Abstract
We present the impact of systematic radiation dose escalation from 64 Gy to 66 Gy to 70 Gy on the outcome after radiation therapy (RT) alone or combined with hormonal treatment (HT) in a series of 494 consecutive localised prostate cancer patients treated during 1990–1999. Prognostic factors for prostate-specific antigen (PSA) failure, overall survival (OS) and prostate cancer specific survival (CSS) were investigated using multivariate analysis. T stage, pre-treatment PSA, grade, radiation dose and HT were found to be independent predictors of PSA failure. T stage, grade and HT were also independent predictors of both OS and CSS, while radiation dose was a significant predictor for OS and indicated a trend (p = 0.07) for CSS. A dose of 70 Gy combined with hormonal treatment improves PSA failure free survival and survival in localised prostate cancer compared with doses of 64–66 Gy.
Prostate cancer is the most common malignancy in Norway, with more than 3 000 new cases a year Citation[1]. Most cases are now diagnosed at a localised stage as a result of the widespread use of prostate-specific antigen (PSA) testing used in Norway since 1987.
External beam radiation therapy (EBRT) has been used as primary therapy for localised prostate cancer for many years in Norway, but was initially regarded as inferior to surgery. Major technological improvements in conformal radiation treatment planning and delivery for prostate cancer allow substantially higher doses to be delivered to the prostate gland with minimal or no additional normal tissue toxicity Citation[2–4]. The use of endocrine therapy further enhances the effect of radiation, and if administered prior to radiotherapy (RT), it may shrink the gland and thereby allow for additional normal tissue shielding Citation[5–7]. Along with the introduction of conformal RT techniques we have stepwise increased the institutional standard tumour dose from 64 Gy to 66 Gy to 70 Gy. Hormonal therapy (HT) was administered according to a regional consensus among urologists referring patients for radiation, mostly initiating HT at the time of referral for RT. We here report the outcome of these three consecutive patient cohorts treated by a systematic dose escalation schedule.
Material and methods
Patients
Between 1990 and 1999, 502 patients with T1-3NxM0 prostate cancer were treated with radical RT with or without adjuvant/neoadjuvant HT at Haukeland University Hospital. In this period the tumour dose was stepwise increased from 64 Gy via 66 Gy to 70 Gy. Eight patients were excluded from the analysis as they for different reasons were given a tumour dose less than 60 Gy, leaving 494 patients available for analysis. The patients were staged by physical examination, PSA testing and isotope bone scan. For most patients a diagnostic transrectal ultrasound and computer tomography (CT) scan was also performed. Surgical lymph node staging and magnetic resonance imaging (MRI) were not routinely performed, but 123 men (25%) underwent pelvic lymphadenectomy for staging prior to RT. In 108 (88%) of these men, the lymphadenectomy did not disclose lymph node metastases. The primary tumour was assigned a T-category based on digital rectal examination Citation[8]. Histology was in the first part of the study based on World Health Organisation (WHO) histological grading (302 patients) Citation[9], later according to the Gleason scoring system (192 patients) Citation[10]. As the treatment decisions were based on the original WHO grading, we have used the original grading in the current analysis. In the uni-and multivariate analysis we converted Gleason score into WHO grading: Gleason score 4–6 to well differentiated, Gleason score 7 to moderately differentiated and Gleason score 8–10 to poorly differentiated Citation[11]. All patients displayed a performance status of 0 or 1 and had a life expectancy of at least five years.
Radiotherapy and hormonal therapy
All patients underwent EBRT with individualised treatment planning, using high-energy photons to a total tumour dose of 64–70 Gy, in 2 Gy fractions five days a week, over 6–7 weeks. Before 1995 the treatment plan was based on a diagnostic CT with adaptation to the patients contour at simulation, later all treatment plans were based on images from our dedicated CT scanner. Until 1995 we applied a four-field box technique (opposing anterior-posterior fields and two opposing lateral fields) with 2 cm uniform margins to 50 Gy before a boost with smaller margins (5 mm for the CTV and 5 mm for ITV) were delivered using four fields or two lateral fields to a total dose of 64 Gy. Field shaping with individually customised blocks was used occasionally in the first part of the study period, and routinely from 1994. In 1996, the use of customised blocks was substituted by multileaf collimation. In addition, the dose was increased, first to a total dose of 66 Gy, and then further increased to 70 Gy in 1997. Details of the 3-D conformal radiotherapy technique have been published elsewhere Citation[12].
Neoadjuvant and adjuvant HT was used in 402 of 494 (81%) patients. Androgen ablations consisted of luteinizing hormone releasing hormone (LhRh) agonist in 53 of these 402 patients (13%), antiandrogen in 2 (1%) and total androgen blockade (TAB) in 347 (86%). In the first part of the study period LhRh agonist was used to downstage tumours before RT for an average of 4–6 months. TAB was used as short-course (≤6 months) treatment in 89% of the cases and as a long-term treatment (between 6 months and 3 years) in 11%. TAB was initiated when the patient was referred for RT, i.e. 8–12 week's prior to start of RT.
Follow-up
Follow-up was individualised according to the standard health care service, which in general implied routine follow-up at local hospitals. The patients were scheduled to be followed at the department of urology at the local hospital, or as a secondary alternative, with the patient's general practitioner (GP). The frequency of follow-up examinations was left to the responsible urologist, but annual reports were sent to the Department of Oncology, reporting on clinical progression, adverse effects and death. The follow-up included physical examination and serum PSA determinations. For all patients seen at a hospital, the clinical charts and hospital records were reviewed; for patients seen by their GP we had communication with the GP when appropriate. All patients were followed to death or to May 5, 2004.
Progression
PSA level was used as a surrogate endpoint for disease activity, using the definition of relapse as a PSA increase of 2 ng/ml greater than the nadir PSA (“Houston criteria”) Citation[13], Citation[14]. All patients with a rising PSA above this level were considered as having biochemical failures (BF). Local recurrence was defined as symptomatic and clinically detectable prostate tumour growth. Bone scan or other radiological procedures were performed in case of a steadily rising PSA or clinical symptoms to define the location of possible tumour metastases.
Adverse effects
Because of the previously described follow-up routines, there were reasons to suspect underreporting of adverse effects by controlling clinicians as no formal scoring system was used. In the present study we focus on the effect on the tumour, while the risk of late effects currently are being investigated prospectively in a later cohort of patients.
Statistical methods
All statistical analyses were performed with the SPSS statistics package (v 12.0, SPSS Inc., Chicago, USA). The primary endpoint was cancer-specific survival (CSS), with overall survival (OS) and BF being secondary endpoints. The time to the relevant events was measured from the start of radiation therapy, analysed by Kaplan-Meier plots and assessed by the log-rank test Citation[15]. Differences between groups were analysed by Kaplan-Meier plots and tested for statistical significance initially using the log-rank test, while Cox regression was used for univariate analyses of continuous covariates. Multivariate analysis was conducted using the Cox proportional hazard regression model. The variables entered into univariate and multivariate analyses were T stage, PSA (log scale), histological grade, radiation dose and year of treatment. PSA was markedly skewed, but approximately normal distributed after log transformation. The statistical significance of the variables entered into the multivariate analysis was assessed using likelihood ratio tests. The project complies with the national research ethics committee's requirements.
Results
Pretreatment characteristics
summarises the clinical characteristics and treatment of the 494 patients included in the three groups given 64 Gy (Group 1), 66 Gy (Group 2) and 70 Gy (Group 3). Nearly half of the cases had T3 disease (42%). The mean initial PSA level (iPSA) was 15.4 ng/ml (range: 0.5–298 ng/ml). The median age was 67 years (range: 47–85 years). Only 91 patients (18%) had EBRT alone, the rest had a combination of HT and EBRT (82%). The median age was different in the three consecutive cohorts, 68 years in Group 1, 66 years in Group 2 and 67 years in Group 3, respectively (p < 0.001). The median pre-treatment PSA was lower in the last cohort: 13.1 ng/ml in Group 3, compared with 20.5 and 20.8 ng/ml in Group 1 and Group 2, respectively (p < 0.001). T-stage was also generally lower in the last cohort (p = 0.006). There were no statistically significant differences in the distribution of grade among the groups.
Table I. Clinical characteristics and treatment of the 494 patients.
Clinical endpoints
The numbers of patients with biochemical, local and distant failures are shown in . The numbers of deaths are also included. Due to the sequential design of the study, differences in follow-up time clearly exist between the cohorts, with the longest follow-up at the lowest dose level. The median follow-up was 107 (2–171) months for those treated with 64 Gy, 81 (13–131) months for those receiving 66 Gy and 61 (8–135) months for those given 70 Gy.
Table II. Outcome.
Biochemical failure
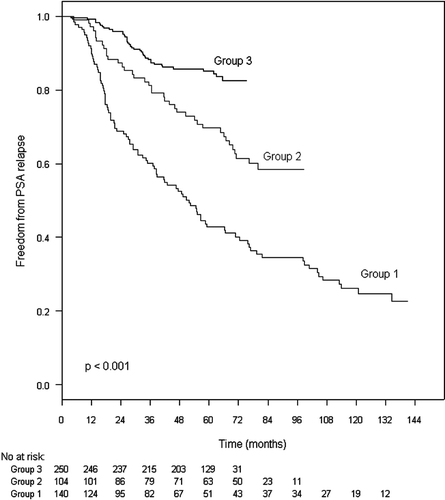
Of the 494 patients, 175 (35%) had PSA failure at a median interval of 29 months (range: 4–150 months) after start of RT. PSA failure was observed for 97 patients (69%) in Group 1, compared to 39 (37%) in Group 2 and 39 (15%) in Group 3. Of the 175 cases with PSA failure, 88 (47%) died, vs. only, 52 (16%) of the 320 cases without PSA failure. The 5 years PSA relapse free survival for Group 1 was 43%, 70% for Group 2 and 85% for Group 3 (p < 0.001) ().
shows the results of the Cox proportional hazards univariate and multivariate analysis of factors affecting PSA relapse. In univariate analysis T stage, pre-treatment PSA, grade, radiation dose, HT and year of therapy were independent predictors of PSA failure. All of these factors except year of therapy remained independent predictors of PSA failure in the multivariate analysis. When analysed as risk groups Citation[16], there were significant effects of radiation dose on PSA failure both in moderate and high risk groups (p < 0.001).
Table III. Results from univariate and multivariate Cox regression, assessing influence of different parameters on PSA recurrence.
Overall survival
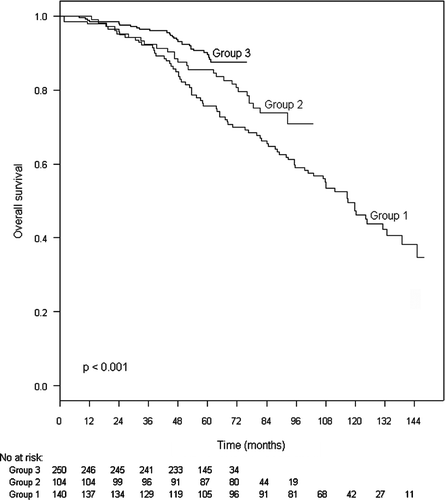
The 5-year OS rate for the entire group was 85% (95 % CI: 81–88 %). The 5-year OS was 76% in Group 1, 85% in Group 2 and 90 % in Group 3 ().
shows the results of the Cox proportional hazards univariate and multivariate analysis of factors affecting overall survival. T stage, grade, radiation dose, HT and year of therapy were significant predictors of survival in the univariate analysis, but only T stage, grade, radiation dose and HT remained independent predictors of survival in the multivariate analysis.
Table IV. Results from univariate and multivariate Cox regression, assessing influence of different parameters on overall survival.
Cancer specific survival
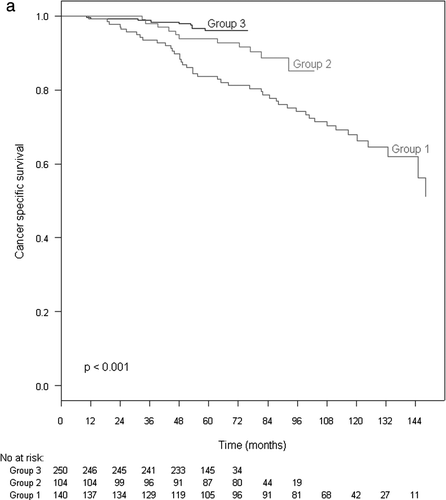
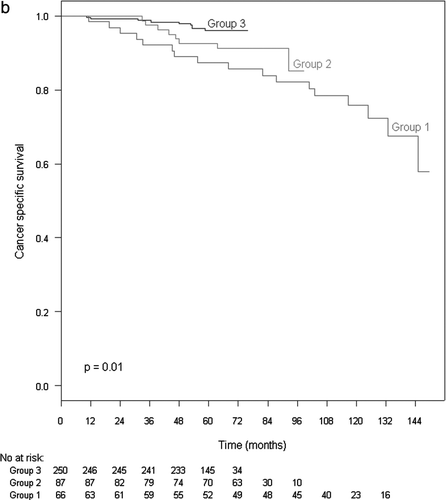
The 5-year CSS rate for all cases was 92%. The 5-year CSS for Group 1 was 83%, 93% for Group 2 and 96% for Group 3 (p < 0.001) when assessed with or without HT (). When assessed in patients having both endocrine therapy and radiation the differences in CSS between the groups was still significant: 87% in Group 1, 91% in Group 2 and 96% in Group 3 (p = 0.01) (). Only a slightly higher CSS was found for patients receiving both EBRT and HT compared to those receiving EBRT alone in Group 1, but this was not statistically significant (p = 0.12).
Figure 3b. Cancer specific survival in patients receiving both EBRT and HT, stratified by radiation.
shows the results of the Cox proportional hazards univariate and multivariate analysis of factors affecting cancer specific survival. On univariate analysis T stage, PSA, radiation dose, grade, year of therapy and HT were significant predictors of survival. T stage, grade and HT remained independent predictors of survival in multivariate analysis, while there was a trend (p = 0.07) for radiation dose. When analysed as risk groups, there were significant effect of radiation dose in high risk patients (p<0.001), but only a trend for moderate risk patients (p = 0.09), on CSS.
Table V. Results from univariate and multivariate Cox regression, assessing influence of different parameters on cancer specific survival.
Time periods
To disclose a possible time effect, we repeated the multivariate analysis stratified by period after deleting the dose variable as it is almost completely determined by era. The stratified analysis did not show a significant relationship between cancer-specific survival and year of therapy (p = 0.09). Also, separate analyses within each era failed to show significant relationships with year of therapy. However, similar analyses for total survival showed a significant relationship (p = 0.004, HR = 0.81) and a significant relationship only in separate analysis for year 1990–1995 (p = 0.041, HR = 0.85). Finally, for PSA-free survival there was a significant relationship in overall stratified analysis (p = 0.047, HR = 1.15) but no significant relationship in separate analyses.
Discussion
Optimal therapy for localised prostate cancer remains a challenge despite the fact that this is the most frequent malignancy in males in many countries. In the early 1990s radiation therapy was considered inferior to radical prostatectomy which remained the recommended first choice for young and healthy patients with a life expectancy of 10–15 years Citation[17]. In the present analysis of the impact of escalating the radiation dose, we observed an improved freedom from PSA rise, as previously reported by others Citation[18] and also better cancer specific and overall survival. There were only slight modifications of our radiation technique during the study, and essentially, the RT remained a local treatment throughout the observation period. The changes in treatment volumes are unlikely to explain our findings.
The natural history of the disease may have changed the last decade Citation[19] with earlier diagnosis due to more or less systematic PSA screening Citation[20], which by itself may cause increased lead times and overdetection Citation[21]. But only a minority of incidentally detected prostate cancers with a PSA value below 3 ng/ml remains insignificant cancers Citation[22]. Better criteria to select those in need of therapy from those with innocent tumours are necessary. In a previous study the effect of radiation dose on overall mortality disappeared when year of treatment was included in the model Citation[23]. In our cohorts, on the other hand, year of treatment was not a significant factor for overall survival, while radiation dose remained a significant factor in the Cox model. The improved outcome after higher radiation dose can therefore hardly reflect a better case mix alone. We have also analysed the data by consecutive time periods, excluding dose as this also reflects the time period. Unfortunately we cannot present a randomized study where period of treatment can be separately analysed in a model containing also the dose. We can therefore not exclude that stage migration contribute to our findings as there is several significant differences between the groups as presented in . However, as multivariate Cox regression represents a scientific approach to weight for the unbalanced factors, the results indicate that dose is an important factor for outcome for patients treated by radiation therapy combined with hormone suppression.
The role of initial active therapy has been challenged Citation[24]. Therefore currently active surveillance and treatment on demand has emerged as a popular therapy option. Previously a similar policy was followed in Denmark where a conservative palliative approach prevailed Citation[25]. However, 62% of their prostate cancer patients died primarily from their malignancy, while only one third neither suffered nor died from their disease. In Scandinavia the majority (80%) of newly diagnosed patients in the period 1995–1998 still did not receive primary therapy with curative intent Citation[26]. However, a Swedish pivotal randomised study indicates a clear reduction in cancer specific mortality after radical prostatectomy compared with watchful waiting, whereas there was no difference in overall survival Citation[27]. After eight years of follow-up, the cancer-specific mortality and development of distant metastases were reduced by about 50% in the surgery group. This finding largely supports earlier studies which indicate that even presumed local cancers, i.e. stage T1 (which are impalpable), might prove to be significant due to progression outside the capsule at time of surgery, clinical progress or pathologic characteristics Citation[28]. In this context it is relevant that T1c tumours with PSA < 10 ng/ml also have a biochemical failure free survival of 97 % after 5 years follow-up after radiation Citation[29] similar to radical prostatectomy Citation[30].
We have decided not to specifically report side effects in this paper as we did not strictly apply fixed forms for recording of side effects and thus there is probably an underreporting of adverse effects in the current series. We have carefully recorded acute and late side effects in a later consecutive series of 250 patients treated at our institution, in which the acute side effects have already been reported Citation[12].
Biochemical PSA failure is widely used as an endpoint for therapy in prostate cancer, with various definitions being applied. Some authors have, however, not found an association Citation[31] or questioned its relationship with increased mortality Citation[32]. Interestingly, using the “Houston criteria” (PSA relapse is scored when PSA is 2 ng/ml greater than nadir), we found a relation between PSA failure and overall survival similar to previous studies with other definitions Citation[33], Citation[34].
The effect of radiation therapy depends on adequate cover of the primary tumour and risk areas for occult spread. We have applied a relatively homogenous tumour coverage during the study period using CT-based planning, but CT scans consistently overestimate the prostate volume Citation[35] and therefore more precise coverage of the tumour volume may reduce side effects. A recent paper reported that radiation dose was the most significant factor for freedom from PSA rise after radiation therapy, rather than radiation technique or use of hormones Citation[36]. We systematically increased the dose in accordance with other centres from 64 Gy Citation[37] via 66 Gy Citation[38] to 70 Gy. Our results confirm that the two lowest doses yielded suboptimal tumour control Citation[39]. Higher radiation doses can be used, and particularly for localised tumours in the high-risk group have doses in the range of 74–81 Gy improved the tumour control rate Citation[3], Citation[40]. Since 2002, we have further escalated the tumour dose for T1c –T3 tumours up to 78 Gy, using the BeamCath® system Citation[41].
The addition of hormone therapy improved the effect of the lowest dose, 64 Gy, but we cannot assess its role at the highest dose level as most patients had started with hormones at the time of referral. There is now general acceptance for addition of long-term hormonal suppression for locally advanced and high risk prostate cancer Citation[42–46], but short- term hormonal therapy can not substitute for radiation dose in high-risk patients Citation[47]. Short-term hormone therapy was given to most patients in the current series in order to maximise the effect of radiation. The finding that short term hormonal ablation was most beneficial in patients with Gleason score 2–6 Citation[48] has not been verified Citation[49]. It is of interest that the dose used in D'Amico's recent study in T1b-2b patients with PSA > 10 ng/ml or Gleason score at least 7 (range 7–10) had a similar radiation dose, 70 Gy, and 6 months of hormonal therapy as used in our study. Our data seems to confirm the excellent results in the combined arm. A similar dose escalation effect was reported in a randomised phase III trial comparing 70 Gy with 78 Gy radiation alone as freedom from failure increased from 64% to 70% Citation[50]. Recently a randomised pilot study suggested a radiation dose escalation effect on biochemical PSA control of disease from 59% to 71% when the dose was increased from 64 Gy to 74 Gy combined with hormone therapy Citation[51].
There is still no results from a randomised study comparing primary surgery versus radiation Citation[52]. Until randomised data are available, patients with prostate cancer stage T1c–T3 should be offered a minimum dose of 70 Gy with short term hormonal therapy, at least for intermediate and high risk patients as recently recommended in USA Citation[53] as an alternative to surgery.
This study was supported by a generous grant from the Norwegian Cancer Society. We acknowledge the contribution by the late consultant Sigmund Vaage, our initial collaborator in Stavanger.
References
- Cancer Registry of Norway. Cancer in Norway 2000. 2002.
- Dahl O, Kardamakis D, Lind B, Rosenwald JC. Current status of conformal radiotherapy. Acta Oncol 1996; 35(Suppl 8)41–57
- Vicini FA, Abner A, Baglan KL, Kestin LL, Martinez AA. Defining a dose-response relationship with radiotherapy for prostate cancer: Is more really better?. Int J Radiat Oncol Biol Phys 2001; 51(5)1200–8
- Zelefsky MJ, Fuks Z, Hunt M, Lee HJ, Lombardi D, Ling CC, et al. High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J Urol 2001; 166(3)876–81
- Horwich A, Wynne C, Nahum A, Swindell W, Dearnaley DP. Conformal radiotherapy at the Royal Marsden Hospital (UK). Int J Radiat Biol 1994; 65(1)117–22
- Lilleby W, Dale E, Olsen DR, Gude U, Fossa SD. Changes in treatment volume of hormonally treated and untreated cancerous prostate and its impact on rectal dose. Acta Oncol 2003; 42(1)10–4
- Shearer RJ, Davies JH, Gelister JS, Dearnaley DP. Hormonal cytoreduction and radiotherapy for carcinoma of the prostate. Br J Urol 1992; 69(5)521–4
- TNM classification of malignant tumours. 4th ed., Geneva, Schweiz: International Union Against Cancer; 1992.
- Mostofi FK, Sesterhenn IA, Sobin LH. Histological typing of prostate tumours. World Health Organization, Geneva 1980
- Mellinger GT, Gleason D, Bailar J. The histology and prognosis of prostatic cancer. J Urology 1967; 97: 331–8
- Van der Kwast TH, Roobol MJ, Wildhagen MF, Martikainen PM, Maatnanen L, Pihl CG, et al. Consistency of prostate cancer grading results in screened populations across Europe. BJU Int 2003; 92(Suppl 2)88–91
- Karlsdottir A, Johannessen DC, Muren LP, Wentzel-Larsen T, Dahl O. Acute morbidity related to treatment volume during 3D-conformal radiation therapy for prostate cancer. Radiother Oncol 2004; 71(1)43–53
- Pickles T, Kim-Sing C, Morris WJ, Tyldesley S, Paltiel C. Evaluation of the Houston biochemical relapse definition in men treated with prolonged neoadjuvant and adjuvant androgen ablation and assessment of follow-up lead-time bias. Int J Radiat Oncol Biol Phys 2003; 57(1)11–8
- Kestin LL, Vicini FA, Martinez AA. Practical application of biochemical failure definitions: What to do and when to do it. Int J Radiat Oncol Biol Phys 2002; 53(2)304–15
- Altman D. Practical statistics for medical research. Chapman and Hall, London and New York 1991; 611
- D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998; 280(11)969–74
- Peschel RE, Colberg JW. Surgery, brachytherapy, and external-beam radiotherapy for early prostate cancer. Lancet Oncol 2003; 4(4)233–41
- Parker CC, Dearnaley DP. Radical radiotherapy for prostate cancer. Cancer Treat Rev 2003; 29(3)161–9
- D'Amico AV, Chen M-H, Oh-Ung J, Renshaw AA, Cote K, Loffredo M, et al. Changing prostate-specific antigen outcome after surgery or radiotherapy for localized prostate cancer during the prostate-specific antigen era. Int J Radiat Oncol Biol Phys 2002; 54(2)436–41
- Cooperberg MR, Lubeck DP, Meng MV, Mehta SS, Carrol PR. The changing face of low-risk prostate cancer: Trends in clinical presentation and primary management. J Clin Oncol 2004; 22(11)2141–9
- Draisma G, Boer R, Otto SJ, Van der Cruijsen IW, Damhuis RA, Schroder FH, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst 2003; 95(12)868–78
- Lujan M, Paez A, Miravalles E, Fernandez I, Llanes L, Berenguer. A prostate cancer detection is also relevant in low prostate specific antigen ranges. Eur Urol 2004; 45(2)155–9
- Jacob R, Hanlon AL, Horwitz EM, Movsas B, Uzzo RG, Pollack A. The relationship of increasing radiotherapy dose to reduced distant metastases and mortality in men with prostate cancer. Cancer 2004; 100(3)538–43
- Parker C. Active surveillance: Towards a new paradigm in the management of early prostate cancer. Lancet Oncol 2004; 5(2)101–6
- Borre M, Nerstrom B, Overgaard J. The natural history of prostate carcinoma based on a Danish population treated with no intent to cure. Cancer 1997; 80(5)917–28
- Iversen P, Tammela TL, Vaage S, Lukkarinen O, Lodding P, Bull-Njaa T, et al. A randomised comparison of bicalutamide (‘Casodex') 150 mg versus placebo as immediate therapy either alone or as adjuvant to standard care for early non-metastatic prostate cancer. First report from the Scandinavian Prostatic Cancer Group Study No. 6. Eur Urol 2002; 42(3)204–11
- Holmberg L, Bill-Axelson A, Helgesen F, Salo JO, Folmerz P, Haggman M, et al. A randomized trial comparing radical prostatectomy with watchful waiting in early prostate cancer. N Engl J Med 2002; 347(11)781–9
- Douglas TH, McLeod DG, Mostofi FK, Mooneyhan R, Connelly R, Moul JW, et al. Prostate-specific antigen-detected prostate cancer (stage T1c): An analysis of whole-mount prostatectomy specimens. Prostate 1997; 32(1)59–64
- Hanks GE, Hanlon AL, Pinover WH, al-Saleem TI, Schultheiss TE. Radiation therapy as treatment for stage T1c prostate cancers. World J Urol 1997; 15(6)369–72
- Pound CR, Walsh PC, Epstein JI, Chan DW, Partin AW. Radical prostatectomy as treatment for prostate-specific antigen-detected stage T1c prostate cancer. World J Urol 1997; 15(6)373–7
- Kupelian PA, Buchsbaum JC, Patel C, Elshatkh M, Reddy CA, Zippe C, et al. Impact of biochemical failure on overall survival after radiation therapy for localized prostate cancer in the PSA era. Int J Radiat Oncol Biol Phys 2002; 52(3)704–11
- Sandler HM, Dunn RL, McLaughlin PW, Hayman JA, Sullivan MA, Taylor JM. Overall survival after prostate-specific-antigen-detected recurrence following conformal radiation therapy. Int J Radiat Oncol Biol Phys 2000; 48(3)629–33
- Vicini FA, Kestin LL, Martinez AA. The correlation of serial prostate specific antigen measurements with clinical outcome after external beam radiation therapy of patients for prostate carcinoma. Cancer 2000; 88(10)2305–18
- Pollack A, Hanlon AL, Movsas B, Hanks GE, Uzzo R, Horwitz EM. Biochemical failure as a determinant of distant metastasis and death in prostate cancer treated with radiotherapy. Int J Radiat Oncol Biol Phys 2003; 57(1)19–23
- Hoffelt SC, Marshall LM, Garzotto M, Hung A, Holland J, Beer TM. A comparison of CT scan to transrectal ultrasound-measured prostate volume in untreated prostate cancer. Int J Radiat Oncol Biol Phys. 2003; 57(1)29–32
- Khuntia D, Reddy CA, Mahadevan A, Klein EA, Kupelian PA. Recurrence-free survival rates after external-beam radiotherapy for patients with clinical T1–T3 prostate carcinoma in the prostate-specific antigen era: What should we expect?. Cancer 2004; 100(6)1283–92
- Duchesne GM. Radiation for prostate cancer. Lancet Oncol 2001; 2(2)73–81
- Fossa SD, Lilleby W, Waehre H, Berner A, Torlakovic G, Paus C, et al. Definitive radiotherapy of prostate cancer: The possible role of staging lymphadenectomy. Int J Radiat Oncol Biol Phys 2003; 57(1)33–41
- Zagars GK, Pollack A, Smith LG. Conventional external-beam radiation therapy alone or with androgen ablation for clinical stage III (T3, NX/N0, M0) adenocarcinoma of the prostate. Int J Radiat Oncol Biol Phys 1999; 44(4)809–19
- Kupelian PA, Potters L, Khuntia D, Ciezki JP, Reddy CA, Reuther AM, et al. Radical prostatectomy, external beam radiotherapy = 72 Gy, permanent seed implantation, or combined seeds/external beam radiotherapy for stage T1–T2 prostate cancer. Int J Radiat Oncol Biol Phys 2004; 58(1)25–33
- Bergstrom P, Lofroth PO, Widmark A. High-precision conformal radiotherapy (HPCRT) of prostate cancer–a new technique for exact positioning of the prostate at the time of treatment. Int J Radiat Oncol Biol Phys 1998; 42(2)305–11
- Maximum androgen blockade in advanced prostate cancer: An overview of the randomised trials. Lancet 2000;355(9214):1491–8.
- Bolla M, Collette L, Blank L, Warde P, Dubors JB, Mirimanoff RO, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): A phase lll randomised trial. Lancet 2002; 360: 103–8
- Kupelian PA. External beam radiation therapy: Role of androgen deprivation. World J Urol 2003; 21(4)190–9
- Gottschalk AR, Roach M 3rd. The use of hormonal therapy with radiotherapy for prostate cancer: Analysis of prospective randomised trials. Br J Cancer 2004; 90(5)950–4
- D'Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6-Month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: A randomized controlled trial. JAMA 2004; 292(7)821–7
- Nguyen KH, Horwitz EM, Hanlon AL, Uzzo RG, Pollack A. Does short-term androgen deprivation substitute for radiation dose in the treatment of high-risk prostate cancer?. Int J Radiat Oncol Biol Phys 2003; 57(2)377–83
- Pilepich MV, Winter K, John MJ, Mesic JB, Sause W, Rubin P, et al. Phase III radiation therapy oncology group (RTOG) trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys 2001; 50(5)1243–52
- Pollack A, Hanlon A, Horwitz EM, Feigenberg S, Uzzo RG, Price RA. Radiation therapy dose escalation for prostate cancer: A rationale for IMRT. World J Urol 2003; 21(4)200–8
- Pollack A, Zagars GK, Starkschall G, Antolak JA, Lee JJ, Huang E, et al. Prostate cancer radiation dose response: Results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys 2002; 53(5)1097–105
- Dearnaley DP, Hall E, Lawrence D, Huddart RA, Eeles R, Nutting CM, et al. Phase III pilot study of dose escalation using conformal radiotherapy in prostate cancer: PSA control and side effects. Br J Cancer 2005; 92(3)488–98
- Nilsson S, Norlen BJ, Widmark A. A systematic overview of radiation therapy effects in prostate cancer. Acta Oncol 2004; 43(4)316–81
- DeWeese TL. Radiation therapy and androgen suppression as treatment for clinically localized prostate cancer: The new standard?. JAMA 2004; 292(7)864–6