Abstract
In order to increase the availability of adjuvant radiotherapy of breast cancer patients and make it more convenient and cheaper, in numerous cancer centres, the dose per fraction has been increased from 2 Gy to 2.25–2.75 Gy and the total dose has been decreased from 50 Gy to 40–45 Gy. The risk of developing any late complications after conventionally fractionated megavoltage radiotherapy is estimated to be below 1%. The aim of this review is to determine whether hypofractionated regimens increase the risk of damage to the brachial plexus. A review of the published literature shows that the use of doses per fraction in the range from 2.2 Gy to 4.58 Gy with the total doses between 43.5 Gy and 60 Gy causes a significant risk of brachial plexus injury which ranged from 1.7% up to 73%. The risk of radiation induced brachial plexopathy was smaller than 1% using regimens with doses per fraction between 2.2 and 2.5 Gy with the total doses between 34 and 40 Gy. Surgical manipulations in the axilla and chemotherapy have to be taken into account as additional factors which may increase the risk of brachial plexopathy.
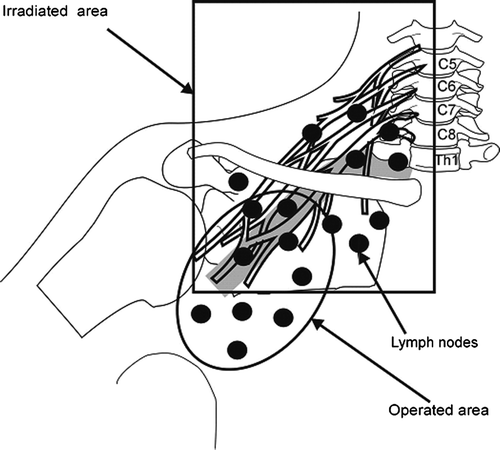
In breast cancer patients brachial plexus morbidity may result from trauma during axillary surgery or from postoperative irradiation Citation[1–4]. shows schematically the topography of the brachial plexus and outlined the operated and irradiated axillary, supra- and infraclavicular nodes area. The irradiated volume includes, among other tissues, the brachial plexus.
Figure 1. Schematic representation of the brachial plexus in relation to the borders of irradiated field and operated area.
The effectiveness of postoperative radiotherapy in diminishing the risk of local relapse in breast cancer patients has been well established Citation[5], Citation[6]. Three recent randomised trials showed that long-term survival was significantly better in postmastectomy patients treated with adjuvant radiation and chemo- or hormonotherapy than in non-irradiated women receiving only systemic treatment Citation[7–9]. This benefit was confirmed in the meta-analysis of the Early Breast Cancer Trialists’ Collaborative Group Citation[6]. The overall survival benefit in postoperative irradiated patients is assured provided that modern radiotherapy techniques are used Citation[10]. Therefore, adjuvant irradiation is considered as routine practice for high risk breast cancer patients with more than 3 positive nodes. Some studies however showed an even higher benefit with postmastectomy radiotherapy in patients with 1–3 positive nodes Citation[11–13]. Following publication of these studies the number of patients referred for postoperative irradiation has risen abruptly. To shorten the waiting time for postoperative irradiation and to make the treatment more convenient for patients and cheaper in many cancer centres, hypofractionation is used. However, there is concern that a short-course fractionation schedule may be associated with the higher risk of late complications to the brachial plexus. The risk of developing brachial plexopathy after conventionally fractionated megavoltage radiotherapy is estimated to be below 1% Citation[14], Citation[15].
The aim of this literature review is to determine whether shortened fractionation regimens are associated with an increased risk of radiation induced brachial plexopathy.
The association between irradiation schedule and radiation-induced brachial plexopathy
Kori et al. Citation[1] reviewed 22 cancer patients with radiation induced brachial plexopathy (RIBP). The interval between radiotherapy and the appearance of first symptoms ranged from 3 months to 26 years with a median of 4 years. In patients who received the total dose over 60 Gy, the signs and symptoms of plexopathy appeared during the first year post therapy, while in those who were received less than 60 Gy, the interval exceeded one year. Stoll et al. and Bentzen et al. Citation[16], Citation[17] found that the interval between radiotherapy and the beginning of RIBP depends on the total dose and the dose per fraction. This interval becomes shorter when dose per fraction and/or total dose increases. Earlier reports described brachial plexus damage with high fraction doses-more than 4 Gy (). Stoll et al. Citation[16] diagnosed RIBP in 73% and 15% of patients, who were treated by a 4 MV photons with a total dose of 55 Gy in 4.58 Gy per fraction and 51 Gy in 4.25 Gy per fraction to the brachial plexus accordingly. Patients were treated with one field to the supraclavicular lymph node region after total mastectomy. The biological equivalent dose (BED) in both radiation regimens was 90 Gy and 80 Gy respectively. The calculation was made for the α/β model, where α/β was 2 Gy. The third group of 139 patients presented by Stoll et al. Citation[16] were treated with orthovoltage irradiation giving a total dose 43.5 Gy to the brachial plexus in 10 fractions. In 14 (10%) of them developed symptoms of brachial plexopathy. The BED was 69 Gy given in 2 Gy per fraction. The follow-up was up to 30 months Citation[16]. Johansson et al. Citation[18] reported a series of 71 patients who underwent radical mastectomy with subsequent 60Co irradiation to the peripheral lymph nodes to a prescribed total dose of 44 Gy in 4 Gy per fraction. Only two of the three fields were irradiated daily: supraclavicular, axillary or parasternal. Because of an overlap of the treated volume, the total dose delivered to the brachial plexus was 57 Gy in 16–17 fractions over 3–4 weeks. The BED was 78 Gy given in 2 Gy per fraction. Within 34 years after completion of the therapy full plegia of the extremity developed in 11 of 12 patients-92%. Massive fibrosis was found in the irradiated volume. Severe radiation-related complications were due to the high doses per fraction and the hot spots produced by fields overlapping owing to changes in the patient's position between treatment of the supraclavicular and axillary fields.
Table I. The incidence of radiation-induced brachial plexopathy (RIBP) in respect to dose per fraction and total dose to brachial plexus.
An independent review of patients with RIBP in the United Kingdom performed by Royal College of Radiologists also highlighted changes in patient position during radiotherapy as a major factor increasing the risk of inadvertent field overlap Citation[19]. Barr et al. Citation[20] reported on the postirradiation complications following breast-conserving surgery. The total radiotherapy dose received by the brachial plexus was 51 Gy in 3.4 Gy per fraction. The BED was 69 Gy given in 2 Gy per fraction. RIBP was found in 2.4% of the women. Powell et al. Citation[21] studied the incidence of RIBP in a series of 449 patients randomised into two arms: the first one irradiated with mean total dose of 45 Gy in 15 fractions (mean dose per fraction 3 Gy – BED = 56 Gy) and the second one with 54 Gy in 27–30 fractions (dose per fraction 1.8–2 Gy – BED = 51 Gy). The incidence of plexopathy was 5.9% in the low total and high fractional dose arm and 1% in the high total and low fractional dose arm. This confirmed the thesis that dose per fraction is an important risk factor for the development of plexopathy. Basso-Ricci et al. Citation[22] diagnosed RIBP in 16 of 490 patients (3.2%) in whom the total dose to the plexus was 60 Gy in 2 Gy per fraction. The technique used was characterized by a high risk of field overlap and a dose to the plexus higher than expected from the prescribed dose. No RIBP was observed after changes in the irradiation technique and lowering the total dose to the supraclavicular nodes to 49 Gy with 1.96 Gy per fraction in a group of 200 patients. Bajrovic et al. Citation[23] observed RIBP in 19 of 140 (14%) patients who were irradiated with 60Co on supraclavicular field with a total dose 52 Gy with 2.6 Gy per fraction to the brachial plexus. Delouche et al. Citation[24] observed RIBP in 2 of 117 patients (1.7%) irradiated to the total given dose of 60 Gy in 2 Gy per fraction.
Nowadays it is believed that RIBP can be avoided using three-dimensional planning and techniques which deliver the proper dose to the brachial plexus and irradiate all volumes without a change in patient's position during treatment. The irradiation of all fields during one fraction using megavoltage equipment with isocentric techniques and multi-leaf asymmetric collimators may decrease the risk of RIBP and other serious late side effects Citation[18], Citation[25].
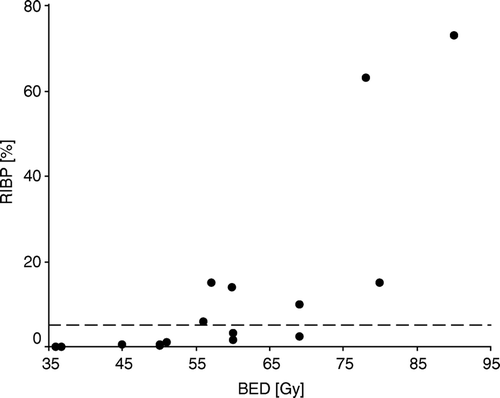
The main controversy now surrounds differences in fractionation schedules. In the majority of radiotherapy centers worldwide the total dose of radical adjuvant irradiation in breast cancer is 46–50 Gy delivered over 4.5–5 weeks with 2 Gy per fraction. However, in numerous radiotherapy centers, particularly in the United Kingdom and Canada, for 20–30 years, fractional doses have been higher: 2.25–2.75 Gy, and total doses lower: 40–45 Gy Citation[26–28]. As a result of such an approach, the duration of therapy is shorter, falling in the range of 3–4 weeks. It has been estimated that in the United Kingdom 2/3 of breast cancer patients irradiated with curative intent are treated with this schedule Citation[29]. The START randomised controlled trial in the United Kingdom has compared different fractionation schedules in breast cancer: 50 Gy in 25 daily fractions, 40 Gy in 15 daily fractions, 39 and 42 Gy in 13 fractions in 5 weeks Citation[30]. Results are awaited. and summarize the published data on the risk of RIBP in relation to the delivered dose and fractionation schedule with respect to BED. When the total dose to the plexus was between 43.5 and 60 Gy with dose per fraction between 2.17 and 4.58 Gy, the incidence of RIBP ranged from 1.7% to 90% and increased with longer time of follow-up. Even when the dose per fraction was low and ranged between 2 and 2.17 Gy, but the total dose was not decreased, between 54.25 and 60 Gy and BED was higher than 55 Gy, the RIBP rose steeply with the dose from 1.7% to 15%. Higher than expected rates of RIBP observed in some groups of patients were probably due to errors in irradiation technique and the higher doses to the brachial plexus than those prescribed.
Figure 2. Relationship between incidences of radiation-induced brachial plexopathy (RIBP) and biological effective dose (BED). The reference line indicates the 5% level of RIBP.
Retrospective studies by Livsey et al. from Manchester Citation[31] and Fairchild et al. from Vancouver Citation[26] reported that shortened radiotherapy courses using higher doses per fraction in the range of 2.27 and 2.5 Gy and smaller total doses of 34–40 Gy (BED 36–45 Gy), were associated with an the incidence of RIBP below 1%, thus similar to that observed after total dose 50 Gy with 2 Gy per fraction Citation[14], Citation[15]. There were no cases of brachial plexopathy during the median follow-up of 12.5 years among 164 irradiated patients in the randomised study reported by Ragaz et al. Citation[9]. The total dose to the brachial plexus was 35 Gy with 2.19 Gy dose per fraction (BED – 36.7 Gy).
These studies strongly suggest that short-course radiotherapy with a small increase of dose per fraction from 2 Gy to 2.19–2.5 Gy and decrease of the total dose from 50 Gy to 34–40 Gy is safe for the brachial plexus, provided the BED is not higher than 51 Gy (). Moreover, retrospective clinical studies with long-term results revealed that the effectiveness of shortened radiotherapy in lowering the risk of local recurrence is comparable to that obtained with conventional fractionation Citation[26–28]. However, in some studies the median time period was relatively short (5–8 years) which have let to the underestimation of the rate of incidence of RIBP.
The addition of adjuvant chemotherapy to radiotherapy may result in increased risk of brachial plexopathy (). RIBP developed in 20 of 1117 (1.8%) women who were irradiated using 4 MV X-rays to supraclavicular and/or axillary nodes with total dose of 50 Gy with 2 Gy per fraction with or without chemotherapy at the Joint Centre for Radiation Therapy in Boston Citation[15]. In 17 patients (85%) the signs and symptoms of brachial plexus injury resolved completely. In the remaining three patients with severe brachial plexus impairment, the dose to the axilla estimated at 5 cm depth was 51, 52 and 53.5 Gy. The main factor in the development of plexopathy was adjuvant systemic treatment given at the discretion of the medical oncologist. The following cytostatic drugs were used in different combinations and sequencing with radiotherapy: cyclophosphamide, methotrexate, 5-fluorouracil, vincristine, prednisone, and rarely doxorubicin. The incidence of brachial plexopathy was found in 4.5% (15/330) of patients treated with radiation and chemotherapy compared to 0.6% (5/787) of those who were treated with radiation alone (p < 0.0001). Analysis of the total radiation dose and use of chemotherapy showed that brachial plexopathy developed in 1.3% (13/991) of patients who received 50 Gy or less (0.4% without chemotherapy versus 3.7% with chemotherapy) and in 5.6% (7/126) who were given total dose over 50 Gy (3.2% without chemotherapy versus 7.9% with chemotherapy) to the brachial plexus.
Table II. The incidence of radiation-induced brachial plexopathy (RIBP) in respect to total axillary radiation dose (TD) and chemotherapy CT.
The Danish Breast Cancer Cooperative Group (DBCG) conducted another study which compared the complications in breast cancer patients following postmastectomy irradiation treated with and without adjuvant chemotherapy Citation[32]. One hundred and sixty one relapse free patients were invited to undergo neurological examination. Mean follow-up was 50 months (range 13–99). One hundred twenty-eight patients (79%) responded. The mean dose to the supraclavicular-axillary area was 54.25 Gy in 2.17 Gy per fraction with 8–14 MV photons. In 82/128 patients (64%), intravenous CMF chemotherapy was given. Radiotherapy was given sequentially between first and second course of chemotherapy. Brachial plexopathy was observed in 19/128 (15%) of patients. Seven of them (5.5%) developed permanent disability due to brachial plexus damage and twelve (9.5%) mild and transient plexopathy. Among these 19 women with RIBP, in 13% (17/128) chemotherapy had been given, compared to 1.6% (2/128) patients without chemotherapy (p = 0.01) (). The tolerance of the brachial plexus to chemo-radiation and the timing of combined radiotherapy with newer regimens of chemotherapy such as the taxanes require further study.
Conclusions
It has been found that the use of doses per fraction in the range 2.2 Gy and 4.58 Gy with the total doses between 43.5 Gy and 60 Gy causes a significant increase of the risk of brachial plexus injury from 1.7% up to 73%. The risk of radiation induced brachial plexopathy was smaller than 1% after administrating of doses per fraction between 2.2 and 2.5 Gy with the total dose between 34 and 40 Gy. When biological effective dose was above 55 Gy, the risk of radiation-induced brachial plexopathy increased rapidly. Surgical manipulations in the axilla and chemotherapy have to be taken into account as additional factors which may increase the risk of brachial plexopathy.
The authors are grateful to Dr Brian Magee from the Christie Hospital in Manchester and Dr Krzysztof Bujko from the Cancer Center in Warsaw for critical comments on the manuscript.
References
- Kori SH, Foley KM, Posner JB. Brachial plexus lesions in patients with cancer 100 cases. Neurology 1981; 31: 45–50
- Cherny NC, Folley KM. Brachial plexopathy in patients with breast cancer. Diseases of the breast, JR Harris, et al. Lippincott-Raven Publishers, Philadelphia 1996; 796–808
- Ferrante MA, Wilbourn AJ. Plexopathies. Comprehensive Clinical Neurophysiology, KH Levin, HO Luders. WB Sounders Company. 2000; 202
- Bentzen SM, Dische S. Morbidity related to axillary irradiation in the treatment of breast cancer. Acta Oncol 2000; 39: 337–47
- Rutqvist LE, Pettersson D, Johansson H. Adjuvant radiation therapy versus surgery alone in operable breast cancer: long-term follow-up of a randomised clinical trial. Radiother Oncol 1993; 26: 104–9
- Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 2000;20:1757–70.
- Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive an adjuvant. N Engl J Med 1997; 337: 949–55
- Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999; 15: 1641–8
- Ragaz J, Jackson SM, Le N, Plenderleith IH, Spinelli JJ, Basco VE, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med 1997; 337: 956–62
- Van de Steene J, Vinh-Hung V, Cutuli B, Storme G. Adjuvant radiotherapy for breast cancer: effects of longer follow-up. Radiother Oncol 2004; 72: 35–43
- Marks LB, Prosnitz LR. One to three” or “four or more”? – Selecting patients for postmastectomy radiation therapy. Cancer 1997; 79: 668–70
- Korzeniowski S. One to three” or Four or more”? – Selecting patients for postmastectomy radiation therapy – correspondence. Cancer 1997; 80: 1357–58
- Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with 4 or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b & c randomized trials. Radiother Oncol ;A 2004; 73(Supp 1)S14
- Fowble BL, Solin LJ, Schultz DJ, Goodman RL. Ten-year results of conservative surgery and irradiation for stage I and II breast cancer. Int J Radiat Oncol Biol Phys 1991; 21: 269–77
- Pierce SM, Recht A, Lingos TI, Abner A, Vicini F, Silver B, et al. Long-term radiation complication following conservative surgery (CS) and radiation therapy (RT) in patients with early stage breast cancer. Int J Radiat Oncol Biol Phys 1992; 23: 915–23
- Stoll BA, Andrews JT. Radiation-induced peripheral neuropathy. Br Med J 1966; 1: 834–7
- Bentzen SM, Thames HD, Travis EL, Ang KK, Van der Schueren E, Dewit L, et al. Direct estimation of latent time for radiation injury in late responding normal tissues: gut, lung at spinal cord. Int J Radiat Biol 1989; 55: 27–32
- Johansson S, Svensson H, Denekamp J. Timescale of evolution of late radiation injury after postoperative radiotherapy of breast cancer patients. Int J Radiat Oncol Biol Phys 2000; 48: 745–50
- Bates T, Evans RG. Audit of brachial plexus neuropathy following radiotherapy. Clin Oncol (R Coll Radiol) 1995; 7(4)236
- Barr LC, Kissin MW. Radiation-induced brachial plexus neuropathy following breast conservation and radical radiotherapy. Br J Surg 1987; 74: 855–6
- Powell S, Cooke J, Parsons C. Radiation induced brachial plexus injury: follow-up of two different fractionation schedules. Radiother Oncol 1990; 18: 213–20
- Basso-Ricci S, della Costa C, Viganotti G, Ventafridda V, Zanolla R. Report on 42 cases of postirradiation lesions of the brachial plexus and their treatment. Tumori 1980; 66: 117–22
- Bajrovic A, Rades D, Fehlauer F, Tribius S, Hoeller U, Rudat V, et al. Is there a life-long risk of brachial plexopathy after radiotherapy of supraclavicular lymph nodes in breast cancer patients?. Radiother Oncol 2004; 71: 297–301
- Delouche G, Bachelot F, Premont M, Kurtz JM. Conservation treatment of early breast cancer. Long-term results and complications. Int J Radiat Oncol Biol Phys 1987; 13: 29–34
- Dobbs HJ. Radiation therapy for breast cancer at millennium. Radiother Oncol 2000; 54: 191–200
- Fairchild AM, Weir LM, Mates D, Olivotto IA. Loco-regional radiation for high-risk breast cancer-results of short fractionation. Breast Cancer Research Treatment 2000; 64Abstract 38: 35
- Magee B, Ribeiro GG, Williams P, Swindell R. Use of electron beam for post-mastectomy radiotherapy: 5-year follow-up of 500 cases. Clin Oncol 1991; 3: 310–4
- Vallis KA, Pintilie M, Chong N, Holowaty E, Douglas PS, Kirkbride P, et al. Assessment of coronary heart disease morbidity and mortality after radiation therapy for early breast cancer. J Clin Oncol 2002; 20: 1036–42
- Yarnold JR, Price P, Steel GG. Treatment of early breast cancer in the United Kingdom: radiotherapy fractionation practices. Clin Oncol 1995; 7: 223–6
- Yarnold J, Ashton A, Bliss J, Homewood J, Harper C, Hanson J, et al. Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: long term results of a randomised trial. Radiother Oncol 2005; 75: 9–17
- Livsey JE, Magee B, Stewart AL, Swindell R. Auxillary recurrence following conservative surgery and radiotherapy in early breast cancer. Clin Oncol 2000; 12: 309–14
- Olsen NK, Pfeiffer P, Johannsen L, Schroder H, Rose C. Radiation-induced brachial plexopathy: neurological follow-up in 161 recurrence free breast cancer patients. Int J Radiat Oncol Biol Phys 1993; 26: 43–9
