Abstract
Proton therapy may offer potential clinical advantages compared with conventional radiation therapy for many cancer patients. Due to the large investment costs for building a proton therapy facility, however, the treatment cost with proton radiation is higher than with conventional radiation. It is therefore important to evaluate whether the medical benefits of proton therapy are large enough to motivate the higher costs. We assessed the cost-effectiveness of proton therapy in the treatment of four different cancers: left-sided breast cancer, prostate cancer, head and neck cancer, and childhood medulloblastoma. A Markov cohort simulation model was created for each cancer type and used to simulate the life of patients treated with radiation. Cost and quality adjusted life years (QALYs) were used as primary outcome measures. The results indicated that proton therapy was cost-effective if appropriate risk groups were chosen. The average cost per QALY gained for the four types of cancer assessed was about €10 130. If the value of a QALY was set to €55 000, the total yearly net benefit of treating 925 cancer patients with the four types of cancer was about €20.8 million. Investment in a proton facility may thus be cost-effective. The results must be interpreted with caution, since there is a lack of data, and consequently large uncertainties in the assumptions used.
Economic evaluations in health care provide important information for medical decision-making. Information about cost-effectiveness is used more often today in pricing and reimbursement decisions, and in decisions about investments in new technologies. Economic evaluations are also important for rational choices between different existing technologies. It is therefore of increasing importance to demonstrate clinical utility and cost-effectiveness associated with medical technologies, particularly for very expensive ones. An economic evaluation weighs the increased cost against costs avoided in other areas, improved effectiveness of the new technology, or both.
Proton beam therapy may offer clinical advantages compared with conventional radiation therapy with x-rays (photons) or electrons for many cancer patients, mainly as a result of a more favourable distribution of the radiation dose Citation[1], Citation[2]. The risk of damage to normal tissues is decreased, which in turn may permit dose escalation and increased probability of cure. If the treatment effect in the patients who receive radiation against cancer was the only concern, proton therapy would in some cases be superior to conventional radiation, since either a higher curative dose with the same side effects, or the same curative dose with lower side effects, can be achieved in some patients compared with conventional radiation therapy. However, proton therapy is expensive, since large investments are required for building accelerators, beam transport systems and gantries. Since scarce health care resources have alternative uses, we therefore need to evaluate whether the medical benefits of proton therapy are large enough to motivate the higher costs.
The major problem with evaluating proton therapy is the limited number of clinical studies. In practice, an economic evaluation of proton therapy will have to be based on models and more or less well-founded assumptions rather than hard evidence from randomised clinical trials. This dilemma is not unique for proton therapy. Without investments in a new medical technology it is hard to gather enough evidence to reach a definitive conclusion about how worthwhile those investments really are.
This study presents an economic evaluation of proton radiation therapy. We will start with a presentation of the cancer types included in the evaluation, and then the methodology applied in the evaluations will be briefly introduced. Thereafter, the costs of proton therapy will be considered. We will focus on the cost difference between proton therapy and therapy with x-rays or electrons. The clinical advantages were measured in terms of survival (or risk reductions) and increased quality of life. Finally, everything will be put together and an assessment of the cost-effectiveness of proton therapy, and the expected health economic consequences of investing in a proton therapy facility, will be made based on findings in the literature.
Materials and methods
Choice of tumour types
An important question is in which types of cancer the medical advantages are enough to motivate the higher investment and treatment cost of proton therapy. One of the forms selected in the present study, prostate cancer, is commonly referred to as an interesting target for proton therapy since the tumour control probability may increase at the same time as the normal tissue complication probability decreases, and patients are being treated at existing proton facilities. The three other selected forms are diagnoses where model studies indicate that the normal tissue complication probability can decrease, i.e. the long-term risk of complications of survivors can be reduced. We have deliberately not focused on cancers that traditionally have been treated with protons with definitive gains, since the purpose of this study is to get an idea of the cost-effectiveness of new indications, motivating a proton facility for both routine treatment and clinical research. We have chosen to study the following four tumour types:
Breast cancer
Left-sided breast cancer is a potential target for proton therapy, mainly since protons may achieve a lower risk for the development of radiation-induced cardiac diseases and radiation pneumonitis Citation[3–8]. It is likely that the effect on tumour control is the same with proton therapy as with photons or electrons, but the dose in sensitive tissues in the heart and the lung may be reduced considerably, so that very low toxicity is likely to develop later on Citation[5], Citation[6].
Prostate cancer
There are indications that radiation therapy against some stages of prostate cancer is effective in reducing the risk of recurrences, but radiation is also associated with adverse reactions due to radiation damage to normal tissues. Complications in the rectal and urinary tracts are the most common problems after radiation Citation[9–13]. Most of the problems are short-term but the morbidity may also be sustained a long time after radiation. The toxicity from radiation limits the radiation dose that can be delivered to the tumours and therefore, by using proton therapy, the tumour dose could potentially be increased without exceeding the tolerance of the surrounding tissue Citation[14], Citation[15].
Head and neck cancer
Head and neck cancers are potential targets for proton therapy, but since this group of cancers is diverse, it is necessary to distinguish between those that would be of primary interest for proton therapy and those that would not. Cancer in the larynx would not be of interest, for example, since it is frequently treated with small radiation volumes and the prognosis is generally favourable. Cancer in the hypofarynx, by contrast, has a much poorer prognosis since it can not be surgically removed and is often treated with radiation to larger volumes Citation[16]. Since higher curative doses would often be needed than is currently possible to achieve given the side effects, proton therapy could be a better alternative than conventional radiation therapy Citation[17]. Radiation in the head and neck region often affects the salivary glands, the facial skin, the mucous membranes, the spinal cord, and the temporal lobes of the brain. Neurological problems are not uncommon, and this risk often sets a limit on the dosages that can be used in radiation of head and neck cancers.
Medulloblastoma
Medulloblastoma is a rare form of cancer, but still accounts for about 20–25% of all central nervous system tumours in children Citation[18]. Radiation therapy is the commonly used treatment for medulloblastoma, but is associated with risk of several adverse events Citation[18], Citation[19]. Children are, for example, particularly sensitive to radiation of the brain and, as a result, they carry a substantial risk of developing neurological side effects after radiation. There is also a risk for other late adverse events, such as thyroid dysfunction, cardiac diseases, and secondary tumours, e.g. sarcomas and breast cancer. Compared with conventional radiation using photons and electrons, proton therapy would probably not improve local tumour control in most of these patients, but it could decrease the risk of complications as a result of late radiation toxicity in vital organs Citation[20].
Models for estimating cost-effectiveness
Methods for economic evaluations
An economic evaluation can be defined as a comparison between the costs and outcomes of two or more different alternatives. In health economic evaluations there are different ways to assess the clinical outcomes, but similar techniques are almost always used for assessing the costs Citation[21]. In economic evaluations of medical treatments costs are usually divided between direct and indirect costs. Direct costs are costs for treatment, detection, prevention and care of an illness, and are often further separated into medical cost, occurring in the health care sector, and non-medical costs. Indirect costs mainly refer to production losses, i.e. changes in productivity resulting from changes in health status, because of absenteeism, lower productivity at work, or premature death.
In cost-effectiveness analysis, the clinical consequences (i.e. effectiveness) of an intervention are related to the costs. Health effects can be measured in many different ways. The measure of effectiveness can be general in its nature, such as life years gained, or it can be disease-specific, such as adverse events avoided or asthma attacks avoided. For a serious condition like cancer, the number of life years gained is an appropriate measure. However, in order to take not only the length of life but also the quality of life into account, a quality weight (utility) can be introduced, whereby all states of health are placed on a scale between 0 and 1, with 0 signifying death and 1 full health. Each life year is then multiplied with an appropriate quality weight, resulting in a measure called Quality Adjusted Life Year, or QALY for short Citation[21].
By taking the ratio of the difference in costs (ΔCost) to the difference in effectiveness (ΔEffect) between two different treatments (or health programmes), an incremental cost-effectiveness ratio can be obtained (ΔCost/ΔEffect). The incremental cost-effectiveness ratio can be interpreted as the cost for one unit of the measure of effectiveness gained, e.g. cost per life year gained or cost per QALY gained.
A societal viewpoint is often taken in cost-effectiveness analysis. This mainly concerns the costs, which from a societal viewpoint should include all costs to all parties that are affected by a disease, i.e. individuals themselves, their friends and relatives, the health care sector, third-party payers, and possibly employers. In practice, however, all relevant costs and effects are not always identified, and some may be hard to measure, e.g. psychosocial costs of pain and suffering.
Models for radiation therapy of cancer
Simulation models were developed for the four different types of cancers to estimate costs and effects of proton versus conventional therapy. The models were populated with findings in the literature. The selection of data from the literature were made based on characteristics such as perceived quality, methodology, size of patient population, country, and how recent the studies were. In general, we compared with the best alternative current treatment.
QALY was the primary effect outcome since many of the advantages of proton therapy are assumed to increase the quality of life of the patients rather than the life expectancy Citation[21]. The valuation of QALYs has been discussed quite extensively, and there are indications available about society's willingness to pay for gains in QALYs Citation[22].
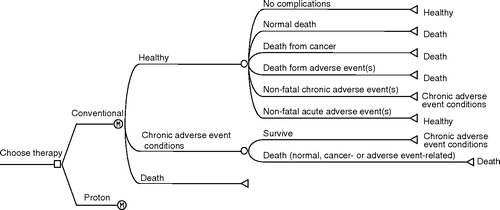
Four Markov cohort models were developed to simulate the life of patients diagnosed with the different types of cancers Citation[23]. The models simulate the course of events in individual patients from diagnosis until death or 100 years of age. In a Markov model, the patients are classified into a number of different health states, each one associated with a certain cost and utility. As time progresses in the model, the patients can move between different states, e.g. cancer in different stages, and with different events or complications, according to a set of transition probabilities. These probabilities are estimated from epidemiological and clinical studies.
The basic structure of the four different Markov models used in the present evaluation was similar and is presented in . The simulations were conducted in 1-year cycles. During each cycle patients were at risk of different events or to die. Total accumulated lifetime costs and QALYs were estimated. Costs and effects were discounted with 3% annually. All costs are in EUR (€) 2002 values (€1 = 9.2 SEK), updated using the Swedish consumer price index Citation[24].
Results
Cost of radiation therapies
Since the present study compares the cost and effects of conventional and proton radiation, we are interested in the difference in cost between these two techniques. A recent study by Goitein and Jermann in Switzerland estimated the costs of proton and x-ray radiation therapy Citation[25]. The investment cost for a proton therapy facility was calculated to about €62.5 million (about 580 million SEK) Citation[25]. The study estimated the operation cost for proton and x-ray therapy per patient, excluding business costs, to €14 700 and €7600 per patient, respectively, i.e. a difference of €7100 which corresponds to a 93% increase in costs for proton therapy.
A Swedish study, based on an inquiry sent to 16 units of radiotherapy, estimated the total cost of radiation therapy in Sweden during 2000 at €46.4 million, corresponding to an average total cost of €57.6 per radiation field, or €2587.0 per patient Citation[26]. The mean operating cost per patient, excluding capital costs, was estimated at €1913.0. However, since we focused only on patients who were complicated to treat and where the aim is long-term cure, the costs in our assessment should be higher than this, but in the absence of more accurate information we used the average operation cost per fraction for all radiation treatments, including palliative treatments, in Sweden.
In the standard case we assumed an operation cost of €123.9 per fraction for conventional radiation Citation[26]. The operation cost for proton therapy was estimated at €239.1, which is in line with both the study by Goitein and Jermann Citation[25] and cost estimations made by the SPTC (Swedish Proton Therapy Centre) project group Citation[27].
The investment cost for a proton facility with the aim to annually treat about 960 patients has, as mentioned above, been estimated to about €63 million. The corresponding cost for an IMRT facility for conventional radiation has been estimated to €16.8 million Citation[25]. To be able to distribute the investment cost to each patient, we translated the one-time investment to a yearly cost during the lifetime of the proton facility, using an annuity cost. The radiation facilities were assumed to have lifetimes of 30 years, and the interest rate was estimated at 5%.
An additional cost for proton therapy that should be included in the calculations is transportation and hotel accommodation, since a majority of the patients treated at a national proton therapy facility would be living far from the facility. Based on population statistics and treatment praxis of the cancer types considered here, we assume that between 0–70% of the patients would have extra travel and transportation costs during the treatment period. We assumed that most patients with medulloblastoma would be treated outside of their home region in any case and no extra costs for travel and accommodation were added for them. For prostate and breast cancer we assumed that 70% of the patients would have extra travel and hotel costs, while the corresponding figure for head and neck cancer patients was 35%. A daily cost of €52.2 Citation[28] for on average 20 days per patient was assumed for those patients who had accommodation costs. A travel cost of €217.4 was also added for these patients.
The total cost of radiation therapy per patient was estimated as the sum of operation cost, capital cost, and travel/hotel cost. The cost depends on the number of fractions and thus varies with the type of tumour. Breast cancer and medulloblastoma patients were assumed to be treated with 25 fractions, which gave a total estimated radiation cost of €4239 for both diseases with conventional therapy, and €10 218 for medulloblastoma and €11 101 for breast cancer with proton therapy. The difference in costs for proton therapy was a result of different assumptions regarding the costs for travel and accommodation.
Prostate and head & neck cancer patients were assumed to be treated with 35 fractions, which gave a total estimated radiation cost of €5477 for both diseases with conventional therapy, and €13 491 for prostate cancer and €13 049 for head & neck cancer with proton therapy.
Model assumptions
Breast cancer
Radiation-induced complications after radiation of the breast are important problems in the treatment of breast cancer Citation[5], Citation[7], Citation[8], Citation[29–32] and it was assumed that proton therapy could reduce the risk of these complications Citation[4], Citation[33], Citation[34]. A population of 55-year old women with left-sided breast cancer was simulated in the standard case analysis Citation[35].
Patients in the model were assumed to be at risk of cardiac and pulmonary adverse events. We did thus not include other adverse effects, like the risk of secondary malignancies, also potentially differing between treatment techniques Citation[36]. Each year, the patients were at risk of normal death, and during the first 10 years, patients were also at risk of tumour-related death. Additionally, patients in the model were at risk of both fatal and non-fatal cardiac diseases. The increased risk of cardiac disease for patients receiving radiation therapy was estimated from a study by Darby et al., which studied the incidence of cardiac disease in about 90 000 Swedish women with breast cancer Citation[37]. It is likely, however, that protons would only be used for a subgroup of patients having greater risk of cardiac mortality than others because based upon the distance of the heart within the radiation field, the individual risks can be estimated Citation[29]. Therefore, a patient population with higher risk than the average breast cancer patient would probably be the target for proton therapy. The estimated increased risk of cardiac diseases was then applied to aged- and sex-matched general population risks of the diseases. The risk of pneumonitis was mainly based on a Swedish retrospective record study by Lind et al., in which the incidence of severe pulmonary complications was estimated to be 14% Citation[38].
Cardiac diseases were assumed to be associated with both increased costs and reductions in health-related utility, while pulmonary disease only was assumed to incur costs. This assumption was made since there are no data available for the utility loss from pneumonitis. The costs and utility reductions were estimated based on findings in the literature Citation[38–46]. The estimated risk reduction of cardiac events and pneumonitis for proton radiation compared to photon and electron radiation was based on findings in a study by Johansson et al. Citation[33].
Prostate cancer
A population of 65-year old men with prostate cancer was followed in standard case analyses. The model included two types of adverse events: gastrointestinal (GI) and urogenital (UG) events. A yearly excess mortality rate due to prostate cancer was estimated at 2.5% per year, based on findings in a study by Sandblom Citation[47]. We applied this mortality rate for the first 15 years after diagnosis since the mortality increased during the first 15 years in the Sandblom study. The incidence GI and UG adverse events were based on various findings in the literature Citation[48–53].
The cost of GI and UG events were also based on findings in the literature and on assumptions of standard treatments for the adverse events Citation[41], Citation[42], Citation[54–57]. It was further assumed that patients with GI and UG events would have a 7% reduction in health utility, based on findings in previous studies Citation[58–60].
Very little information on the relative risks and effects of proton therapy in prostate cancer patients was available. It was assumed that proton therapy would be delivered at a higher target radiation dose than conventional radiation, and thereby increases the tumour control. A recent report by the Swedish Council on Technology Assessment in Health Care concluded that there is evidence that patients with localized, intermediate and high risk prostate cancer may benefit from higher radiation doses, at least in terms of freedom from failure or metastases Citation[61]. There is, however, inconclusive evidence on the overall survival effect of radiation therapy at different doses for primary prostate cancer. In the standard case we assumed a 20% reduction in cancer recurrence for proton therapy, based upon PSA free survival reported in studies by Slater et al., Hanks, and Valicenti et al. Citation[62–64]. We assumed a direct relation between cancer recurrences and cancer mortality, which means that we assumed a 20% reduction in mortality from prostate cancer with protons. The assumption was supported by a recent study by Pollack et al., in which biochemical failure was an early surrogate for distant metastasis and prostate cancer death Citation[65]. Proton therapy was also assumed to be associated with a lower risk of adverse events. The relative risk of adverse events was estimated to 0.6 Citation[66].
Head and neck cancer
A population aged 65 years at diagnosis was used as a basis for the analysis. The mortality during the first 9 years in the model was based on data from the Swedish cancer registry between 1986 and 1995. Patients surviving more than 9 years after diagnosis were assumed to have a normal age-specific mortality rate Citation[67].
In head and neck cancer, only dose planning studies are available for direct comparisons of proton therapy and conventional therapy Citation[17]. We used studies concerning alternative fractionation schedules, above all hyperfractionation, as a basis for the effects of the radiation dose on survival Citation[68–70]. The studies of hyperfractionation may perhaps provide the best available evidence of the advantages of a higher total dose. The problem is that while it is possible to show that hyperfractionation leads to improved tumour control, it is more difficult show that long-term overall survival is significantly higher Citation[70], Citation[71]. However, since many studies report better long-term survival with hyperfractionated schedules, it seems likely that this is largely due to the higher total dose. Based on data from Horiot et al. and Fu et al. Citation[69], Citation[70], a mortality risk reduction of 24% was assumed for proton radiation.
Two of the most bothersome side effects arising from irradiation of the head and neck region are acute mucositis and acute and chronic xerostomia. Radiation treatment that is focused near the salivary glands may inflict temporary or permanent damage to them. Xerostomia, which leads to dry mouth, can produce serious negative effects on the patient's quality of life, causing difficulties in tasting, chewing, swallowing, and speaking. It also increases susceptibility to dental caries and tooth loss with osteonecrosis as a secondary risk Citation[72–74].
There are many studies measuring the quality of life after radiation therapy in head and neck cancer, but few, if any, which can be used directly in health economic calculations. Based on available studies the utility score of head and neck patients two to three years after diagnosis was estimated to 0.75 Citation[75–78]. In the standard case analysis, however, we did not assume any gains in quality of life, since our calculations indicate that the potential cost-effectiveness of raising the curative dose is better.
An important cost saving is the reduced need for dentistry, since patients undergoing radiation therapy normally are referred to dentistry before the start of treatment and are seen regularly during treatment. The estimated cost reduction was based on assumptions about the reduction in the number of dental visits that head and neck patients would have. Over the remaining lifetime, the dentistry costs savings for head and neck patient were calculated to be about €3261 Citation[79], Citation[80].
Medulloblastoma
The analyses were conducted for a cohort of 5-year old children in Sweden diagnosed with medulloblastoma Citation[81]. Children surviving brain cancers have an increased mortality compared to the general population. Some of this increased mortality is due to the increased risk of secondary tumours and some is due to an increased risk of other fatal diseases Citation[82], Citation[83]. Surviving children with brain tumours also have high risk for a variety of other, non-fatal, late adverse events Citation[20], Citation[84–88].
Risks of death were in the model divided into normal death, death due to tumour recurrence, treatment related cardiac death, treatment related subsequent tumour death and treatment related other death. The additional mortality risks, compared to the general population, were based on findings in a study by Mertens et al.Citation[82]. Six types of adverse events were included in the model: hearing loss, IQ loss, hypothyroidism, growth hormone deficiency, osteoporosis, cardiac disease and subsequent cancers.
The risk of hearing loss was estimated at 13%, based on a study by Huang et al. Citation[85]. The average IQ loss was estimated at 17 points based on several studies estimating predicted IQ losses to be between 17 and 26 points Citation[86], Citation[89–91]. It is, however, not known to what extent the IQ loss can be attributed to radiation or if the risk of IQ loss is lower when using proton radiation. In the standard case it was assumed that only 25% of the IQ loss could be related to radiation therapy, but the assumption was varied in sensitivity analyses. The risks of the other adverse events were based on findings in the literature search Citation[20], Citation[82], Citation[87], Citation[88], Citation[92]. Proton therapy may also have the advantage of improving tumour control due to the possibility of giving a higher radiation dose Citation[93]. This effect was, however, not included in the present analyses since there are no clinical data available on this.
Costs and reductions in health utility due to the adverse events were calculated based on findings in the literature Citation[42], Citation[54], Citation[57], Citation[94–97]. Costs for the different adverse events were in most cases assumed to be additive, but cost for lost productivity was only assumed for IQ loss. This assumption was made to avoid double counting of the lost productivity, since several of the possible adverse events are associated with reduced productivity.
Patients receiving proton radiation were assumed to have a risk reduction compared to conventional radiation of 52% for treatment-related subsequent cancer and a reduction of 33% for treatment-related cardiac and other deaths, respectively Citation[82]. The risk reduction for hearing loss, hypothyroidism, growth hormone deficiency, IQ loss and osteoporosis was estimated to 88% Citation[20], Citation[88].
presents the standard case assumptions for the key parameters used in the four simulation models.
Table I. Standard case assumptions used in simulation models.
Cost-effectiveness of proton therapy
The cost-effectiveness of a proton radiation facility depends on the total patient population that can be treated at the facility, and hence on the number of patients with the different types of cancers. The number of patients was based on an estimation of the potential patient population targeted for proton therapy.
Breast cancer
We assumed that 300 breast cancer patients per year would be target for proton therapy. We also assumed that this population would have a higher risk of cardiac events. The increased total cost for this patient population was calculated to about €5920 per patient. The total numbers of gained QALYs were calculated to 0.17 per patient, corresponding to a cost per QALY of about €34 200.
Prostate cancer
We assumed that 300 prostate cancer patients per year would be treated with proton therapy, although it is possible that more prostate cancer patients could be target for proton therapy. The increased total cost in the standard case was calculated to about €7953 per patient and the total numbers of gained QALYs were calculated to 0.30 per patient, corresponding to a cost per QALY of about €26 800.
Head and neck cancer
We assumed that 300 head and neck cancer patients would be target for proton therapy. The increased total cost for these patients was in the standard case calculated to about €3887 per patient and the total numbers of gained QALYs were calculated to 1.02 per patient, corresponding to a cost per QALY of about €3800.
Medulloblastoma
We assumed that 25 medulloblastoma patients would be target for proton therapy. The reduced total cost for these patients was in the standard case calculated to about €23 600 per patient. The total numbers of gained QALYs were calculated to 0.68 per patient. Proton therapy thus dominated (i.e. had both lower cost and better effect) conventional therapy.
presents the standard case results and a selection of sensitivity analyses for the four types of cancers.
Table II. Results from simulation models.
shows the total estimated costs and benefits of a proton therapy facility, based on the results from the simulation models. The table shows that the proton therapy increases the cost with about €4.7 million per year, and leads to about 464 gained QALYs per year. This corresponds to an average cost-effectiveness ratio of about €10 130 per QALY gained.
Table III. Proton versus conventional therapy outcome.
If the value of a gained QALY was estimated at €55 000 Citation[22], the total yearly net benefit (total value – total costs) could be calculated to about €20.8 million. This therefore indicates that investment in a proton facility may be considered cost-effective under the assumptions used in the simulations. It should be noted that the result was based on the assumption that the four types of cancers were the only ones treated. In reality, this will not be the case, which means that the cost-effectiveness may potentially increase if other, more cost-effective, patients could be identified.
Discussion
This study presents an assessment of the potential benefits and cost-effectiveness of proton therapy compared to conventional radiation. The analysis was limited to four types of cancers that might be suitable as targets for proton therapy: left-sided breast cancer, prostate cancer, head and neck cancer and childhood medulloblastoma. Despite an extensive radiotherapeutic literature on each type of cancer, the information about the clinical effects of proton therapy was very limited. There was also a lack of information on health economic data, i.e. costs and quality of life in patients treated with radiation therapy. As a consequence, the estimates used in the assessment had to be based on more or less uncertain assumptions.
In economic evaluations, a comparison should always be made with the most relevant alternative to the treatment that is being evaluated. For proton therapy the most relevant alternative would be IMRT, but since long-term studies by necessity are based on older technologies, it was not always possible to compare with the latest technology.
Another complication is that the assessment was based on an assumed lifetime of 30 years for the proton therapy facility. If new and effective alternative treatments would be introduced during this period of time, it could affect the validity of the assumptions used in the assessment. Improvements may also be seen in proton therapy, and it is possible that a proton therapy facility may last for well beyond 30 years, which would reduce the capital cost per patient. It is thus unknown whether further developments in cancer therapy will change the cost-effectiveness relations in either direction.
The results of this study indicate that proton therapy may be a cost-effective treatment if appropriate risk groups are chosen as targets for proton therapy, and that an investment in a proton therapy facility may be cost-effective compared to using conventional radiation. It is plausible that patients targeted for proton therapy would in practice be selected even more for high risk of adverse reactions, which would mean that the benefits from proton therapy would increase.
The large uncertainty around many variables included in the assessment makes it necessary to perform extensive sensitivity analyses to see how different assumptions could affect the results. It is, however, complicated to analyse the total uncertainty for a large number of variables. The results of the selection of different sensitivity assumptions were fairly stable. A couple of key assumptions could be distinguished, e.g. the importance of the risk of cardiac events in breast cancer patients and the effect of proton therapy on prostate and head and neck cancer mortality. These factors had a very large effect on the cost-effectiveness, and the results found in this study thus rely on the validity of these key assumptions. These key factors also stress the importance of identifying appropriate targets for proton therapy, where the potential benefits described in this study can be realised.
Of the four cancer types selected for this investigation, only one, prostate cancer, is by tradition considered as a suitable target for proton therapy. The three other sites all have a potential to be treated more effectively with protons, but clinical evidence of favourable effects is not documented, only assumed. Classical proton therapy targets, like tumours in the base of the skull, were not included.
This analysis was part of the Swedish Proton Therapy Centre investigation and financial support was received from Uppsala University, Uppsala County Council and the Swedish Cancer Society.
References
- Suit H, Goldberg S, Niemierko A, Trofimov A, Adams J, Paganetti H, et al. Proton beams to replace photon beams in radical dose treatments. Acta Oncol 2003; 42(8)800–8
- Glimelius B, Isacsson U, Blomquist E, Grusell E, Jung B, Montelius A. Potential gains using high-energy protons for therapy of malignant tumours. Acta Oncol 1999; 38(2)137–45
- Rutqvist L, Rose C, Cavallin-Ståhl E. A systematic overview of radiation therapy effects in breast cancer. Acta Oncol 2003; 42(5–6)532–545
- Lomax AJ, Cella L, Weber D, Kurtz JM, Miralbell R. Potential role of intensity-modulated photons and protons in the treatment of the breast and regional nodes. Int J Radiat Oncol Biol Phys 2003; 55(3)785–92
- Gagliardi G, Lax I, Ottolenghi A, Rutqvist LE. Long-term cardiac mortality after radiotherapy of breast cancer—application of the relative seriality model. Br J Radiol 1996; 69(825)839–46
- Gagliardi G, Bjohle J, Lax I, Ottolenghi A, Eriksson F, Liedberg A, et al. Radiation pneumonitis after breast cancer irradiation: analysis of the complication probability using the relative seriality model. Int J Radiat Oncol Biol Phys 2000; 46(2)373–81
- Hernberg M, Virkkunen P, Maasilta P, Keyrilainen J, Blomqvist C, Bergh J, et al. Pulmonary toxicity after radiotherapy in primary breast cancer patients: results from a randomized chemotherapy study. Int J Radiat Oncol Biol Phys 2002; 52(1)128–36
- Hurkmans CW, Borger JH, Bos LJ, van der Horst A, Pieters BR, Lebesque JV, et al. Cardiac and lung complication probabilities after breast cancer irradiation. Radiother Oncol 2000; 55(2)145–51
- Cho KH, Khan FM, Levitt SH. Cost-benefit analysis of 3D conformal radiation therapy—treatment of prostate cancer as a model. Acta Oncol 1999; 38(5)603–11
- Tralins K, Wallner K. Follow-up costs after external radiation for low risk prostate cancer. Int J Radiat Oncol Biol Phys 1999; 44(2)323–6
- Altwein J, Ekman P, Barry M, Biermann C, Carlsson P, Fossa S, et al. How is quality of life in prostate cancer patients influenced by modern treatment? The Wallenberg Symposium. Urology 1997; 49((4A Suppl))66–76
- Fransson P, Damber JE, Tomic R, Modig H, Nyberg G, Widmark A. Quality of life and symptoms in a randomized trial of radiotherapy versus deferred treatment of localized prostate carcinoma. Cancer 2001; 92(12)3111–9
- Zelefsky MJ, Cowen D, Fuks Z, Shike M, Burman C, Jackson A, et al. Long term tolerance of high dose three-dimensional conformal radiotherapy in patients with localized prostate carcinoma. Cancer 1999; 85(11)2460–8
- Shipley WU, Verhey LJ, Munzenrider JE, Suit HD, Urie MM, McManus PL, et al. Advanced prostate cancer: the results of a randomized comparative trial of high dose irradiation boosting with conformal protons compared with conventional dose irradiation using photons alone. Int J Radiat Oncol Biol Phys 1995; 32(1)3–12
- Slater JD, Yonemoto LT, Rossi CJ, Jr, Reyes-Molyneux NJ, Bush DA, Antoine JE, et al. Conformal proton therapy for prostate carcinoma. Int J Radiat Oncol Biol Phys 1998; 42(2)299–304
- Zackrisson B, Mercke C, Strander H, Wennerberg J, Cavallin-Stahl E. A systematic overview of radiation therapy effects in head and neck cancer. Acta Oncol 2003; 42(5–6)443–61
- Cozzi L, Fogliata A, Lomax A, Bolsi A. A treatment planning comparison of 3D conformal therapy, intensity modulated photon therapy and proton therapy for treatment of advanced head and neck tumours. Radiother Oncol 2001; 61(3)287–97
- Freeman CR, Taylor RE, Kortmann RD, Carrie C. Radiotherapy for medulloblastoma in children: a perspective on current international clinical research efforts. Med Pediatr Oncol 2002; 39(2)99–108
- Kiltie AE, Lashford LS, Gattamaneni HR. Survival and late effects in medulloblastoma patients treated with craniospinal irradiation under three years old. Med Pediatr Oncol 1997; 28(5)348–54
- Miralbell R, Lomax A, Russo M. Potential role of proton therapy in the treatment of pediatric medulloblastoma/primitive neuro-ectodermal tumors: spinal theca irradiation. Int J Radiat Oncol Biol Phys 1997; 38(4)805–11
- Drummond M, O'Brien B. Methods for the Economic Evaluation of Health Care Programmes. Oxford University Press. Oxford. 1997
- Ekman M. Studies in Health Economics. EFI, Stockholm School of Economics, Stockholm 2002
- Sonnenberg F, Beck J. Markov models in medical decision making: a practical guide. Med Decis Making 1993; 13(4)322–38
- Statistics Sweden. Sweden's Statistical Databases. 2005.
- Goitein M, Jermann M. The relative costs of proton and X-ray radiation therapy. Clin Oncol (R Coll Radiol) 2003; 15(1)S37–50
- Strålbehandling vid cancer. Linköping:The Swedish Council on Technology Assessment in Health Care (SBU); 2003.
- SPTC. Summary report from the project group for the Swedish Proton Therapy Centre (SPTC). 2003.
- Pricelist, patient hotel at Akademiska hospital, Uppsala. 2003.
- Hurkmans CW, Cho BC, Damen E, Zijp L, Mijnheer BJ. Reduction of cardiac and lung complication probabilities after breast irradiation using conformal radiotherapy with or without intensity modulation. Radiother Oncol 2002; 62(2)163–71
- Lind PA, Pagnanelli R, Marks LB, Borges-Neto S, Hu C, Zhou SM, et al. Myocardial perfusion changes in patients irradiated for left-sided breast cancer and correlation with coronary artery distribution. Int J Radiat Oncol Biol Phys 2003; 55(4)914–20
- Gyenes G, Rutqvist LE, Liedberg A, Fornander T. Long-term cardiac morbidity and mortality in a randomized trial of pre- and postoperative radiation therapy versus surgery alone in primary breast cancer. Radiother Oncol 1998; 48(2)185–90
- Gyenes G. Radiation-induced ischemic heart disease in breast cancer—a review. Acta Oncol 1998; 37(3)241–6
- Johansson J, Isacsson U, Lindman H, Montelius A, Glimelius B. Node-positive left-sided breast cancer patients after breast-conserving surgery: potential outcomes of radiotherapy modalities and techniques. Radiother Oncol 2002; 65(2)89–98
- Lomax AJ, Boehringer T, Coray A, Egger E, Goitein G, Grossmann M, et al. Intensity modulated proton therapy: a clinical example. Med Phys 2001; 28(3)317–24
- Lundkvist J, Ekman M, Ericsson Rehn S, Isacsson U, Jönsson B, Glimelius B. Economic evaluation of proton radiation therapy in the treatment of breast cancer. Radiother Oncol 2005; 75(2)179–85
- Travis LB. Therapy-associated solid tumors. Acta Oncol 2002; 41(4)323–33
- Darby S, McGale P, Peto R, Granath F, Hall P, Ekbom A. Mortality from cardiovascular disease more than 10 years after radiotherapy for breast cancer: nationwide cohort study of 90 000 Swedish women. BMJ 2003; 326(7383)256–7
- Lind PA, Gagliardi G, Wennberg B, Fornander T. A descriptive study of pulmonary complications after postoperative radiation therapy in node-positive stage II breast cancer. Acta Oncol 1997; 36(5)509–15
- Zethraeus N, Molin T, Henriksson P, Jonsson B. Costs of coronary heart disease and stroke: the case of Sweden. J Intern Med 1999; 246(2)151–9
- National inpatient care statistics. Stockholm:National Board of Health and Welfare (Socialstyrelsen, EpC); 2002
- Price list, Lund University hospital, Lund, Sweden. 2002.
- LINFO, FASS Lakemedel i Sverige (Swedish drug prices). 2001, Oslo: Lakemedelsinformation AB.
- National statistics. Stockholm:Statistic Sweden; 2003
- Nicholson T, McGuire A, Milne R. Cost-utility of enoxaparin compared with unfractionated heparin in unstable coronary artery disease. BMC Cardiovasc Disord 2001; 1(1)2
- Capomolla S, Febo O, Ceresa M, Caporotondi A, Guazzotti G, La Rovere M, et al. Cost/utility ratio in chronic heart failure: comparison between heart failure management program delivered by day-hospital and usual care. J Am Coll Cardiol 2002; 40(7)1259–66
- Cohen DJ, Taira DA, Berezin R, Cox DA, Morice MC, Stone GW, et al. Cost-effectiveness of coronary stenting in acute myocardial infarction: results from the stent primary angioplasty in myocardial infarction (stent-PAMI) trial. Circulation 2001; 104(25)3039–45
- Sandblom G, Dufmats M, Varenhorst E. Long-term survival in a Swedish population-based cohort of men with prostate cancer. Urology 2000; 56(3)442–7
- Lilleby W, Fossa SD, Waehre HR, Olsen DR. Long-term morbidity and quality of life in patients with localized prostate cancer undergoing definitive radiotherapy or radical prostatectomy. Int J Radiat Oncol Biol Phys 1999; 43(4)735–43
- Dearnaley DP, Khoo VS, Norman AR, Meyer L, Nahum A, Tait D, et al. Comparison of radiation side-effects of conformal and conventional radiotherapy in prostate cancer: a randomised trial. Lancet 1999; 353(9149)267–72
- Widmark A, Fransson P, Tavelin B. Self-assessment questionnaire for evaluating urinary and intestinal late side effects after pelvic radiotherapy in patients with prostate cancer compared with an age-matched control population. Cancer 1994; 74(9)2520–32
- Kupelian PA, Reddy CA, Carlson TP, Altsman KA, Willoughby TR. Preliminary observations on biochemical relapse-free survival rates after short-course intensity-modulated radiotherapy (70 Gy at 2.5 Gy/fraction) for localized prostate cancer. Int J Radiat Oncol Biol Phys 2002; 53(4)904–12
- Zelefsky MJ, Fuks Z, Hunt M, Yamada Y, Marion C, Ling CC, et al. High-dose intensity modulated radiation therapy for prostate cancer: early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys 2002; 53(5)1111–6
- Gardner BG, Zietman AL, Shipley WU, Skowronski UE, McManus P. Late normal tissue sequelae in the second decade after high dose radiation therapy with combined photons and conformal protons for locally advanced prostate cancer. J Urol 2002; 167(1)123–6
- Stockholm County Council, StockholmSweden. 2003.
- Crook J, Esche B, Futter N. Effect of pelvic radiotherapy for prostate cancer on bowel, bladder, and sexual function: the patient's perspective. Urology 1996; 47(3)387–94
- Johnstone PA, Gray C, Powell CR. Quality of life in T1-3N0 prostate cancer patients treated with radiation therapy with minimum 10-year follow-up. Int J Radiat Oncol Biol Phys 2000; 46(4)833–8
- Price list, Labmedicin, Huddinge hospital, Stockholm. 2003.
- Krahn M, Ritvo P, Irvine J, Tomlinson G, Bremner KE, Bezjak A, et al. Patient and community preferences for outcomes in prostate cancer: implications for clinical policy. Med Care 2003; 41(1)153–64
- Saigal CS, Gornbein J, Reid K, Litwin MS. Stability of time trade-off utilities for health states associated with the treatment of prostate cancer. Qual Life Res 2002; 11(5)405–14
- Albertsen PC, Nease RF, Jr, Potosky AL. Assessment of patient preferences among men with prostate cancer. J Urol 1998; 159(1)158–63
- Nilsson S, Widmark A, Norlen BJ. A systematic overview of radiation therapy effects in prostate cancer. Acta Oncol 2004; 43: 316–81
- Zelefsky MJ, Leibel SA, Gaudin PB, Kutcher GJ, Fleshner NE, Venkatramen ES, et al. Dose escalation with three-dimensional conformal radiation therapy affects the outcome in prostate cancer. Int J Radiat Oncol Biol Phys 1998; 41(3)491–500
- Perez CA, Michalski J, Lockett MA. Radiation therapy in the treatment of localized prostate cancer: an alternative to an emerging consensus. Mo Med 1995; 92(11)696–704
- Hanks GE, Hanlon AL, Pinover WH, Horwitz EM, Price RA, Schultheiss T. Dose selection for prostate cancer patients based on dose comparison and dose response studies. Int J Radiat Oncol Biol Phys 2000; 46(4)823–32
- Pollack A, Hanlon AL, Movsas B, Hanks GE, Uzzo R, Horwitz EM. Biochemical failure as a determinant of distant metastasis and death in prostate cancer treated with radiotherapy. Int J Radiat Oncol Biol Phys 2003; 57(1)19–23
- Cella L, Lomax A, Miralbell R. Potential role of intensity modulated proton beams in prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys 2001; 49(1)217–23
- Statistical Yearbook of Sweden. Statistics Sweden 2001.
- Mendenhall W, Riggs C, Amdur R, Hinerman R, Villaret D. Altered fractionation and/or adjuvant chemotherapy in definitive irradiation of squamous cell carcinoma of the head and neck. Laryngoscope 2003; 113(3)546–51
- Horiot JC, Le Fur R, N'Guyen T, Chenal C, Schraub S, Alfonsi S, et al. Hyperfractionation versus conventional fractionation in oropharyngeal carcinoma: final analysis of a randomized trial of the EORTC cooperative group of radiotherapy. Radiother Oncol 1992; 25(4)231–41
- Fu K, Pajak T, Trotti A, Jones C, Spencer S, Phillips T, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys 2000; 48(1)7–16
- Jeremic B, Shibamoto Y, Milicic B, Nikolic N, Dagovic A, Aleksandrovic J, et al. Hyperfractionated radiation therapy with or without concurrent low-dose daily cisplatin in locally advanced squamous cell carcinoma of the head and neck: a prospective randomized trial. J Clin Oncol 2000; 18(7)1458–64
- Brizel DM, Wasserman TH, Henke M, Strnad V, Rudat V, Monnier A, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol 2000; 18(19)3339–45
- Henson BS, Inglehart MR, Eisbruch A, Ship JA. Preserved salivary output and xerostomia-related quality of life in head and neck cancer patients receiving parotid-sparing radiotherapy. Oral Oncol 2001; 37(1)84–93
- Lin A, Kim HM, Terrell JE, Dawson LA, Ship JA, Eisbruch A. Quality of life after parotid-sparing IMRT for head-and-neck cancer: a prospective longitudinal study. Int J Radiat Oncol Biol Phys 2003; 57(1)61–70
- Hammerlid E, Silander E, Hornestam L, Sullivan M. Health-related quality of life three years after diagnosis of head and neck cancer—a longitudinal study. Head Neck 2001; 23(2)113–25
- Hammerlid E, Taft C. Health-related quality of life in long-term head and neck cancer survivors: a comparison with general population norms. Br J Cancer 2001; 84(2)149–56
- Epstein JB, Emerton S, Kolbinson DA, Le ND, Phillips N, Stevenson-Moore P, et al. Quality of life and oral function following radiotherapy for head and neck cancer. Head Neck 1999; 21(1)1–11
- Epstein JB, Robertson M, Emerton S, Phillips N, Stevenson-Moore P. Quality of life and oral function in patients treated with radiation therapy for head and neck cancer. Head Neck 2001; 23(5)389–98
- Hospital dentist Marta Roing, personal communication. 2003.
- Statistical Yearbook of Health and Medical Care. National Board of Health and Welfare 2002.
- Lundkvist J, Ekman M, Ericsson SR, Jonsson B, Glimelius B. Cost- effectiveness of proton radiation in the treatment of childhood medulloblastoma. Cancer 2005; 103(4)793–801
- Mertens AC, Yasui Y, Neglia JP, Potter JD, Nesbit ME, Jr, Ruccione K, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol 2001; 19(13)3163–72
- Moller TR, Garwicz S, Barlow L, Falck Winther J, Glattre E, Olafsdottir G, et al. Decreasing late mortality among five-year survivors of cancer in childhood and adolescence: a population-based study in the Nordic countries. J Clin Oncol 2001; 19(13)3173–81
- Grau C, Overgaard J. Postirradiation sensorineural hearing loss: a common but ignored late radiation complication. Int J Radiat Oncol Biol Phys 1996; 36(2)515–7
- Huang E, Teh BS, Strother DR, Davis QG, Chiu JK, Lu HH, et al. Intensity-modulated radiation therapy for pediatric medulloblastoma: early report on the reduction of ototoxicity. Int J Radiat Oncol Biol Phys 2002; 52(3)599–605, 3rd
- Copeland DR, deMoor C, Moore BD, 3rd, Ater JL. Neurocognitive development of children after a cerebellar tumor in infancy: A longitudinal study. J Clin Oncol 1999; 17(11)3476–86
- Gurney JG, Kadan-Lottick NS, Packer RJ, Neglia JP, Sklar CA, Punyko JA, et al. Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood Cancer Survivor Study. Cancer 2003; 97(3)663–73
- Miralbell R, Lomax A, Cella L, Schneider U. Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumors. Int J Radiat Oncol Biol Phys 2002; 54(3)824–9
- Ris MD, Packer R, Goldwein J, Jones-Wallace D, Boyett JM. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children's Cancer Group study. J Clin Oncol 2001; 19(15)3470–6
- Palmer SL, Goloubeva O, Reddick WE, Glass JO, Gajjar A, Kun L, et al. Patterns of intellectual development among survivors of pediatric medulloblastoma: a longitudinal analysis. J Clin Oncol 2001; 19(8)2302–8
- Walter AW, Mulhern RK, Gajjar A, Heideman RL, Reardon D, Sanford RA, et al. Survival and neurodevelopmental outcome of young children with medulloblastoma at St Jude Children's Research Hospital. J Clin Oncol 1999; 17(12)3720–8
- Ricardi U, Corrias A, Einaudi S, Genitori L, Sandri A, di Montezemolo LC, et al. Thyroid dysfunction as a late effect in childhood medulloblastoma: a comparison of hyperfractionated versus conventionally fractionated craniospinal radiotherapy. Int J Radiat Oncol Biol Phys 2001; 50(5)1287–94
- Archambeau JO, Slater JD, Slater JM, Tangeman R. Role for proton beam irradiation in treatment of pediatric CNS malignancies. Int J Radiat Oncol Biol Phys 1992; 22(2)287–94
- Mohr PE, Feldman JJ, Dunbar JL. The societal costs of severe to profound hearing loss in the United States. Policy Anal Brief H Ser 2000; 2(1)1–4
- Horapparat for vuxna-nytta och kostnader. Linkoping:The Swedish Council on Technology Assessment in Health Care (SBU); 2003
- Schwartz J. Societal benefits of reducing lead exposure. Environ Res 1994; 66(1)105–24
- Läkemedelsboken. Lund:Apoteket AB; 2001