Abstract
Corticosteroids are commonly used in patients with advanced cancer on both specific and non-specific indications. They are potent drugs with potentially serious adverse side effects. We have in two separate surveys collected answers from 302 physicians and data from 1292 patients on corticosteroid prescription attitudes and clinical practice in Swedish palliative care. Corticosteroids were used in more than 50% of the cancer patients and with high response rates when treating appetite loss, nausea, fatigue or poor wellbeing. The positive response came within the first week of treatment and persisted beyond four weeks. Patients with prostate cancer had a significantly better treatment response of corticosteroids on fatigue as compared to patients with lung cancer. Few physicians had guidelines on the use of corticosteroids in advanced cancer and there were differences in the attitudes between different medical specialties. Guidelines based on prospective clinical trials are needed.
Corticosteroids are frequently prescribed to patients with advanced cancer for symptom relief. The indications are wide in this patient group ranging from treatment of specific conditions like spinal cord compression and raised intracranial pressure, to non-specific indications like anorexia, general weakness and other symptoms. There is still a controversy upon their use for non-specific indications, although earlier studies have shown positive effect on appetite, strength and well being Citation[1–5].
Treatment with corticosteroids is associated with potentially serious adverse effects. Close monitoring of the patient using the lowest effective dose and discontinuing if no benefit is obtained is recommended Citation[6], Citation[7].
Several studies outside the Nordic countries have examined the use of corticosteroids in individual palliative care units on both specific and non-specific indications Citation[6–11]. Only one study used a multicentre setup Citation[12]. In summary, the studies show that between one third and a half of all palliative patients being enrolled in palliative care are treated with corticosteroids for symptom control. Dexamethasone is the drug of choice and the doses varies between 0.5 mg on alternate days to 16 mg daily Citation[6–8], Citation[10], Citation[11]. Most of the studies show a positive but short lasting effect in a majority of treated patients, however one study claimed positive beneficial effect in less than 30% of the patients Citation[6]. Is this widespread use of corticosteroids in palliative care based on evidence or does it merely reflect treatment traditions in lack of controlled trials?
The aim of our study was to 1) look at attitudes and practice among physicians in Sweden regarding treatment with corticosteroids on non-specific indications in patients with advanced cancer, 2) examine the use of corticosteroids in patients enrolled in palliative care in Sweden, and 3) to relate these findings to existing evidence.
Material and methods
A questionnaire on physicians’ attitudes and practice regarding treatment with corticosteroids on non-specific indications in advanced cancer was sent by mail in the autumn of 2000 to all members of the Swedish Society of Oncology and the Swedish Association for Palliative Care, in total 573 physicians (Survey 1). The selection was made upon the assumption that these members constituted a representative sample of physicians treating patients with advanced cancer. Six physicians with long experience of treatment with corticosteroids participated in the development of the questionnaire and adjustments were made according to their comments. The questionnaire focused on treatment of appetite loss, fatigue, nausea and poor wellbeing and comprised 18 questions on the availability of local guidelines, the number of patients being treated, preferred drugs and doses, estimation and evaluation of effect, tapering of doses, gastroprotection and side effects. Most of the questions were either a single or multiple choice model. The respondents answered anonymously but were asked to state their medical speciality. They were explicitly requested to answer the questions out of their daily practice, avoiding looking for answers in textbooks. Members who did not answer the questionnaire within one month received a reminding letter by mail. All answers were entered into an Excel database and subsequently transferred to the program package SPSS 10.0 for descriptive statistical analyses.
The second survey, on the use of corticosteroids in palliative care, was sent in the autumn of 2004 to all members of the palliative research network in Sweden (PANIS) which at that time comprised physicians on 37 specialised palliative care units all over the country. The network was established in 2002 and uses a web based survey generator to regularly collect data from patients regarding questions of symptom prevalence, treatment traditions and current problems in palliative care Citation[13], Citation[14]. Each participating unit received a questionnaire by e-mail on the use of corticosteroids in patients enrolled in palliative care (Survey 2). The questionnaire comprised 10 questions on age, gender, diagnosis, whether the patient had ongoing treatment with systemic corticosteroids, drug, dosage, indication for treatment, effect, gastroprotection and side effects. Patients using corticosteroids only for inhalation or for topical use were registered as not having ongoing treatment with systemic corticosteroids. All patients at each participating unit were registered on a specific day, and the registering physician or nurse had the opportunity to choose the day most appropriate for registration within a time interval of three weeks. The registration was based on the patient record, and evaluation of treatment effect and side effects was based on the clinical impression of the physicians and/or nursing staff caring for the patient. There were no individual self-assessments made by the patients. All registrations were entered into the web based survey generator at the respective participating unit. Reports for analyses were generated within the survey generator by the researcher after the survey was closed and were subsequently transferred to an Excel database for descriptive statistical analyses. Comparing statistical analyses where performed with STATISTICA (data analysis software system) StatSoft, Inc. (2005, version 7.1). As the assessments were measured on an ordinal scale, non-parametric statistics were used. The Mann-Whitney U test was used to test for differences in treatment effect on symptoms between two different cancer diagnoses. Spearman rank correlation was used to test for correlations between assessments of two different symptoms and Friedman ANOVA was used to test for differences in assessments of multiple symptoms. All tests were considered two sided.
Both surveys were approved by the local ethics committee.
Results
Survey 1
The first survey was answered by 338 physicians (59%). Thirty-six answers were excluded due to incomplete answering (n = 12) or statements from the respondents that they lacked experience of the actual treatment (n = 24). The remaining 302 completed questionnaires were collected from all over the country. Almost half of the respondents were oncologists (in Sweden the oncologists are both medical and radiation oncologists). Together with geriatricians, surgeons, internists and general practitioners they constituted 85% of all the respondents (). Ninety-seven percent of the responding surgeons worked in a surgical department and 83% of the oncologists worked in an oncology department. Sixty-eight percent of the general practitioners and 59% of the geriatricians worked in a palliative care unit.
Table I. Items from Survey 1 as answered by the different medical specialities.
One third of the physicians had local guidelines on treatment with corticosteroids in advanced cancer on their clinic. Two thirds answered that they prescribed corticosteroids to more than 50% of their cancer patients with appetite loss, fatigue, nausea or poor wellbeing. Poor chances of improvement, fear for side effects or simply forgetting the treatment were the main reasons for not starting treatment.
Drugs, dosage and effect
Betamethasone (equipotent to dexamethasone and approximately seven times more potent than prednisolone) was the most commonly prescribed drug followed by prednisolone. Dexamethasone was used only by a few physicians. When asked to state which dose they commenced on, the mean starting daily dose for treating anorexia, fatigue or low mood was 3.5 mg of betamethasone or 17 mg of prednisolone respectively. The mean starting dose for treating nausea was 4.8 mg or 19 mg daily respectively. Seventy-five percent of the respondents answered that they tapered the dose to maintenance dose if possible. Eighty-three percent stated that more than 50% of their patients had a positive effect of the treatment and 97% of the respondents experienced that the positive effect came within five days. Sixty-three percent answered that the positive effect usually lasted between 3–6 weeks.
When asked to state which symptom(s) had the most positive response to corticosteroid treatment, 71% of the physicians answered poor wellbeing. The corresponding figure for appetite loss was 53%, nausea 45% and fatigue 40%. Sixty-eight percent of the respondents evaluated the treatment effect within one week, and if no positive effect was seen the treatment was stopped without tapering by one third of the physicians. The rest lowered the dose gradually.
Gastroprotection and side effects
Nearly half of the physicians had local guidelines on the use of gastroprotection in patients treated with corticosteroids and 68% of all respondents used preferably proton pump inhibitors (PPI). Twenty-seven percent of the respondents answered that they prescribed gastroprotectors to 75–100% of their patients treated with corticosteroids. The corresponding figure for patients treated concomitantly with corticosteroids and non-steroidal anti-inflammatory drugs (NSAID) was 65%.
Two thirds of the respondents did not see side effects related to treatment with corticosteroids as a problem. The side effects most often seen were oral candidosis, aggravated or triggered diabetes mellitus, moon face and fragile skin/purpura. The respondents were also asked to state which potential side effects they gave most attention to in the last days of the patient's life. These were oral candidosis, aggravated or triggered diabetes mellitus, negative psychiatric effects and insomnia.
summarizes the differences in answers between the different medical specialities in five of the questions.
Survey 2
The second survey was sent to 37 palliative care units of whom 30 units (81%) participated in the study. Thirty-five physicians registered answers in the survey generator and 18 of them had four years earlier received the questionnaire in Survey 1. The actual number of physicians participating in both surveys was not possible to calculate due to the anonymous set up in Survey 1. A total of 1292 patients where registered in the survey generator and a majority were enrolled in advanced home care. The mean age was 67 years and there was a predominance of women. One thousand one hundred and sixteen patients had cancer, with breast-, lung-, prostate-, colorectal-, pancreatic- and ovarian cancer being the most frequent diagnoses. A total of 608 patients (47%) had ongoing treatment with systemic corticosteroids, 582 (96%) of them had a cancer diagnosis. Twenty-six patients with a non-malignant diagnosis used systemic corticosteroids and 62% of them had a chronic pulmonary disease. More than 60% of the patients with lung or prostate cancer used corticosteroids whereas only 28% of the patients with ovarian cancer had ongoing treatment. A higher proportion of patients in in-patient units used corticosteroids as compared to patients in advanced home care. summarizes the demographical findings.
Table II. Demographical data for patients in Survey 2.
Drugs and dosage
Eighty-five percent of the cancer patients on corticosteroids used betamethasone. Eighteen of the non-malignant patients (69%) used prednisolone, seven used betamethasone and one patient used hydrocortisone.
Oral administration was used in more than 90% of the cancer patients and 85% received a single daily dose of their corticosteroid. Two thirds of the cancer patients on betamethasone had a daily dose below 3.5 mg, whereas only 33 patients had a daily dose over 8 mg. In 24 of these 33 patients (73%) the indication for treatment was a primary brain tumour or brain metastases. Two thirds of the cancer patients had used corticosteroids for more than four weeks. summarizes the details on drugs and dosage of corticosteroids in the cancer patients.
Table III. Drugs and dosage in cancer patients treated with corticosteroids in Survey 2.
Indications and effects
The indications for treatment with corticosteroids in cancer patients are shown in . It was possible for the responder to specify multiple indications for a patient. The non-specific indications dominated with appetite loss, fatigue and poor wellbeing being the most frequent. In 101 patients’ appetite loss, fatigue and poor well being were registered as indications for treatment with corticosteroids. In these patients the registering physician made a separate assessment of each individual symptom as shown by the Friedman test, with Friedman ANOVA χ2=21.4, p < 0.001. Treatment of symptoms related to brain tumours- or metastases was the most common specific indication in this survey.
Table IV. Indications for treatment and assessment of effect for cancer patients treated with corticosteroids in Survey 2.
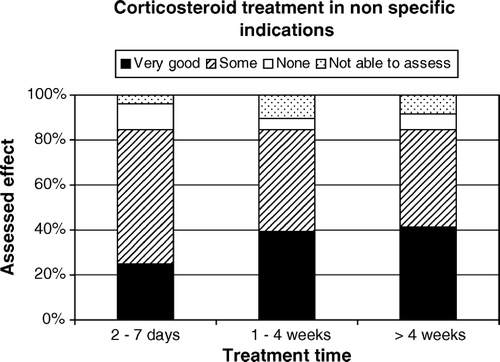
The assessed effect of the treatment for each indication in cancer patents is presented in . shows the effect in the five most common indications for all cancer patients treated with corticosteroids. Most patients with non-specific indications had a positive response to corticosteroids with less than 10% of the patients assessed as having no effect of the treatment. Although the number of patients treated was low, the results indicated an inferior effect on specific indications like spinal cord compression and intestinal obstruction. In 23 patients with spinal cord compression, 13 were assessed as having very good or some effect of corticosteroids. Three patients assessed as having no effect of corticosteroids had all been treated fore more than four weeks. In seven patients the respondents were not able to assess the effect. Two of these patients had been treated for less than two days and four patients had received corticosteroids for more than four weeks. There was a strong positive correlation in cancer patients between the assessed effect of corticosteroid treatment on appetite loss and nausea (rs=0.60, p < 0.001). The same applied for the effect of treatment on poor well being and fatigue (rs=0.73, p < 0.001). The differences in response to corticosteroid treatment for appetite loss, fatigue, poor wellbeing, nausea and pain in the four most common cancer diagnoses are shown in Figures. The difference in treatment effect on fatigue between patients with lung cancer and prostate cancer was statistically significant (z = 2.6, p = 0.02 two-sided). The mean age in the treated patients with lung cancer was 66 years, for patients with prostate cancer it was 76 years. When analyzing the treatment effect over time for all cancer patients using corticosteroids for appetite loss, fatigue, nausea or poor wellbeing, shows that the positive response came within a week and the response was stable over time. Patients treated more than four weeks retained the positive effect.
Figure 1. Assessment of effect on different symptoms for cancer patients treated with corticosteroids (Survey 2).
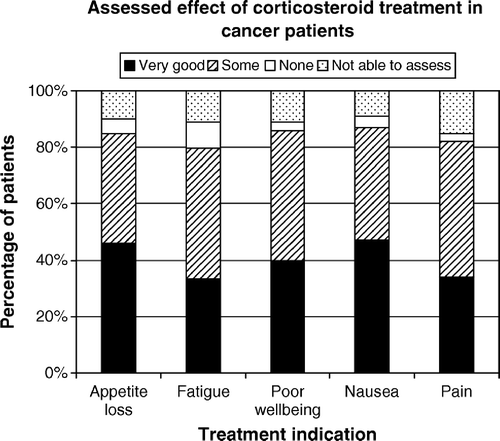
Figure 2–5. Response to corticosteroid treatment in the four most common cancer diagnoses in Survey 2.
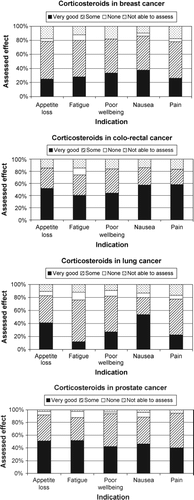
Figure 6. Assessed effect of treatment with corticosteroids over time in cancer patients using corticosteroids for appetite loss, fatigue, nausea or poor wellbeing (Survey 2).
Dyspnoea was the indication for treatment in 15 of the 26 patients with a non-malignant diagnosis. Fourteen of these 15 patients had a diagnosis of chronic pulmonary disease. Two patients were assessed as having a very good response, nine had some response and in four patients the respondent could not assess the effect.
Gastroprotection and side effects
Gastroprotection was used in 75% of the patients treated with corticosteroids. When comparing the group of patients which received gastroprotection with the untreated group, there was no difference in the dosage of corticosteroid, or the duration of steroid treatment. Corticosteroid treatment itself was stated as the reason for prescribing gastroprotectors in 53% of the patients on gastroprotection, another 38% had accompanying risk factors which motivated treatment. Proton pump inhibitors constituted 95% of all prescribed gastroprotectors.
In 181 (31%) of the cancer patients treated with corticosteroids the respondent experienced troublesome side effects. Eighty-one percent of these patients had used corticosteroids for more than four weeks and ninety percent were on betamethasone, with daily doses less than 3 mg in a majority of the patients. When comparing the different drugs, 33% of all cancer patients on betamethasone and 20% of the cancer patients on prednisolone were assessed as having troublesome side effects. The five most common side effects among patients judged as having troublesome side effects were moon face, myopathy/muscle weakness, skin purpura, oral candidosis and aggravated/triggered diabetes mellitus. The frequency of these symptoms are listed in .
Table V. The most common side effects in the 181 cancer patients in Survey 2 assessed as having troublesome side effects related to treatment with corticosteroids.
Discussion
These two surveys include answers from 302 physicians and data from 1292 patients on corticosteroid prescription attitudes and clinical practice in Swedish palliative care. Two-thirds of the physicians treating patients with advanced cancer claimed that they prescribed corticosteroids to more than 50% of their patients, and 52% of the cancer patients were actually treated. The proportion of patients on corticosteroids in this study is in accordance with earlier studies from other countries Citation[8], Citation[9], Citation[11] and reflects the wide range of indications for this treatment. The finding that almost 70% of the patients in in-patient units had ongoing treatment with corticosteroids raises the question on whether the use of these potent drugs has become a matter of routine rather than a closely monitored specific treatment. Few physicians had local guidelines on treatment with corticosteroids in advanced cancer. Earlier studies have stressed the need for guidelines, and given the frequency of use and the potentially serious adverse side effects associated with corticosteroid treatment, implementation of guidelines are important Citation[6], Citation[7], Citation[11].
The results in both surveys were coherent regarding the proportion of patients treated, drugs and dosages, assessed effects and side effects. Put together, there was in general a good accordance between practice and existing evidence.
The study sample in Survey 2 included 608 patients enrolled in palliative care which received treatment with systemic corticosteroids. This is the largest sample of this type published to date. All participating units were explicitly told to register all patients at the unit regardless of ongoing corticosteroid treatment or not. This is in accordance with the methodology used in earlier studies within the network Citation[13], however there were no monitoring at the units ensuring adherence to this.
Physician attitudes and clinical practice showed that betamethasone was the preferred drug in this patient group. This is in contrast to other countries where dexamethasone often is the first drug of choice; however both drugs are similar in many aspects. The preference for betamethasone in Sweden is based on tradition and accessibility to a convenient supply.
The high response rates to corticosteroid treatment for the most common indications in Survey 2 are of considerable clinical significance in this group of patients with advanced cancer. Approximately 80% of the cancer patients were assessed as having some or very good response to the treatment on different non-specific indications. The results exceeds the earlier findings by Hanks, Needham, and Mercadante, but resembles those found by Hardy Citation[6–8], Citation[10]. The results of the two surveys do not indicate that this could be explained by the use of higher doses of corticosteroids as compared to the above mentioned studies. An advanced malignancy could be seen as a chronic stressful condition in which treatment with corticosteroids would result in symptom relief. This view is supported in a previous study by our group where patients with advanced cancer were found to have a covariation between high levels of endogenous cortisol and more pronounced symptoms Citation[15].
Assessment of treatment effect and severity of side effects in Survey 2 were based on the evaluation of the responsible physician and/or nursing staff rather than individual patient ratings. This design ensured the collection of data from a large number of patients in order to reflect clinical practice. However, this influenced the reliability regarding assessment of effects and side effects of corticosteroid treatment. These assessments were based on impressions from experienced physicians and nurses with an inherent lack of sensitivity. Although the respondents were asked to evaluate the effect of corticosteroids specifically, no account was taken to concomitant medication or influences of other treatments against symptoms which might have been undertaken.
The physicians in Survey 1 apprehended fatigue as the symptom least affected by corticosteroid treatment. In the study of clinical practice we found that the effect of corticosteroids on fatigue in prostate cancer was superior to that in lung cancer. This could be explained by a stronger effect of corticosteroids in hormone related cancers.
The results in Survey 2 indicated an inferior effect off corticosteroid treatment on spinal cord compression compared to treatment for other indications. In a recent review on the diagnosis and management of spinal cord compression the author summarized that high-dose dexamethasone could be an effective adjunct to radiotherapy but carried the risk of serious toxicity Citation[16]. In our study seven of ten patients with no effect, or where the respondent were not able to assess the effect, had received treatment with corticosteroids for more than four weeks. The relatively high proportion of not assessable patients with spinal cord compression could be explained by the complex clinical situation, disease progression and possible care in different clinical settings during corticosteroid treatment. The strong positive correlation between the assessed effect of corticosteroid treatment on appetite loss and nausea was expected, as was the positive correlation between fatigue and poor well being. The concept of well being is often used but not clearly defined within palliative care. In the design of this study we did not limit the concept and an interpretation of the results is that physicians and/or nurses perceived that fatigue implied poor well being in the individual patient. Both surveys showed that a positive effect of corticosteroid treatment could be expected within a week, and in Survey 2 we found that this effect persisted beyond four weeks. Despite the limitation of the design, the result is interesting and is in contrast to earlier studies which have indicated that symptomatic benefits rarely extends for more than four weeks Citation[1], Citation[3].
Corticosteroids are potent drugs and side effects are common. The observation that two thirds of the respondents in Survey 1 did not see side effects related to treatment with corticosteroids as a problem is notable and raises questions about physicians’ ability to judge adverse effects.
Comparing the results on side effects between the two surveys indicated that the attitudes of the physicians underestimated the risk for myopathy. This is in accordance with the findings by Hardy et al. where proximal myopathy was considered the one most distressing to patients Citation[7]. Over 80% of the patients with troublesome side effects had used corticosteroids for more than four weeks. A majority of these patients on betamethasone had a daily dose lower than 3 mg. Although the doses used before the study were not registered, the results reflect the importance of treatment time when it comes to side effects and the need for monitoring.
The notion that advanced cancer is a condition of chronic stress supports the extensive use of gastroprotectors which was seen in Survey 2. All patients on a combination of NSAID and corticosteroid should be prescribed gastroprotection as there is a 15-fold increase in the risk of gastric irritation Citation[17]. The attitudes among the physicians showed that this was not common knowledge since only 65% of the respondents prescribed gastroprotectors to more than 75% of their patients with this combination.
The divergences in attitudes between the different medical specialities in Survey 1 are probably explained by the differences in the patient population exposed to the respondents and the traditions within the specialities, again pointing at the need for guidelines. A majority of the answering geriatricians and general practitioners worked in palliative care units and had attitudes in better accordance with existing guidelines. Few surgeons worked outside a surgical department and their attitudes revealed that they started fewer patients on corticosteroids and used less gastroprotectors in patients on both corticosteroids and NSAID's.
In conclusion, we have shown that corticosteroids are commonly prescribed in Swedish palliative care, often without guidelines. There is a high response rate to corticosteroid treatment and the results indicate that the positive effect can persist beyond four weeks. There is a need for implementation of guidelines based on solid evidence to assure patients optimal effect of corticosteroids and to minimize the risk for side effects. Future studies, preferably prospective clinical trials, should therefore focus on finding the optimal dose for symptom relief and the optimal dose regimen, i.e. continuous vs. pulsed treatment, for maintenance of the positive effect.
We thank Annika Norman, R. N. and Cathrine Kjellberg, R. N. for assistance with data collection in Survey 1. We thank all participating units in PANIS for their contribution with patient data in Survey 2. Survey 1 was supported by the county council in Stockholm. Survey 2 was supported by the Swedish Cancer Society.
References
- Moertel CG, Schutt AJ, Reitemeier RJ, Hahn RG. Corticosteroid therapy of preterminal gastrointestinal cancer. Cancer 1974; 33(6)1607–9
- Willox JC, Corr J, Shaw J, Richardson M, Calman KC, Drennan M. Prednisolone as an appetite stimulant in patients with cancer. Br Med J (Clin Res Ed) 1984; 288(6410)27
- Bruera E, Roca E, Cedaro L, Carraro S, Chacon R. Action of oral methylprednisolone in terminal cancer patients: A prospective randomized double-blind study. Cancer Treat Rep 1985; 69(7–8)751–4
- Della Cuna GR, Pellegrini A, Piazzi M. Effect of methylprednisolone sodium succinate on quality of life in preterminal cancer patients: A placebo-controlled, multicenter study. The Methylprednisolone Preterminal Cancer Study Group. Eur J Cancer Clin Oncol 1989; 25(12)1817–21
- Popiela T, Lucchi R, Giongo F. Methylprednisolone as palliative therapy for female terminal cancer patients. The Methylprednisolone Female Preterminal Cancer Study Group. Eur J Cancer Clin Oncol 1989; 25(12)1823–9
- Needham PR, Daley AG, Lennard RF. Steroids in advanced cancer: Survey of current practice [see comments]. BMJ 1992; 305(6860)999
- Hardy JR, Rees E, Ling J, Burman R, Feuer D, Broadley K, et al. A prospective survey of the use of dexamethasone on a palliative care unit. Palliat Med 2001; 15(1)3–8
- Hanks GW, Trueman T, Twycross RG. Corticosteroids in terminal cancer–a prospective analysis of current practice. Postgrad Med J 1983; 59(697)702–6
- Adunsky, A, Berkowitz, M, Waller, A. Corticosteroids in terminal cancer. Harefuah 1995;128(5):278–80, 335.
- Mercadante S, Fulfaro F, Casuccio A. The use of corticosteroids in home palliative care. Support Care Cancer 2001; 9(5)386–9
- Gannon C, McNamara P. A retrospective observation of corticosteroid use at the end of life in a hospice. J Pain Symptom Manage 2002; 24(3)328–34
- Nauck F, Ostgathe C, Klaschik E, Bausewein C, Fuchs M, Lindena G, et al. Drugs in palliative care: results from a representative survey in Germany. Palliat Med 2004; 18(2)100–7
- Lundstrom S, Strang P. Establishing and testing a palliative care research network in Sweden. Palliat Med 2004; 18(2)139
- Lundstrom S, Bystrom E, Gyllenhammar E, Martinsson U, Norrstrom B, Strang P. Palliative medicine network promotes research and development. Lakartidningen 2005; 102(16)1235–8
- Lundstrom S, Furst CJ. Symptoms in advanced cancer: relationship to endogenous cortisol levels. Palliat Med 2003; 17(6)503–8
- Loblaw DA, Perry J, Chambers A, Laperriere NJ. Systematic review of the diagnosis and management of malignant extradural spinal cord compression: the Cancer Care Ontario Practice Guidelines Initiative's Neuro-Oncology Disease Site Group. J Clin Oncol 2005; 23(9)2028–37
- Piper JM, Ray WA, Daugherty JR, Griffin MR. Corticosteroid use and peptic ulcer disease: Role of nonsteroidal anti-inflammatory drugs. Ann Intern Med 1991; 114(9)735–40
