the role of demographic and epidemiological shifts in end-of-life care policy. Palliat Med. 2017;31(10):964–974. doi:10.1177/0269216317691259.
- Driller B, Talseth-Palmer B, Hole T, et al. Cancer patients spend more time at home and more often die at home with advance care planning conversations in primary health care: a retrospective observational cohort study. BMC Palliat Care. 2022;21(1):61. doi:10.1186/s12904-022-00952-1.
- Anderson A, Chojnacka I. Benefits of using the liverpool care pathway in end of life care. Nurs Stand. 2012;26(34):42–50. doi:10.7748/ns.26.34.42.s47.
- Haukeland UH. Livets siste dagar – plan for lindring i livets sluttfase. In: Competence Senter for Palliative Care HBHT, editor. https://helse-bergen.no/kompetansesenter-i-lindrande-behandling/palliasjon-verktoy-for-helsepersonell/livets-siste-dagar-plan-for-lindring-i-livets-sluttfase. Competence Senter for Palliative Care, Helse Bergen Hospital Trust; 2021.
- HMR. www.palliativplan.no. Helse Møre og Romsdal.
- Groenvold M, Petersen MA, Aaronson NK, et al. The development of the EORTC QLQ-C15-PAL: a shortened questionnaire for cancer patients in palliative care. Eur J Cancer. 2006;42(1):55–64. doi:10.1016/j.ejca.2005.06.022.
- Costantini M, Higginson IJ, Boni L, et al. Effect of a palliative home care team on hospital admissions among patients with advanced cancer. Palliat Med. 2003;17(4):315–321. doi:10.1191/0269216303pm744oa.
- Bekelman JE, Halpern SD, Blankart CR, et al. Comparison of site of death, health care utilization, and hospital expenditures for patients dying With cancer in 7 developed countries. JAMA. 2016;315(3):272–283. doi:10.1001/jama.2015.18603.
- Assareh H, Stubbs JM, Trinh LTT, et al. Variation in out-of-hospital death among palliative care inpatients across public hospitals in New South Wales, Australia. Intern Med J. 2019;49(4):467–474. doi:10.1111/imj.14045.
- Pinderhughes ST, Lehn JM, Kamal AH, et al. Expanding palliative medicine across care settings: one health system experience. J Palliat Med. 2018;21(9):1272–1277. doi:10.1089/jpm.2017.0375.
- Federman AD, Soones T, DeCherrie LV, et al. Association of a bundled hospital-at-home and 30-Day postacute transitional care program With clinical outcomes and patient experiences. JAMA Intern Med. 2018;178(8):1033–1040. doi:10.1001/jamainternmed.2018.2562.
- Handley NR, Bekelman JE. The oncology hospital at home. J Clin Oncol. 2019;37(6):448–452. doi:10.1200/JCO.18.01167.
- Dixon J, King D, Knapp M. Advance care planning in England: is there an association with place of death? Secondary analysis of data from the national survey of bereaved people. BMJ Support Palliat Care. 2019;9(3):316–325. doi:10.1136/bmjspcare-2015-000971.
- Pellizzari M, Rolfini M, Ferroni E, et al. Intensity of integrated cancer palliative care plans and end-of-life acute medical hospitalisation among cancer patient in Northern Italy. Eur J Cancer Care. 2018;27(1):27. doi:10.1111/ecc.12742.
- Flierman I, van Seben R, van Rijn M, et al. Health care providers’ views on the transition between hospital and primary care in patients in the palliative phase: a qualitative description study. J Pain Symptom Manage. 2020;60(2):372–380 e1. doi:10.1016/j.jpainsymman.2020.02.018.
- Orlovic M, Callender T, Riley J, et al. Impact of advance care planning on dying in hospital: evidence from urgent care records. PLoS One. 2020;15(12):e0242914. doi:10.1371/journal.pone.0242914.
- Miccinesi G, Bianchi E, Brunelli C, et al. End-of-life preferences in advanced cancer patients willing to discuss issues surrounding their terminal condition. Eur J Cancer Care. 2012;21(5):623–633. doi:10.1111/j.1365-2354.2012.01347.x.
- McCaffrey N, Ratcliffe J, Currow D, et al. What aspects of quality of life are important from palliative care patients’ perspectives? A framework analysis to inform preference-based measures for palliative and end-of-life settings. Patient. 2024;17(1):39–52. doi:10.1007/s40271-023-00651-w.
- Alsirafy SA, Hammad AM, Ibrahim NY, et al. Preferred place of death for patients With incurable cancer and their family caregivers in Egypt. Am J Hosp Palliat Care. 2019;36(5):423–428. doi:10.1177/1049909118813990.
- Beccaro M, Costantini M, Giorgi Rossi P, et al. Actual and preferred place of death of cancer patients. Results from the italian survey of the dying of cancer (ISDOC). J Epidemiol Community Health. 2006;60(5):412–416. doi:10.1136/jech.2005.043646.
- Yamagishi A, Morita T, Miyashita M, et al. Preferred place of care and place of death of the general public and cancer patients in Japan. Support Care Cancer. 2012;20(10):2575–2582. doi:10.1007/s00520-011-1373-8.
- Detering KM, Hancock AD, Reade MC, et al. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340(mar23 1):c1345–c1345. doi:10.1136/bmj.c1345.
- Skorstengaard MH, Jensen AB, Andreassen P, et al. Advance care planning and place of death, hospitalisation and actual place of death in lung, heart and cancer disease: a randomised controlled trial. BMJ Support Palliat Care. 2020;10(4):e37–e37. doi:10.1136/bmjspcare-2018-001677.
- Tang ST, McCorkle R. Determinants of congruence between the preferred and actual place of death for terminally ill cancer patients. J Palliat Care. 2003;19(4):230–237. doi:10.1177/082585970301900403.
- Shanbhag D, Graham ID, Harlos K, et al. Effectiveness of implementation interventions in improving physician adherence to guideline recommendations in heart failure: a systematic review. BMJ Open. 2018;8(3):e017765. doi:10.1136/bmjopen-2017-017765.