Abstract
We examined whether women's survival from lung cancer is influenced by hormonal factors associated with reproductive events. In all 4235 women and 4797 men born on 1 January 1935 or later with lung cancer diagnosed in 1978–1999 were identified in the Danish Cancer Registry and followed up to 31 December 2002 by linkage to the Central Population Registry. Cox regression analysis was used to estimate hazard rate ratios (HRs), and survival probabilities were calculated. Both nulliparous women and men without children had worse prognoses than those with children (women: HR 1.14; CI 1.03–1.26; men: HR 1.24; CI 1.15–1.34). The 5-year survival rate of nulliparous women with adenocarcinoma was 20.3%, while that for parous women was 20.5%; the corresponding rates for men were 13.0% and 16.6%. The number of children affected the risk for death in both sexes, indicating that the finding is not due to hormonal factors but to unmeasured elements such as socio-economic status or lifestyle factors related to parenthood.
It is well known that women with lung cancer have better survival rates than men, given the same extent of disease and the same histological type Citation[1–3]. Female sex has been associated with better survival in clinical trials of chemotherapy and newer, biologically targeted therapy of lung cancer, and a survival advantage was also observed in large surgical series Citation[4–13]. The reason for this disparity is unknown. We considered it conceivable that hormonal factors play a role, as lung cancer in women differs in some aspects from lung cancer in men. The most marked difference is in the relative distribution of the histological subtypes of lung cancer: adenocarcinoma has always been the most frequent histological subtype in women, while squamous cell carcinoma is the most frequent histological subtype in men, although adenocarcinoma is becoming more frequent among men Citation[14]. Small cell, squamous cell and large cell carcinomas are more strongly associated with smoking than adenocarcinoma, while adenocarcinoma is the most frequent histological subtype in both male and female non-smokers, indicating that risk factors other than smoking play a role in the development of this histological subtype Citation[15]. The difference in the distribution of histological subtypes among men and women has been attributed to difference in gender-related smoking habits; however, other factors, such as endogenous sex hormones, cannot be excluded Citation[16].
As the levels of endogenous female sex hormones are modified by reproductive history, we examined whether the survival of women from lung cancer is influenced by major reproductive events before diagnosis, such as age at birth of first child, number of full-term pregnancies and the offspring sex ratio. Estrogen hormone receptors alpha and beta are expressed in normal and malignant lung tissue to different degrees in men and women, but the function of estrogens in the lung is still not clear Citation[17]. If estrogens have a favorable influence on the progression of lung cancer in women, by slowing the progression of the tumor, it might be assumed that higher serum levels of estrogen are associated with better prognosis. Nulliparous women are exposed to higher average levels of estrogen throughout their reproductive life than parous women owing to a longer ovulation time. We therefore hypothesized that childless women would have better prognoses than women with children and that this survival benefit would be most evident for adenocarcinoma of the lung. As it is clearly important to distinguish between a true biological effect and lifestyle factors correlated to reproductive history, such as tobacco habits, occupation and diet, we replicated the analysis in male patients, assuming an equal effect of reproductive history on survival in male and female patients if the effect is due to lifestyle factors but an effect only in women if it is due to hormonal or other female-specific biological factors.
Methods
All 65 776 cases of lung cancer diagnosed in Denmark between 1978 and 1999 were identified in the Danish Cancer Registry database, which contains name, personal identification number, date of birth, sex, date of diagnosis, site of tumor, extent of disease (localized, regional or metastatic) and histological type for each case. A system of multiple reports, from hospital, pathology and forensic medicine departments and private medical clinics, ensures a high degree of completeness of the information in the registry. Unreported cancer cases, listed only on death certificates, are added to the Registry annually after verification by the treating hospital. Since 1987, the information in the Registry has been supplemented by an annual search for cancer cases in the files of the National Hospital Discharge Registry; when additional cases are found, the treating hospitals are requested to send a standardized notification to the Registry for verification.
Since 1978, the Danish Cancer Registry has used the International Classification of Diseases for Oncology (ICD-O) for registration; this is composed of a four-digit topography code and a four-digit histology code supplemented by a fifth digit for tumor behavior Citation[18]. The quality of the histological codes for lung cancer was considered acceptable from 1978 and high since 1983 Citation[19]. The cases were categorized by the WHO classification of lung tumors Citation[20] into five histological groups: squamous cell carcinoma, small cell carcinoma, adenocarcinoma, large cell carcinoma and other histological types, including tumors with no histological verification.
To obtain information on reproductive events before the date of diagnosis of lung cancer, we linked information on all patients born on or after 1 January 1935 to the files of the Central Population Registry. This Registry was established on 1 April 1968, when all Danish residents who were alive at that time were assigned a unique 10-digit personal identification number which includes date of birth and a four-digit running number indicating sex. Links between family members were established by means of addresses Citation[21]. Since that time, the record of each individual has been continuously updated for a number of basic demographic variables including vital status, number of children, children's identification number and children's date of birth, which were collected for this study. The year 1935 was chosen because most of the reproductive period of individuals born in that year or later would be covered. The registrations of children born in the early 1950s are less than complete, but the completeness was over 90% for children born in the late 1950s or later Citation[22]. Although the Registry does not differentiate between legal adoptions and natural births, only about 1500 children are adopted annually in Denmark, while there are about 65 000 births; the 2.3% of adoptions should therefore not distort our results Citation[23]. All patients were followed up to 31 December 2002.
All statistical analyses were performed with STATA 7 (Stata Corporation, Texas, USA). Cox regression analysis was used to estimate hazard rate ratios (HRs), a measure of the relative risk of dying, with the Breslow method for ties and a time scale of years from diagnosis (based on recorded calendar years). Two-sided 95% confidence intervals (CIs) for the hazard rate ratios were calculated with Wald's test of the Cox regression parameter. Observed survival probabilities were calculated by the Kaplan-Meier method. As age is an important predictor of survival from lung cancer, all estimates of the survivor function were initially adjusted for age at diagnosis; as the effect of age was not important in this cohort of relatively young patients, we therefore we show unadjusted Kaplan-Meier estimates in the figures.
Results
We included 9032 patients with lung cancer, 4797 men and 4235 women (), in the analyses. The hazard ratio was significantly lower for women than for men (HR, 0.91; 95% CI, 0.87–0.95) after adjustment for age, extent of disease and histological type. Age was not a strong predictor of survival in this relatively young cohort (mean age at diagnosis, 52.3 for men and 51.8 years women), but, as expected, extent of disease was an important predictor of survival. Large cell carcinoma had the worst prognosis of the four histological subgroups, also after adjustment for sex, age and extent of disease. There was no statistically different survival among the three other histological groups after adjustment, although a trend towards better survival among small cell carcinoma was observed.
Table I. Relative risk for dying from lung cancer (hazard ratio, HR) by sex, age, extent of disease and histological type.
Most of the patients had one or more children; slightly more men (18.7%) than women (11.5%) were childless (). The women were generally younger than the men at the time their first child was born: 27.0% of the women and only 6.1% of the men were 19 years or younger. The sex ratio of the patients’ children showed a slight overrepresentation of boys (about 40% of patients had more boys than girls) but was similar for both sexes.
Table II. Selected reproductive characteristics of Danish lung cancer patients, 1978–99.
We estimated the hazard of dying by number of children for people of each sex and adjusted our analyses for age, extent of disease and histological type (). Nulliparous women had a worse prognosis than women who had one to three children (the reference group) (HR, 1.14; 95% CI, 1.03–1.26). The hazard ratio for women who had four or more children did not differ significantly from that for the reference group. Men without children also had a worse prognosis than those with one to three children (HR 1.24; 95% CI, 1.15–1.34), and having four or more children did not have a significant effect on the prognosis. Neither age at the time the first child was born nor the sex ratio of the children had an important effect on the hazard of dying from lung cancer for either sex.
Table III. Hazard ratios for selected reproductive factors in Danish male and female lung cancer patients, 1978–99, by sex.
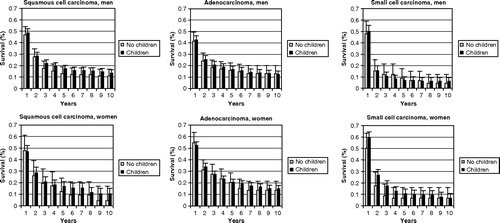
shows the histological type-specific survival of patients with localized or regional lung cancer by number of children, presented in two categories: patients with and without children. Because few data were available, there is no figure for patients with large cell carcinoma. Overall, for all histological groups, patients with children had better survival than patients without children, although the differences were not large. As adjustment for age or extent of disease did not change the estimates appreciably, we present the crude survival estimates. The five-year survival rates from adenocarcinoma were 20.3% for nulliparous women, 20.5% for parous women, 13.0% for childless men and 16.6% for male patients who had children.
Discussion
In our cohort of 9032 Danish patients with lung cancer, female patients had better survival than male patients, given the same age, extent of disease and histological type. Patients with large cell carcinoma had poorer prognosis than patients with squamous cell carcinoma, adenocarcinoma and small cell carcinoma, but the prognosis of patients with the three latter histologies were similar, although a trend towards better prognosis of small cell carcinoma was observed after adjustment of sex, age and extent of disease. These findings indicate that the well-known poor prognosis of patients with small cell carcinoma is largely due to the extent of disease at diagnosis. The number of children had no clear effect on the hazard of dying. Contrary to our hypothesis, nulliparous women had a higher risk for dying than parous women. The effect of number of children on the risk for dying was stronger in men than in women, indicating that this finding is not due to factors associated with childbearing and childbirth but rather to other, unmeasured elements, such as socio-economic status or lifestyle factors related to parenthood.
The age at which the first child was born did not influence the hazard of dying. When we examined the hazard by the sex ratio of the patients’ children, we also found no effect, but we observed that 40% of both male and female patients had more boys than girls. It has been hypothesized that parental hormone concentrations around the time of conception might determine the sex of the child to some extent, low concentrations of testosterone and estrogen being associated with the birth of girls Citation[24]. Some studies have suggested that smoking reduces estrogen levels in women and testosterone levels in men Citation[25], Citation[26]. On the basis of these arguments, we would have expected to find a lower male:female ratio among the patients’ children.
Women have higher circulating estrogen levels than men, and it has been suggested that this might contribute to the higher susceptibility of women to lung cancer. That suggestion has probably been refuted, however, by the finding in a prospective cohort study in the USA of 85 000 persons that women were not more susceptible to lung cancer Citation[27]. The authors reviewed all previously published prospective analyses on the same subject and came to the same conclusion.
Several reports have been published on the possible association between hormonal factors, estrogen replacement therapy and lung cancer risk in women, with conflicting results Citation[28–35]. The effect of these factors on survival from lung cancer has not, however, been studied extensively. In a study by Moore et al. of data from the Surveillance, Epidemiology, and End Results database for 1992–1997 on 14 676 women with primary non-small cell lung cancer, the cases were categorized into two groups, pre-menopausal (≤50 years) and postmenopausal (≥51 years) Citation[36]. The authors found that pre-menopausal women more often presented with advanced disease and underwent more extensive resection yet had better survival than postmenopausal women after adjustment for stage, histological type, tumor size and grade and extent of surgery. Whether this survival advantage is due simply to the age difference between the two groups or whether there is really a less malignant entity called pre-menopausal lung cancer remains to be determined.
It can also be argued that the higher androgen levels of men than women could stimulate the growth of lung cancer cells and lead to a poorer prognosis. This hypothesis was tested by Johnson et al. in a study of 44 men with lung cancer who had undergone orchiectomy between 6 months before and 6 months after a diagnosis of lung cancer, and 88 control subjects with lung cancer, matched for age, race and region Citation[37]. The patients who had undergone orchiectomy had significantly better survival than those who had not; survival five years after the diagnosis was 53% for the former group and 34% for the latter. The authors did not state whether the diagnoses of lung cancer had been verified histologically or why the patients had undergone orchiectomy, and misclassification of prostate cancer might have influenced the survival rates to some extent.
Our findings indicate that reproductive pattern cannot explain the better survival of female than male lung cancer patients. One might speculate that women, who generally have longer life expectancy than men, are less burdened with co-morbidity; alternatively, the fact that women have higher smoking cessation rates than men at the time of diagnosis of lung cancer and afterwards might be associated with a better prognosis Citation[38], Citation[39].
We could not assess the effect of co-morbidity in our cohort. Calculation of relative survival rates, by dividing the observed overall survival rates of the patients by the survival rates for the background population of the same age and sex composition, can sometimes provide an adjustment for deaths due to causes that are not related to the cancer under study. We chose not to report relative survival for two main reasons: (1) as our study subjects were relatively young (mean age, 52 years), the relative survival rate is close to the observed rate, because there is minimal mortality in the background population of the same age and sex; (2) the relative rate is useful only if the cancer is the only variable that separates the cancer cohort from the background population, which is not the case here owing to the added excess risk of dying because of smoking Citation[40]. Different dietary habits might also explain some of the survival difference. Women generally have higher intake of fruit and vegetables than men. Although fruit and vegetables are believed to reduce the risk for lung cancer, the mechanism by which they would exert their protective effects and the specific factors involved remain unresolved. Whether dietary factors also have beneficial effects after the development of lung cancer and improve survival also remains to be explored. Further research is warranted. The answers to these and other important questions, such as the role of metabolic and genetic factors, should improve the survival of future lung cancer patients.
References
- Skuladottir H, Olsen JH. Conditional survival of patients with the four major histologic subgroups of lung cancer in Denmark. J Clin Oncol 2003; 21: 3035–40
- Sant M, Aareleid T, Berrino F, Bielska Lasota M, Carli PM, Faivre J, et al. EUROCARE-3: survival of cancer patients diagnosed 1990–94—results and commentary. Ann Oncol 2003; 14(Suppl 5)V61–V118
- Gloeckler Ries LA, Reichman ME, Lewis DR, Hankey BF, Edwards BK. Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (SEER) program. Oncologist 2003; 8(6)541–52
- Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. N Engl J Med 1999; 341: 1198–205
- de Perrot M, Licker M, Bouchardy C, Usel M, Robert J, Spiliopoulos A. Sex differences in presentation, management, and prognosis of patients with non–small cell lung carcinoma. J Thorac Cardiovasc Surg 2000; 119: 21–6
- O'Connell J, Kris MG, Gralla RJ, Groshen S, Trist A, Fiore JJ, et al. Frequency and prognostic importance of pre-treatment clinical characteristics in patients with advanced non–small cell lung cancer treated with combination chemotherapy. J Clin Oncol 1986; 4: 1604–14
- Albain KS, Crowley JJ, LeBlanc M, Livingston RB. Survival determinants in extensive-stage non–small cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol 1991; 9: 1618–26
- Radzikowska E, Glaz P, Roszkowski K. Lung cancer in women: age, smoking, histology, performance status, stage, initial treatment and survival: population based study of 20 561 cases. Ann Oncol 2002; 13: 1087–93
- Johnson BE, Steinberg SM, Phelps R, Edison M, Veach SR, Ihde DC. Female patients with small cell lung cancer live longer than male patients. Am J Med 1988; 85: 194–6
- Kris M, Natale R, Herbst R, Lynch TT, Jr, Prager D, Belani CP, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer. JAMA 2003; 290: 2149–58
- Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non–small cell lung cancer. J Clin Oncol 2003; 21: 2237–46
- Alexiou C, Onyeaka CV, Beggs D, Akar R, Beggs L, Salama FD, et al. Do women live longer following lung resection for carcinoma?. Eur J Cardiothorac Surg 2002; 21: 319–25
- Ferguson MK, Karrison T. Does pneumonectomy for lung cancer adversely influence long-term survival?. J Thorac Cardiovasc Surg 2000; 119: 440–8
- Skuladottir H, Olsen JH, Hirsch FR. Incidence of lung cancer in Denmark: historical and actual status. Lung Cancer 2000; 27: 107–18
- Wingo PA, Ries LA, Giovino GA, Miller DS, Rosenberg HM, Shopland DR, et al. Annual report to the nation on the status of cancer, 1973–1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst 1999; 91: 675–90
- Wynder EL, Hoffmann D. Smoking and lung cancer: scientific challenges and opportunities. Cancer Res 1994; 54: 5284–95
- Fasco MJ, Hurteau GJ, Spivack SD. Gender-dependent expression of alpha and beta estrogen receptors in human nontumor and tumor lung tissue. Mol Cell Endocrinol 2002; 188: 125–40
- World Health Organization. International classification of diseases for oncology (ICD-O). World Health Organization, Geneva 1976
- Krasnik M, Frølund CHL, Rosenstock SJ, Franzmann M-B, Storm HH. Forekomsten af lungekræft i Danmark 1943–1986. Cancerregisterets pålidelighed. Ugeskr Laeg 1994; 156: 3021–5
- World Health Organization. Histologic typing of lung cancer2nd ed. World Health Organization, Geneva 1981
- Danish Ministry of Interior Affairs and Health. Civil registration system in Denmark. Copenhagen: Central Office of Civil Registration; 2001 ( http://www.cpr.dk/).
- Westergaard, T. Birth order and cancer risk. Ph. D thesis, University of Copenhagen, 1997.
- Statistics Denmark. Statistical Yearbook 2003. Copenhagen: Statistics Denmark; 2003. ( http://www.dst.dk/HomeUK/Statistics/Publications/Yearbook/2003.aspx)
- James WH. Evidence that mammalian sex ratios at birth are partially controlled by parental hormone levels at the time of conception. J Theor Biol 1996; 180: 271–86
- Michnovicz JJ, Naganuma H, Hershcopf RJ, Bradlow HL, Fishman J. Increased urinary catechol estrogen excretion in female smokers. Steroids 1988; 52: 69–83
- Zmuda JM, Cauley JA, Kriska A, Glynn NW, Gutai JP, Kuller LH. Longitudinal relation between endogenous testosterone and cardiovascular disease risk factors in middle aged men: a 13-year follow-up of former Multiple Risk Factor Intervention Trial participants. Am J Epidemiol 1997; 146: 609–17
- Bain C, Feskanich D, Speizer FE, Thun MJ, Hertzmark E, Rosner BA, et al. Lung cancer rates in men and women with comparable histories of smoking. J Natl Cancer Inst 2004; 96: 826–34
- Gao YT, Blot WJ, Zheng W, Ershow AG, Hsu CW, Levin LI, et al. Lung cancer among Chinese women. Int J Cancer 1987; 40: 604–9
- Seow A, Poh WT, Teh M, Pike MC, Henderson BE, et al. Diet, reproductive factors and lung cancer risk among Chinese women in Singapore: evidence for a protective effect of soy in nonsmokers. Int J Cancer 2002; 97: 365–71
- Taioli E, Wynder EL. Endocrine factors and adenocarcinoma of the lung in women. J Natl Cancer Inst 1994; 86: 869–70
- Wu-Williams AH, Dai XD, Blot WJ. Lung cancer among women in north-east China. Br J Cancer 1990; 62: 982–7
- Wu AH, Yu MC, Thomas DC, et al. Personal and family history of lung disease as risk factors for adenocarcinoma of the lung. Cancer Res 1988; 48: 7279–84
- Adami HO, Persson I, Hoover R, Schairer C, Bergkvist L. Risk of cancer in women receiving hormone replacement therapy. Int J Cancer 1989; 44: 833–9
- Ettinger B, Friedman GD, Bush T, Quesenberry CP, Jr. Reduced mortality associated with long term postmenopausal estrogen therapy. Obstet Gynecol 1996; 87: 6–12
- Kreuzer M, Gerken M, Heinrich J, Krienbrocn L, Wickmann ME. Hormonal factors and risk of lung cancer among women?. Int J Epidemiol 2003; 32: 263–71
- Moore KA, Mery CM, Jaklitsch MT, Estocin AP, Bnend R, Swanson SJ, et al. Menopausal effects on presentation, treatment, and survival of women with non-small cell lung cancer. Ann Thorac Surg 2003; 76: 1789–95
- Johnston EM, McIntyre LM, Hoff JA, Bepler G. The effect of orchiectomy on lung cancer survival. Anticancer Res 1999; 19: 5567–70
- de Perrot M, Licker M, Bouchardy C, Usel M, Robert J, Spiliopoulos A. Sex differences in presentation, management, and prognosis of patients with non-small cell lung carcinoma. J Thorac Cardiovasc Surg 2000; 119: 21–6
- Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. Smoking and lung cancer survival. the role of comorbidity and treatment. Chest 2004; 125: 27–37
- Henson DE, Ries LA. The relative survival rate. Cancer 1995; 76: 1687–8