Abstract
The purpose was to review all patients with non-seminomatous germ cell tumours (NSGCT) treated at a single institution in order to evaluate the management and outcome. Patients were prospectively registered. Completed SWENOTECA forms and medical records of all 132 NSGCT patients treated between January 1985 and December 2000 were reviewed. Data on demographic, clinical, histological and biochemical characteristics as well as patient treatment and outcome were registered. The minimum follow-up was 2.2 years (median 8.3 years). In stage I, there was an overall relapse rate of 21%. These relapses were all treated successfully. Among stage II–IV patients, post treatment RPLND/surgical resections were performed in 31 patients (50%), and residual malignant disease was found in 23%. Relapse (N = 3) in metastatic disease patients, were seen in stage IV only. In stage II–IV, 5 died from germ cell malignancy of whom 3 never achieved CR. Five-year overall and disease-specific survivals were 95% and 96%. For stage I, II, III, and IV, the 5-year disease-specific survivals were 100%, 98%, 100%, and 69% respectively. Grouped according to the International Germ Cell Consensus Classification, the 5-year overall survivals were 100%, 92%, and 60% for the good, intermediate, and poor prognosis groups of stage II–IV patients, respectively. This report is a complete review of NSGCT patients treated in our minor university clinic. Survival rates are comparable to recently published data, due to a commitment to multicentre protocol and research collaboration.
Testicular cancer is a relatively rare neoplasm, but the most common malignancy among young men, comprising about 45% of all cancers in the age group 15–29 years. The testicular cancer incidence rate increased 4-fold between 1955 and 2000 and has recently exceeded 10 per 100 000 in Norway Citation[1], one of the highest incidences in the world. Since 1955, the 5-year disease-specific survival has increased from 60% to 95% Citation[1]. The introduction of new cisplatin-based combination chemotherapy since the 1970s Citation[2], Citation[3], better quality of staging work-up and evaluation as a result of new imaging technology, and more appropriate use of surgery have contributed to the improved prognosis, in particular for metastatic disease. Today, roughly 85% of patients with advanced disease are being cured Citation[4].
In the treatment of metastatic non-seminoma, there is some evidence indicating that centers, which do not treat a certain “critical mass” of patients may not achieve optimal treatment outcomes Citation[5], Citation[6]. Other investigators have not been able to show such evidence Citation[7], and some have demonstrated improved survival when patients were entered in trials Citation[8]. In line with the latter, a previous retrospective study on 98 testicular cancer patients (seminoma and non-seminoma) from our institution demonstrated that good treatment results could be achieved at a minor oncology department provided that the management of these patients followed protocols with internationally recognized treatment regimes Citation[9].
This report is an update with 132 non-seminomatous germ cell tumours (NSGCT) treated in our institution from 1985 to 2000. The aim of this review was 2-fold: 1) to report patient characteristics and treatment results achieved through trials and protocolised treatment schedules in our relatively small oncology department and 2) to discuss whether participating in multicentre co-operative testicular cancer research organisations, such as the Swedish Norwegian Testicular Cancer Study Group (SWENOTECA), may assure state of the art handling and treatment of NSGCT patients and lead to acknowledged outcome rates.
Materials and methods
Patients and registered variables
In our institution, all patients with germ cell tumours have been prospectively registered with a minimum data set for each patient submitted the SWENOTECA data registry. Registered data and medical records of all 132 patients treated for NSGCT at our institution between January 1985 and December 2000 were reviewed. Since the orchiectomy was normally performed at each patient's local hospital in Northern Norway, the number of patients that had been treated at our department was crosschecked with the Cancer Registry of Norway to ensure completeness of our database.
The post-orchiectomy treatment and follow-up of NSGCT patients in Norway are carried out at five oncology departments at university hospitals. The University Hospital of Northern Norway is the smallest university clinic, and about 10 NSGCT patients are yearly treated at our department.
The following variables were registered: Age, date of diagnosis, histology, biochemical parameters including tumour markers, stage, bilateral disease, location of metastasis, treatment, treatment effect, achieved complete remission, relapse and death. Overall survival and disease-specific survivals were estimated.
Guidelines, clinical variables and evaluation
At clinical suspicion of a testicular tumour, a unilateral orchiectomy through an inguinal incision was to be performed, most often at the local hospital. The SWENOTECA guidelines contained detailed technical recommendations for this surgical procedure. After establishing the histological diagnosis, all patients from other hospitals in Northern Norway were referred to our regional oncology centre. The histology diagnoses were according to the WHO classification Citation[10]. The non-seminoma histopathology was grouped according to: 1) embryonal carcinoma, 2) choriocarcinoma, 3) teratoma, 4) mixed tumours including different combinations of non-seminomatous histology or components of seminoma and 5) seminoma with elevated alpha-fetoprotein (AFP). Vascular invasion was defined as tumour cells found within an endothelial confined space, and was routinely described in the histopathology reports from 1991.
After CT scanning of the chest and abdomen/pelvis, patients were clinically staged using the Royal Marsden Hospital (RMH) staging system Citation[11]. Patients with metastatic non-seminomas were additionally classified according to prognostic groups, using the International Germ Cell Consensus Classification (IGCCC) Citation[12]. All, except three patients with extragonadal germ cell tumours (retroperitoneal, N = 2; bladder, N = 1), had their tumour origin in a testicle.
Serum levels of human choriogonadotropin (HCG) ≥5 IU/L, AFP ≥10 ng/mL and lactate dehydrogenase (LD) ≥450 U/L were considered pathological. Patients without evidence of metastatic disease and with normal or normalised tumour markers after orchiectomy were classified as stage I. If the post-orchiectomy levels of AFP and/or HCG did not normalise or increased without other evidence of metastasis, the disease was classified as stage I Mk+. In metastatic disease, a serum marker decline slower than the established respective marker half-life or a tumour size reduction less than 25%, as assessed by CT scanning, indicated inadequate treatment efficacy. Any alterations in treatment strategy as a consequence of inadequate treatment efficacy were registered.
Complete remission was defined as absence of clinical, radiological, biochemical, and/or histological signs of disease. Overall and disease-specific survival was estimated from date of diagnosis to date of death or to the date of last observation. Using the Population Registry of Norway, the database was updated with regard to survival until February 15, 2003, which was the last registration date.
Treatment and follow-up policy according to SWENOTECA
The treatment regimens have changed over time according to new SWENOTECA multicentre studies or revised treatment protocols for NSGCT. The changes are outlined in the following.
In SWENOTECA I (1981–1990), patients with clinical stage I disease were treated with retroperitoneal lymph node dissection (RPLND) Citation[5]. Four courses of cisplatin, vinblastine and bleomycin (CVB) were given to patients with evidence of metastasis. In 1987 CVB was replaced by bleomycin, etoposide and cisplatin (BEP-20) as the standard chemotherapy regimen.
From 1990 to 1994 (SWENOTECA II), patients in clinical stage I were treated according to a relapse risk assessment based on the presence of vascular invasion in the tumour specimen and/or positive preoperative tumour markers Citation[13]. Low-risk patients followed a surveillance program, intermediate-risk patients underwent RPLND, while high-risk patients were treated with three courses of BEP-20. Between 1995 and 2000 (SWENOTECA III), clinical stage I patients were randomised to two courses of adjuvant CVB/BEP-20 or to surveillance alone, based solely on vascular invasion in the primary tumour. After termination of the latter study, all stage I patients with vascular invasion had received one BEP-20 course, while low-risk patients had been followed with surveillance.
Regarding metastatic disease, three courses BEP-20 were considered adequate for low volume disease, while larger volume disease required four courses. Treatment effect evaluation with marker assessments and CT scanning was performed after two cycles. The SWENOTECA IV protocol outlined a strategy for treatment intensification in case of insufficient treatment efficacy. At inadequate response, ifosfamide were added to BEP-20 (BEP-if), stem cell harvesting performed, and high-dose chemotherapy (HDCT) with autologous stem cell support successively carried out if necessary. Grossly, stage II patients with initial retroperitoneal tumours >2 cm went through RPLND after completion of chemotherapy. Patients with residual tumours at completion of chemotherapy were candidates for tumour resection.
Statistical analysis
The SPSS for Windows® software package was used for the statistical analyses. Patients with missing values for a variable were excluded from analysis for that variable.
Product-limit survival estimates were obtained for all levels of each covariate using the method of Kaplan and Meier Citation[14]. Statistical significance between survival curves was assessed using the log-rank test Citation[15]. All continuous variables, except age, were categorised before analysis. The significance level was set at 0.05.
Results
The patient characteristics are presented in . The median age at time of diagnosis was 28 years (range 15–72 years). Embryonal carcinoma was the dominant histological diagnosis, and vascular invasion was demonstrated in 43% of the cases. About half the patients were diagnosed with localised (stage I) and metastatic (stage IMk + -IV) disease, respectively. Ten percent had stage IV disease at diagnosis. Of all patients, 74% had elevated AFP and/or HCG. Sixty-six percent of the tumours were AFP-producing, while 53% produced HCG. Preoperative AFP and HCG data from the remitting hospitals were almost complete. In only one patient were data for both markers missing. For serum LD, however, data were missing in 36% of the cases.
Table I. Patient characteristics of 132 patients treated for NSGCT.
Median follow-up was 8.3 years (range 2.2 to 18 years). Ten patients died during the follow-up period. Five deaths were due to germ cell tumours, whereas the others were caused by: Glioblastoma, N = 1; Snowmobile accident, N = 1; Ischemic coronary heart disease, N = 2; and sudden death without defined cause, N = 1. No patients died as a result of treatment-related toxicity.
Stage I disease
Fifteen (21%) stage I patients relapsed, 30% (10/33) of patients with embryonal carcinoma and 14% (5/37) with non-embryonal tumours. The median time from orchiectomy to relapse was six months (range 2 to 12 months). Due to altered guidelines and new stage I trial protocols, the post orchiectomy treatment and follow-up differed over time, and the relapse-rate varied accordingly (). In patients subjected to RPLND, the relapse rate was 29% (lung metastases, N = 4; retroperitoneal metastases, N = 2; elevated tumour markers alone, N = 1). There were no relapses among 11 high-risk patients administered adjuvant chemotherapy, whereas 22% of the low-risk patients on the surveillance program developed relapse (retroperitoneal metastases, N = 6; elevated tumour markers alone, N = 2). Relapsing patients were treated with three or four courses of CVB or BEP-20. In patients with retroperitoneal relapse, RPNLD or surgical resections was carried out according to standard procedures. Two relapsing patients underwent further surgical resection of lung metastases.
Table II. Treatment and relapse among 70 patients with stage I non-seminomatous germ cell tumours.
No patients with relapse died. Among the non-relapsing stage I patients, there were three deaths due to causes other than NSGCT (glioblastoma, N = 1; sudden death, N = 1; snowmobile accident, N = 1).
Metastatic disease
In 16 patients, clinically considered to be in stage I, post-orchiectomy RPLND revealed retroperitoneal malignancy leading to an upstaging (stage II). All patients with metastatic disease, except one, were treated with combination chemotherapy as first-line treatment (CVB, N = 5; BEP-20, N = 56). Due to old age and marginally renal clearance, one patient with Stage IIA disease was treated with RPLND alone.
Routine post chemotherapy RPLND in stage ≥IIB or surgical resection of residual mass was performed in 45% (20/44) of stage II and 80% (4/5) of stage III. One stage III patient underwent mediastinal lymph node dissection in addition to retroperitoneal resections. Due to persistent malignant cells in resected retroperitoneal specimens, further chemotherapy was needed in four stage II patients (20%). Stage IV patients (N = 13) were distributed according to distant metastatic locations as follows: lung (N = 7); lung and liver (N = 1); lung, liver and bone (N = 1); lung and brain (N = 1); lung, urethra and penis (N = 1); lung and pelvis (N = 1); lung and bladder (N = 1). Of these, seven patients (54%) underwent post chemotherapy retroperitoneal surgical resections, three patients (23%) additional visceral resections (lung, N = 2; liver, N = 1), and three patients (23%) visceral resections alone (lung, N = 3; brain, N = 1). Persistent malignancy was identified in three (23%) of the stage IV patients. Relapses in patients with metastatic disease (brain, N = 1; retroperitoneal, N = 2) were all seen in stage IV patients, and occurred within two years after achieved complete remission. The patient with cerebral relapse died six months after relapse diagnosis, while the other two patients were successfully treated.
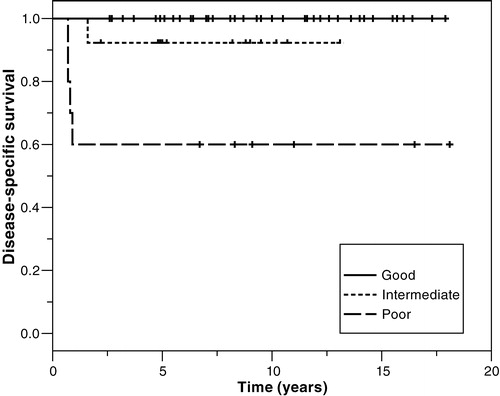
When our patients were classified according to the IGCCC prognostic factor-based staging system (IGCCC97), 63%, 21%, and 16% were classified as having good, intermediate, and poor prognosis, respectively (). Five percent (2/39) of good prognosis patients relapsed, while there was no relapse in the intermediate prognosis group. Of poor prognosis cases, 40% (4/10) either relapsed (N = 1) or never achieved CR (N = 3). These four comprised 80% of those dying from germ cell malignancy.
Table III. Distribution of 62 patients with metastatic germ cell cancers (stages IMk+ to IV) according to the International Germ Cell Consensus Classification Citation[12].
Due to inadequate treatment effect, first-line chemotherapy was intensified by adding ifosfamide in 26% (N = 16) of patients after two courses of BEP-20 (stage II, 8/44; stage III, 3/5; stage IV, 5/13). In three of these patients (19%), the response to PEI chemotherapy was inadequate, thus therapy was consequently altered to third line chemotherapy. From 1995, patients were routinely subjected to autologous stem cells harvesting at signs of inadequate treatment response. In total, only four patients went through high-dose chemotherapy (HDCT) with autologous stem cell support (stage II C-D, N = 2; stage IV treatment resistant retroperitoneal relapse six months after treatment completion, N = 1; stage IV with brain/lung metastases, N = 1). One of the treatment resistant patients in advanced stage II and the stage IV patient with treatment resistant retroperitoneal relapse both were cured. The other stage IID patient developed a treatment resistant primitive neuroectodermal tumour (PNET) of neuroblastoma type in the teratoma and died. So did also the stage IV patient with multiple brain metastases.
Survival
In total, five patients died from germ cell malignancy, all within two years after diagnosis (stage IID with malignant mesenchymal transformation of mature teratoma, N = 1; non-pulmonary stage IV, N = 3; non-pulmonary stage IV with brain relapse, N = 1). These three patients with non-pulmonary visceral metastases (liver, N = 1; bone and liver, N = 1, brain, N = 1) never achieved CR.
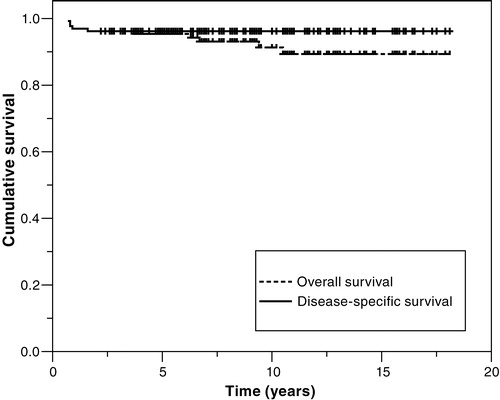
The 5-year overall and 5-year disease-specific survival for all 132 patients were 95% and 96%, respectively (). In stage I, the 5-year overall and 5-year disease-specific survival were 98% and 100% (), respectively. The difference in numbers is related to one death from a snowmobile accident 3 years after diagnosis in a disease-free patient.
Figure 2. Disease-specific survival of patients with NSGCT according to clinical stage (RMH staging system). Stage I, N = 70; stage IMk + /II, N = 44; stage III, N = 5; stage IV, N = 13 (p < 0.0001). Graphs for stage I and stage III are superimposed.
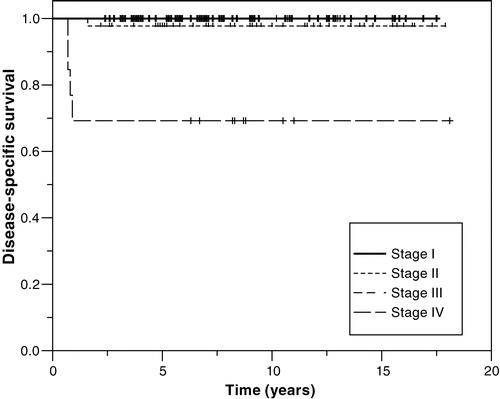
The 5-year overall and disease-specific survival for all patients with metastatic NSGCT was 92%. Respective to stage, the 5-year disease-specific survival was 98%, 100% and 69% for stage II, III and IV, respectively (). Survival data according to the IGCCC prognostic groups are presented in . The 5-year disease-specific survival was 100%, 92% and 60% for the good, intermediate, and poor prognostic groups, respectively.
Discussion
Herein, we focus on treatment results for NSGCT from a single institution to report our experience and in particular to compare and contrast our results with those of others. Our rather small oncology department serves a population of 470 000 and is responsible for all staging, treatment, and follow-up of germ cell tumours in the region of Northern Norway. Our clinical unit has, since its establishment in 1985, been part of the SWENOTECA collaboration. When cross-checking germ cell tumour data since 1985 with the Norwegian Cancer Registry, we found that few patients were treated outside our region and only during the first six years of the observation period. Thus, our NSGCT cohort is near complete and can be regarded as representative and an accurate description of this disease in Northern Norway.
The 132 NSGCT patients had demographic, histological, and biochemical features similar to those reported by others. The age at diagnosis was comparable to previous reports Citation[4], Citation[16–18]. The grouping of histological subdiagnoses differs between reported series. However, our finding that the majority (87%) of patients had either pure embryonal carcinoma or mixed tumours, was consistent with previous reports Citation[16], Citation[17]. Reported vascular invasion in 45–50% of NSGCT Citation[4], Citation[17], is in agreement with our data. Concerning tumour markers, the majority of patients had elevated AFP or HCG and 74% had at least one of these markers elevated, corroborating other studies Citation[16], Citation[17].
The stage distribution of NSGCT patients was slightly different from others. We found 53% with stage I disease, basically comparable to other series Citation[16–19]. But, the frequency of stage IV disease (10%) at our clinic was in the lower range when compared to others (10–20%) Citation[16–20]. A shorter lag time between symptoms and diagnosis or a staging bias due to inadequate examination procedures may explain this difference. The latter explanation would, however, lead to an unfavourable outcome in lower stages, and this was not the case in our series.
Nevertheless, when patients were categorised according to the IGCCC classification, the distribution across prognostic groups were comparable to previously reported data, indicating that our group of patients was not prognostically favourable Citation[12], Citation[16]. The basis for establishing the IGCCC was 5202 metastatic NSGCT patients and 660 metastatic seminoma patients Citation[12]. Among patients with metastatic NSGCT, 60%, 26%, and 14% of cases allocated to the good, intermediate, and poor prognostic group, respectively. In comparison, the respective distribution in our series was 63%, 21%, and 16%. Recently, the Spanish group reported a similar distribution of 63%, 19%, and 18% for good, intermediate and poor prognosis Citation[16].
If stage I patients in general are not administered chemotherapy, relapses will develop in about 30% Citation[13], Citation[21–23]. In our series, 29% of those subjected to RPLND during the early part of the period and 22% of low-risk patients following the surveillance program in the latter part of the period eventually relapsed. In contrast, none of the high-risk stage I patients treated with one or two courses chemotherapy relapsed, corroborating the very low relapse risk after chemotherapy treatment to high risk patients, reported by Klepp and co-workers Citation[13]. All patients relapsing after RPLND or surveillance were treated successfully with chemotherapy and are without evidence of disease. At our institution, RPLND is no longer a treatment option to patients with stage I disease. By offering surveillance to low-risk stage I patients, overtreatment can be reduced by 70%. However, surveillance involves closer follow-ups; a considerable challenge as our institution covers a large geographical area of 110 000 km2.
In metastatic disease (stage IMk + -IV), active undifferentiated malignant elements are reported in about 10% of residual paraaortic masses post treatment Citation[4]. In our series, 23% (N = 7) of those subjected to surgical resections (retroperitoneal and visceral) after completed initial chemotherapy, were found to have persistent malignancy in at least one resected specimen.
Survival outcomes have been evaluated with respect to both clinical stage and IGCCC prognostic groups. Internationally, the 5-year overall survival in patients with NSGCT has been reported between 65% and 94% Citation[16–20], Citation[24], Citation[25]. In comparison, our 5-year overall and 5-year disease-specific survivals were 95% and 96%, respectively. Our 5-year overall survival rate for NSGCT stage II patients was slightly higher when compared to other series, 97% vs. 54–94%, respectively Citation[18], Citation[19], Citation[24]. The 3-year actuarial survivals in the original IGCCC non-seminoma cohort published in 1997 were 92%, 80%, and 48% for the good, intermediate, and poor prognostic groups Citation[12]. Our 5-year actuarial survivals across the same groups were 100%, 92%, and 60%. Germa-Lluch and co-workers Citation[16] reported IGCCC-related survival data similar to ours, except for a better 5-year survival of 72% in the poor prognostic group. This may be explained by their use of a more intensive alternating chemotherapy regimen (BOMP-EPI) in poor prognosis patients (shortened intervals between BOMP and EPI, dose increase of cisplatin). In contrast, treatment intensification according to SWENOTECA was to start after the second chemotherapy course, and even though such treatment included HDCT with autologous stem cell support in eligible patients, treatment intensification started later and was initially less intense.
The importance of centralising management of patients with NSGCT to major cancer centres have been emphasised by several authors, and some argue that size matters Citation[5], Citation[6], Citation[26], Citation[27]. It can easily be agreed that patients with germ cell tumours should be treated by surgical and oncological experts to ensure the highest cure rates for these young patients. Though, based on a published clinical trial performed in 49 European centres Citation[6], treatment volume has been raised as a criterion to select which centres should treat metastatic NSGCT. The authors reported an 85% higher relative risk of death for poor-prognosis patients treated at centres entering <5 patients when compared to those entering ≥5 per four years. Nevertheless, the study was subjected to several potential biases, and there was no difference in risk of death between centres entering 5–9 patients or ≥20 patients per four years. Retrospective series from Scotland Citation[22], Citation[28] and Ireland Citation[24], demonstrating higher survival in large urban centres in comparison to minor rural centres, have also been used to argue for centralisation. Yet, it is noteworthy that at the time these patients were treated, there were no established multicentre or national treatment protocols in these countries. Besides, the data from 667 Australian testicular cancer patients Citation[19], could not demonstrate any difference in survival between patients treated in minor or major centres.
Through research collaboration and the continuous update of treatment protocols, SWENOTECA has become an important tool for achieving optimised treatment outcomes for NSGCT in Norway and Sweden Citation[29]. Although our department is below the volume threshold argued by Collette and co-workers Citation[6], we believe that, provided committed specialists, committing to collaborative research groups treatment volume can be largely compensated for and state of the art results achieved. The interdisciplinary Consensus on diagnosis and treatment of testicular germ cell tumours established by the German Testicular Cancer Study group is another good example for this approach Citation[30]. Through advancing the research field and utterly refining the therapeutic guidelines, cure rates and quality of life in NSGCT patients can be further improved.
The authors disclose no financial or other conflicts of interest. A. Kildahl-Andersen and M. G. Erke have contributed equally to this article. The secretarial assistance by Ann Grethe Nyheim is greatly appreciated. We thank the Norwegian Cancer Society for its financial support.
References
- Cancer Registry of Norway, 2000. www.kreftregisteret.no/forekomst_og_overlevelse_2000/testis/incidence.htm.
- Einhorn LH, Donohue J. Cis-diamminedichloroplatinum, vinblastine, and bleomycin combination chemotherapy in disseminated testicular cancer. Ann Intern Med 1977; 87: 293–8
- Peckham M. Testicular cancer. Acta Oncol 1988; 27: 439–53
- Dearnaley DP, Huddart RA, Horwich A. Managing testicular cancer. BMJ 2001; 322: 1583–8
- Aass N, Klepp O, Cavallin-Ståhl E, Wieklünd H, Unsgoard B, Baldetorp L, et al. Prognostic factors in unselected patients with nonseminomatous metastatic testicular cancer: A multicenter experience. J Clin Oncol 1991; 9: 818–26
- Collette L, Sylvester RJ, Stenning SP, Fossa SD, Mead SM, de Wit R, et al. Impact of the treating institution on survival of patients with “poor-prognosis” metastatic nonseminoma. J Natl Cancer Inst 1999; 91: 839–46
- Begg CB, Carbone PP, Elson PJ, Zelen M. Participation of community hospitals in clinical trials. Analysis of five years experience in the Eastern Cooperative Oncology Group. New Engl J Med 1982; 306: 1076–80
- Stiller CA. Centralised treatment, entry to trials and survival. Br J Cancer 1994; 70: 352–62
- Norum J, Nordøy T, Wist E. Testicular cancer treated in a minor general oncology department. Eur J Cancer 1995; 31A: 293–5
- Mostofi FK, Sobin LH. Histological typing of testis tumours. International Histological Classification of Tumours, No 16. World Health Organization, Geneva 1979; 1–39
- Peckham MJ, McElwain TJ, Barrett A, Hendry WF, et al. Combined management of malignant teratoma of the testis. Lancet 1979; 2: 267–70
- International Germ Cell Consensus Classification. A prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol 1997; 15: 594–603
- Klepp O, Dahl O, Flodgren P, Stierner U, Olsson AM, Olbring J. Risk-adapted treatment of clinical stage 1 non-seminoma testis cancer. Eur J Cancer 1997; 33: 1038–44
- Kaplan EI, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–81
- Peto R, Pike MC, Armitage P, Braslow NE, Cox BR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient II. Analysis and examples. Br J Cancer 1977; 35: 1–39
- Germà-Lluch JR, Garcia del Muro X, Maroto P, Paz-Ares L, Arranz JA, Guma J, et al. Clinical pattern and therapeutic results achieved in 1490 patients with germ-cell tumours of the testis: the experience of the Spanish Germ-Cell Cancer Group (GG). Eur Urol 2002; 42: 553–62
- Rosenthal MA, Stuart-Harris RC, Tiver KW, Langlands AO, Kefford RF. Single institution experience with non-seminomatous germ cell tumours of the testis: local perspectives on a curable cancer. Aust NZ J Med 1991; 21: 857–62
- Gudbjartsson T, Magnusson K, Bergthorsson J, Barkardottir RB, Agnarsson BA, Thoroddsen B, et al. A population-based analysis of increased and improved survival of testicular cancer patients in Iceland. Scan J Urol Nephrol 2003; 37: 292–8
- Toner GC, Neerhut GJ, Schwarz MA, Thursfield IJ, Sandeman TF, Giles GG, et al. The management of testicular cancer in Victoria, 1988–1993. Med J Aust 2001; 174: 328–31
- Steele GS, Richie JP, Stewart AK, Menck HR. The national cancer database report on patterns of care for testicular carcinoma, 1985–1996. Cancer 1999; 86: 2171–83
- Francis R, Bower M, Brunstrom G, Holden L, Newlands ES, Rustin GJ, et al. Surveillance for stage I testicular germ cell tumours: results and cost benefit analysis of management options. Eur J Cancer 2000; 36: 1925–32
- Howard GCW, Clarke K, Elia MH, Hotcheon AW, Kaye SB, Windsor PM, et al. A Scottish national audit of current patterns of management for patients with testicular non-semimatous germ-cell tumors. Br J Cancer 1995; 72: 1303–6
- Foster RS, Roth BJ. Clinical stage I nonseminoma: surgery versus surveillance. Semin Oncol 1998; 25: 145–53
- Thornhill JA, Walsh A, Conroy RM, Fennelly JJ, Kelly DG, Fitzpatrick JM. Physician-dependent prognostic variables in the management of testicular cancer. Br J Urol 1988; 61: 244–9
- Oshima A, Kitagawa T, Ajiki W, Tsukuma H, Takenaka S, Iura A. Survival of testicular cancer patients in Osaka, Japan. Jpn J Clin Oncol 2001; 31: 438–43
- Feuer EJ, Sheinfeld J, Bosl GJ. Does size matter? Association between number of patients treated and patient outcome in metastatic testicular cancer. J Natl Cancer Inst 1999; 91: 816–8
- Bagshawe KD, Begent RHJ, Newlands ES, Rustin GJS. What sort of oncology team should treat testicular teratoma?. Lancet 1985; I: 646
- Harding MJ, Paul J, Gillis CR, Kaye SB. Management of malignant teratoma: does referral to a specialist unit matter?. Lancet 1993; 341: 999–1002
- Bray F, Sankila R, Ferlay R, Parkin DM. Estimates of cancer incidence and mortality in Europe in 1995. Eur J Cancer 2002; 38: 99–166
- Krege S, Souchon R, Schmoll HJ. Interdisciplinary consensus on diagnosis and treatment of testicular germ cell tumours: Result of an update conference on evidence-based medicine (EBM). Eur Urol 2001; 40: 372–91