Abstract
Little is known about the Bcl-2 family members in mesothelioma. These proteins are involved in the control of apoptosis, carrying out both pro- and anti- apoptotic functions. Immunohistochemistry was used to examine the expression of p53 and Bcl-2 family members in 54 archival mesothelioma samples (39 epithelial, 15 sarcomatoid tumours). Overexpression of p53 was observed in 81% (44/54). For anti-apoptotic proteins, overexpression was recorded as follows: Bcl-2 40% (22/54), Bcl-XL 24% (13/54), Mcl-1 92% (50/54). For pro-apoptotic proteins, loss of expression was recorded as follows: Bad 25% (14/54), Bak 24% (13/54), Bax 42% (23/54), Bid 37% (20/54), Bim 18% (10/54). Statistically significant differences between epithelial and sarcomatoid tumours were observed for Bid (p < 0.001), Bad (p = 0.012) and Bcl-XL (p = 0.03). Significant differences in abnormal expression of apoptosis proteins were found between epithelial and sarcomatoid subtypes but histological subtype was the only factor with significant association to patient prognosis.
Mesothelioma is a relatively rare, aggressive disease affecting the mesothelium. There were 1 755 recorded deaths from mesothelioma in the UK in 2002. There are three main types of mesothelioma, each named after the area of tumour development – pleural, peritoneal and pericardial. Pleural disease is more common as it develops from inhalation of irritants e.g. asbestos fibres. Malignant pleural mesothelioma (MPM) has a poor prognosis – survival is generally accepted as 4–12 months Citation[1]. MPM has been classified into three histological subtypes: epithelial tumours which account for approximately 60% of cases, sarcomatoid tumours which account for approximately 10% of cases and biphasic tumours which account for approximately 30% of cases. Biphasic tumours are composed of both epithelial and sarcomatoid components. Prognosis has been reported to be significantly better for epithelial tumours Citation[2–4].
There is no currently established form of treatment that has a major impact on the survival of MPM patients. Historically, treatment of MPM has focused on traditional methods including supportive care, surgery, radiotherapy and chemotherapy. The lack of success with single modality treatment has resulted in increased interest in multimodality approaches, which are showing more favourable outcomes. Attitudes towards therapy have changed from an almost nihilistic approach, due to lack of survival, to the realisation of achievement of symptom relief. Recently, modern chemotherapeutic agents such as Alimta have shown promising results Citation[5]. The aim of radiotherapy or chemotherapy is to induce lethal levels of DNA damage in the tumour cells. Resistance to therapy may be associated with abnormal expression of the proteins involved in apoptosis. When DNA damage is detected, the p53 protein causes the cell cycle to arrest at the G1/S checkpoint, allowing damage to be repaired before replication occurs. If the damage cannot be repaired efficiently, p53 triggers apoptosis by regulating the levels of the Bcl-2 family of proteins. Although resistance to apoptosis in many tumours results from a mutant p53 gene, wild-type p53 is more commonly found in MPM therefore resistance to apoptosis may arise downstream of p53 Citation[6], Citation[7].
The Bcl-2 family of proteins have an important function in the control of apoptosis. The family members consist of pro- and anti-apoptotic members that induce opposing effects on the permeability of the mitochondria. The proteins share at least one Bcl-2 Homology (BH) domain and can be categorised into three classes Citation[8]. The “anti-apoptotic” members (e.g. Bcl-2, Bcl-XL and Mcl-1) share sequence homology with 3–4 BH domains. The “multi-domain pro-apoptotic” members (e.g. Bax and Bak) contain BH1 – BH3 domains. The final class is the “BH3-only pro-apoptotic” members (e.g. Bad, Bid and Bim) which only share sequence homology in the BH3 domain. The pro-apoptotic proteins work by inducing permeability of the mitochondrial membrane resulting in caspase activation. The BH3-only group of proteins initiate cell death signalling and are responsible for the activation of Bax and Bak Citation[9]. The BH3-only proteins act by binding to, and inactivating, anti-apoptotic Bcl-2 family proteins Citation[10]. The anti-apoptotic proteins work by maintaining the permeability of the mitochondrial membrane and thus inhibiting the release cytochrome C. Overexpression of anti-apoptotic proteins have been implicated in resistance to therapy Citation[11], Citation[12].
Little is known about the expression of apoptotic markers in MPM. In this study, immunohistochemistry was used to examine the expression of a panel of apoptotic biomarkers in a series of archival formalin-fixed, paraffin embedded MPM sections. The aim of the study was to establish the expression patterns of apoptosis proteins in MPM and to identify proteins which may be prognostic factors.
Methods
Patients and samples
Local Research Ethical Committee approval was obtained for the research. A list was obtained from the Histopathology records (Hull Royal Infirmary) of all patients diagnosed with MPM (epithelial and sarcomatoid subtypes) from 1995–2001. Histology slides were reviewed by a single Consultant Histopathologist and formalin-fixed, paraffin-embedded blocks were retrieved from pathology archives. A series of samples from 54 patients was established, containing 39 epithelial cases and 15 sarcomatoid cases. Clinicopathological data was collected for all patients (). Survival times were calculated from date of diagnosis to date of death.
Table I. Summary of Patient Details
Immunohistochemistry
The method of staining has been described previously Citation[13]. In brief, 4 µm thick sections were cut onto SuperFrost® Plus microscope slides (Menzel-Glaser, Germany) and incubated overnight at 37°C. Antigen retrieval was achieved by boiling slides in 1500 ml distilled water containing 15 ml Antigen Unmasking Solution (Vector Laboratories Inc., Burlingame, CA, USA) for 3 minutes at 15 psi. Non-specific protein was blocked by incubation with 1x casein (Vector Laboratories) and endogenous avidin and biotin were blocked using the Avidin/Biotin Blocking Kit (Vector Laboratory Inc., Burlingame, CA, USA). One hundred µl of primary antibody was diluted in 0.2x casein in TBS to final concentration for use () and applied to the test sections. A negative control with primary antibody omitted was included in each batch. The slides were incubated at room temperature for 2 hours. Antibody detection was carried out using the avidin-biotin complex (ABC) method using the Duet Kit (K0492, DakoCytomation Ltd, High Wycombe, UK). The slides were incubated with the chromagen 3,3′-diaminobenzidine tetrahydrochloride (DAB), and 0.01% hydrogen peroxide as enzyme substrate until staining was apparent. Sections were counterstained with Harris Haematoxylin. Sections were subjectively scored as either positive or negative by two independent investigators using previously established criteria Citation[14]. Any discrepancies were resolved by further discussion. Sections were scored as positive for p53 overexpression if strong nuclear staining was observed in greater than 10% of tumour cells. As previously described, for anti-apoptotic proteins of the Bcl-2 family (Bcl-2, Bcl-XL and Mcl-1), sections were scored as positive if overexpression was observed in greater than 10% of tumour cells Citation[14]. For pro-apoptotic proteins (Bad, Bak, Bax, Bid and Bim) loss of expression was considered to be significant, with sections scored negative if greater than 50% of tumour cells showed loss of expression Citation[14].
Table II. Details of antibody suppliers and dilutions used.
Statistical analysis
SPSS software version 11.0 (SPSS, Chicago, USA) was used for statistical analysis. Correlation of protein expression with patient survival was calculated by log rank and linear regression analysis. Differences in staining patterns between epithelial and sarcomatoid MPM were calculated by Fisher Two Side Exact Test. Probability values p ≤0.05 were considered statistically significant.
Results
Fifty-four MPM sections were analysed for the expression of p53 and Bcl-2 family protein members. Nuclear staining was observed for p53 and Mcl-1, with the remaining Bcl-2 family proteins demonstrating cytoplasmic staining. summarises details of the number of cases demonstrating over-expression of p53, Bcl-2, Bcl-XL, and Mcl-1 and loss of expression of Bax, Bak, Bid, Bad and Bim. Over-expression of p53 was observed in 44/54 (81%) of cases. The anti-apoptotic proteins Bcl-2, Bcl-XL and Mcl-1 demonstrated over-expression in 22/54 (40%), 13/54 (24%) and 50/54 (92%) of tumours respectively. Loss of expression of the pro-apoptotic family proteins Bad, Bak, Bax, Bid and Bim were observed in 14/54 (25%), 13/54 (24%), 23/54 (42%), 20/54 (37%) and 10/54 (18%) of tumours respectively.
Table III. Details of Expression of p53 and Members of the Bcl-2 Family Proteins in Malignant Pleural Mesothelioma.
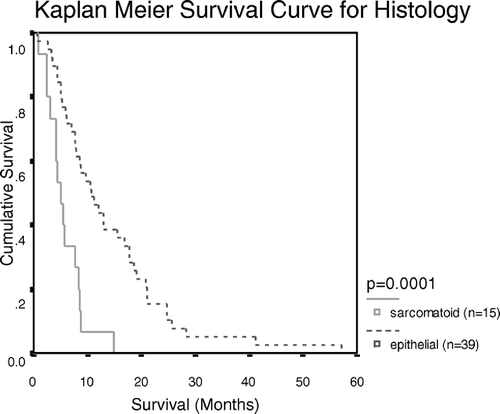
Univariate survival analysis with Log Rank analysis indicated statistical significance with histological subtype (p = 0.0001; ). Multivariant analysis with Linear Regression confirmed that none of the members of the Bcl-2 family of proteins analysed here correlated with survival.
Figure 1. Kaplan Meier Survival Curve showing the survival patterns for sarcomatoid and epithelial MPM
Comparison of apoptotic markers between histological subtypes indicated a statistically significant difference between epithelial MPM and sarcomatoid MPM for loss of expression of Bad (p = 0.012) and Bid (p < 0.001) and for overexpression of Bcl-XL (p = 0.03).
Discussion
This study was designed to analyse the expression of apoptotic proteins in archival MPM. To date there have been few reports in the literature on the expression of apoptotic proteins in MPM using immunohistochemistry on archival samples. Nine proteins were investigated: three anti-apoptotic and five pro-apoptotic Bcl-2 family proteins, in addition to p53. Results were analysed in relation to survival and histological subtype to identify proteins that may be important in assessing prognosis and therapy response in MPM patients.
Previously published results from other MPM studies that assessed p53 overexpression by immunohistochemistry gave values of 70% and 67% positivity Citation[15], Citation[16]. These studies were carried out on samples sizes of 20 and 15 archival MPM sections respectively. There are numerous antibodies against p53 and in our study, using clone DO1, 81% of tumours demonstrated overexpression of p53, with no significant difference between subtypes. It is worth noting that p53 mutations are uncommon in MPM and the overexpression of p53 detected using immunohistochemistry may represent an increase in the wild type protein Citation[7], Citation[17], Citation[18]. The results obtained in this study may give a more comprehensive view of p53 immunohistochemistry in MPM sections than is currently available in the literature as this test series was larger and is the only archival immunohistochemical study to date which presented survival analysis.
The frequency of Bcl-2 expression observed in this study is higher than previously reported in the literature with 40% of section showing overexpression. There was no significant difference between subtype. Previous archival studies reported 20% and 8% Citation[19–21]. The slight discrepancy may be due to differences in scoring criteria, for example, Soini et al. Citation[20] used a cut off value of 25% positive tumour cells whereas our laboratory used 10% positivity as the cut off point for overexpression.
To our knowledge, Bcl-XL expression has not previously been reported in archival MPM. Soini et al., Citation[20] assessed the expression of Bcl-X and reported that 100% of test sections were positive. However, the Bcl-X antibody may detect both Bcl-XL and Bcl-XS and our antibody was specific to Bcl-XL.
Overexpression of Mcl-1 was observed in 92% of cases. This is in agreement with the one previous report in the literature using immunohistochemistry, which reported expression in 100% of 34 test sections Citation[20].
To our knowledge this is the first immunohistochemical study to assess loss of expression of a series of pro-apoptotic Bcl-2 family proteins in archival MPM. Since it is the abnormal loss of expression of pro-apoptotic proteins that may be associated with poor prognosis or poor response to therapy, we specifically noted this feature.
Immunohistochemical analysis of Bax in MPM has not been frequently reported in the literature. Soini et al. Citation[20] reported that 100% of a series of 35 mesothelioma sections expressed bax. We identified loss of expression in 23/54 (42%). The expression of the pro-apoptotic proteins Bad, Bak, Bid and Bim has not been previously described using immunohistochemistry in MPM.
Log Rank analysis indicated that the histological subtype of MPM was the only factor which showed a significant correlation with patient survival. Patients with epithelial tumours survived significantly longer than patients with sarcomatoid tumours. This is in agreement with previously reported studies Citation[2–4]. To our knowledge, the only previous study that analysed the prognostic association of members of the Bcl-2 family of proteins in MPM was performed by Kaarteenaho-Wiik et al. Citation[22] who also reported that Bcl-2 expression did not correlate with survival.
Comparison of expression levels of members of the Bcl-2 family of proteins between histological subtypes in MPM has not been previously reported to our knowledge. Our study found that expression levels of Bad, Bid and Bcl-XL differed significantly between epithelial and sarcomatoid MPM subtypes. The Bcl-XL protein was overexpressed more frequently in sarcomatoid tumours (46% versus 15%, p = 0.03). The Bad and Bid proteins demonstrated loss of expression more frequently in sarcomatoid MPM (53% versus 15%, p = 0.012 and 80% versus 20%, p < 0.001 respectively).
To our knowledge, this is the first immunohistochemical study to analyse the expression of this panel of apoptotic markers in MPM in correlation with survival or histological subtype. This study has shown that the expression levels of p53 and a series of the members of the Bcl-2 family of proteins have no effect on the prognosis of MPM. Variation in the levels of expression of Bad, Bid and Bcl-XL between epithelial and sarcomatoid tumours indicate that expression levels of these apoptotic proteins may be important in the differentiation of the histological subtypes. This optimised panel of apoptosis-related antibodies may be of use in the investigation of chemotherapy response in MPM patients.
This work was funded by an NHS Research and Development Small Grant.
References
- Antman K, Shemin R, Ryan L, Klegar K, Osteen R, Herman T, et al. Malignant mesothelioma: Prognostic variables in a registry of 180 patients, the Dana-Farber Cancer Institute and Brigham and Women's Hospital experience over two decades, 1965–1985. J Clin Oncol 1988; 6: 147–53
- Curran D, Sahmoud T, Therasse P, van Meerbeeck J, Postmus PE, Giaccone G. Prognostic factors in patients with pleural mesothelioma: The European Organization for Research and Treatment of Cancer experience. J Clin Oncol 1998; 16: 145–52
- Herndon JE, Green MR, Chahinian AP, Corson JM, Suzuki Y, Vogelzang NJ. Factors predictive of survival among 337 patients with mesothelioma treated between 1984 and 1994 by the Cancer and Leukemia Group B. Chest 1998; 113: 723–31
- Edwards JG, Abrams KR, Leverment JN, Spyt TJ, Waller DA, O'Byrne KJ. Prognostic factors for malignant mesothelioma in 142 patients validation of CALGB and EORTC prognostic scoring systems. Thorax 2000; 55: 731–5
- Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003; 21: 2636–44
- Ramael M, Lemmens G, Eerdekens C, Buysse C, Deblier I, Jacobs W, et al. Immunoreactivity for p53 protein in malignant mesothelioma and non-neoplastic mesothelium. J Pathol 1992; 168: 371–5
- Mor O, Yaron P, Huszar M, Yellin A, Jakobovitz O, Brok-Simoni F, et al. Absence of p53 mutations in malignant mesothelioma. Am J Resp Cell Mol 1997; 16: 9–13
- Kirkin V, Joos S, Zornig M. The Role of Bcl-2 family members in tumorigenesis. Acta Bioch Bioph 2004; 1644: 229–49
- Huang DC, Strasser A. BH3-Only proteins – essential initiators of apoptotic cell death. Cell 2000; 103: 839–42
- Cory S, Adams JM. The Bcl-2 family: Regulators of the cellular life-or-death switch. Nat Rev Cancer 2002; 2: 647–56
- Reed JC. Mechanisms of apoptosis avoidance in cancer. Curr Opin Oncol 1999; 11: 68–75
- Debatin K-M. Apoptosis pathways in cancer and cancer therapy. Cancer Immunol Immun 2004; 53: 153–9
- Cawkwell L, Gray S, Murgatroyd H, Sutherland F, Haine L, Longfellow M, et al. Choice of management strategy for colorectal cancer based on a diagnostic immunohistochemical test for defective mismatch repair. Gut 1999; 45: 409–15
- Berrieman HK, Smith L, O'Kane SL, Campbell A, Lind MJ, Cawkwell L. The Expression of Bcl-2-Family Proteins Differs Between Non-Small Cell Lung Cancer Sub-Types. Cancer 2005; 103: 1415–9
- Kafiri G, Thomas DM, Shepherd NA, Krausz T, Lane DP, Hall PA. p53 expression is common in malignant mesothelioma. Histopathology 1992; 21: 331–4
- Segers K, Backhovens H, Singh SK, De Voecht J, Ramael M, Von Broeckhoven C, et al. Immunoreactivity for p53 mutations in human malignant mesotheliomas. Virchows Arch 1995; 427: 431–6
- Kitamura F, Araki S, Tanigawa T, Miura H, Akabane H, Iwasaki R. Assessment of mutations of Ha- and Ki-ras oncogenes and the p53 suppressor gene in seven malignant mesothelioma patients exposed to asbestos--PCR-SSCP and sequencing analyses of paraffin-embedded primary tumors. Ind Health 1998; 36: 52–6
- Kitamura F, Araki S, Suzuki Y, Yokoyama K, Tanigawa T, Iwasaki R. Assessment of the mutations of p53 suppressor gene and Ha- and Ki-ras oncogenes in malignant mesothelioma in relation to asbestos exposure: A study of 12 American patients. Ind Health 2002; 40: 175–81
- Segers K, Ramael M, Singh SK, Weyler J, Van Meerbeeck J, Vermeire P, et al. Immunoreactivity for Bcl-2 proteins in malignant mesothelioma and non-neoplastic mesothelium. Virchows Arch 1994; 424: 631–4
- Soini Y, Kinnula V, Kaarteenaho-Wiik R, Kurttila E, Linnainmaa K, Paakko P. Apoptosis and expression of apoptosis regulating proteins bcl-2, mcl-1, bcl-X and bax in malignant mesothelioma. Clin Cancer Res 1999; 5: 3508–15
- Lumb PD, Suvarna SK. Metastasis in pleural mesothelioma. Immunohistochemical markers for disseminated disease. Histopathology 2004; 44: 345–52
- Kaarteenaho-Wiik R, Soini Y, Pollanen R, Paakko P, Kinnula VL. Overexpression of tenascin-C in malignant pleural mesothelioma. Histopathology 2003; 42: 280–91
