Abstract
To study the visual outcome, local tumour control, and eye preservation 5 years after ruthenium/rhodium 106 brachytherapy for choroidal melanoma. The study included 55 consecutive patients treated by 106Ru/Rh brachytherapy for a choroidal melanoma during the period 1988–2000 and followed through 2004. The 5-year probability for not losing at least 5 Snellen lines was 59% (n = 45), for retaining a visual acuity of 0.33 or better was 28% (n = 34), and for retaining better than 0.1 was 40% (n = 45). The 5-year probability for no local recurrence was 73% and for eye preservation 72% (n = 55). 106Ru/Rh brachytherapy for choroidal melanoma resulted in a clinically significant vision loss, no local recurrence, and eye preservation in most patients after 5 years. 106Ru/Rh brachytherapy can be regarded as a good treatment option for small and medium-sized tumours but not for large tumours.
The incidence rate of choroidal and ciliary body melanomas has been estimated to 0.6 per 100 000 person-years Citation[1]. These tumours are primarily handled by enucleation or radiotherapy with some treated by local resection or other modalities. No difference in survival has ever been documented between enucleation and brachytherapy Citation[2], Citation[3] , proton radiation Citation[4], Citation[5] , Helium ion radiation Citation[6], or local resection Citation[7]. Therefore, a change in treatment from enucleation towards eye preserving therapy with retention of some visual function has taken place for choroidal and ciliary body melanomas Citation[8–10]. The indication for eye preserving therapy depends primarily on tumour location and size, but age and general health of the patient and presence of extrascleral extension or distant metastasis are also taken into consideration. Eye preserving treatment is especially relevant for tumours in the only seeing eye of the patient.
In this study, the 5-year visual outcome, local tumour control, and eye preservation of all choroidal melanomas treated by ruthenium/rhodium 106 brachytherapy at a Danish centre since the introduction in 1988 and through 2000 are evaluated.
Material and methods
The study included 55 consecutive patients (28 men and 27 women), who had been treated by 106Ru/Rh brachytherapy at the Department of Ophthalmology, Århus University Hospital, Denmark for a choroidal melanoma during the period 1988–2000. The median age at treatment was 61 years (range 22–87 years). Laterality showed 28 right and 27 left eyes. One patient was retreated by brachytherapy for a local recurrence and was later enucleated for recurrence. Four patients had adjunctive transpupillary thermotherapy and one had adjunctive brachytherapy with none having local recurrence. No ciliary body melanoma had been treated by 106Ru/Rh brachytherapy and none of the choroidal tumours involved the ciliary body.
Five different 106Ru/Rh plaques (Bebig GmbH, Berlin, Germany) were used: CCZ (diameter 12 mm, n = 1), CCA (15 mm, n = 17), CCB (20 mm, n = 30), CCC (25 mm, n = 5), and COB (20 mm with notch for placement close to the optic nerve, n = 2). The treatment dose was in all patients 100 Gy in the design depth (6 mm) of the plaque. The plaque was sutured into place under general anaesthesia in most patients. The diameter of the plaque was chosen to be at least 4 mm larger than the largest basal diameter of the tumour leaving room for tumour not visible at ophthalmoscopy or ultrasonography and for some imprecision in the surgical procedure.
The study design was historical follow-up with information from medical records. The start of follow-up was set at the time of brachytherapy and the end of follow-up was at the last visit at ophthalmologic department at hospital or practising ophthalmologist in 2004 or at enucleation or death. The patients were generally followed every 3–6 months within the first years, thereafter every 6–12 months for some years, and finally followed at their practising ophthalmologist.
The melanoma diagnosis was clinical in all cases with no patient having preoperative intraocular biopsy for histological verification.
Serous retinal detachment was seen in 71% (37/52) and vitreous haemorrhage in 8% (4/54) of patients at presentation.
During the study period, 122 patients with choroidal or ciliary body melanomas were treated at our department. The primary treatment was in other 61 patients enucleation, in five patients cobalt 60 brachytherapy (early in the period), and in one patient local tumour resection.
Tumour size and location
Largest basal diameter was in 43 patients evaluated by B-scan ultrasonography. In patients without ultrasonography, tumour diameter was estimated from ophthalmoscopy in six cases, fundus photo in one, fluorescein angiography in one, diameter of the plaque in one, magnetic resonance imaging in two, and computerised tomography in one case. In one patient there was no information on diameter. Median largest basal diameter was 12.0 mm (n = 54, range 6.0–21.0 mm).
Height was estimated by A-scan ultrasonography in 52 patients, by magnetic resonance imaging in one patient, and by computerised tomography in one patient. In one patient there was no information on height. Scleral thickness was not included in the tumour height. Median height was 5.8 mm (n = 54, range 1.8–11.0 mm).
In the TNM classification Citation[11] 13% (7/54) of tumours were T1, 28% (15/54) were T2, 59% (32/54) were T3, none was T4, and one could not be classified.
The anterior tumour margin was between the ora serrata and equator in 18 cases and posterior to the equator in 37 cases. The median distance from the posterior tumour margin to the optic disc was 5.0 mm (n = 51, range 0.0–15.0 mm) and to the fovea 5.0 mm (n = 50, range 0.0–11.0 mm).
Visual acuity
Preoperative and postoperative visual acuities (best corrected if available) were registered. Visual acuity measured as a decimal number was converted to a Snellen fraction and four further categorical levels were included: counting fingers, hand movements, light perception, and no light perception. Visual acuity after enucleation was set at no light perception. Change in visual acuity was evaluated as a number of Snellen line changes in relation to the pre-treatment vision, e.g. one line was lost from 6/18 to 6/24 or from counting fingers to hand movements. The time point for a given number of Snellen lines loss was the date when vision permanently was registered to have fallen that much; that is, transient vision loss was disregarded.
Change in visual acuity within plus or minus two lines was regarded as equal vision, loss of three or more lines as deterioration, and gain of three or more lines as improvement.
Local tumour control and eye preservation
Local recurrence was defined as any clinical tumour re-growth in basal diameter or height.
It was registered whether the reason for enucleation was local recurrence, secondary complication, or on patient′s request.
Statistics
Visual outcome, local tumour control, and eye preservation over time were estimated by Kaplan-Meier analysis.
Prognostic factors for visual outcome, local tumour control, and eye preservation were evaluated by univariate Cox proportional hazards analysis. The assumption of proportional hazards was examined through log minus log plots for each analysis.
All analyses were performed by the statistical software package SPSS version 10.0–11.5 (SPSS Inc., Chicago, Illinois, USA).
Statistical significance level was set at 0.05 (two-sided).
Results
Visual acuity
Last postoperative visual acuity was known for 47 eyes within one year and for eight eyes more than one year before the end of follow-up, enucleation, or death. The median follow-up time from brachytherapy to last postoperative visual acuity/enucleation was 4.6 years (range 0.2–12.9 years). At the end of follow-up at December 31 2004 seventeen patients had died after a median follow-up time of 3.4 (range 0.3–13.0) years. Follow-up time was shorter than 5 years in 29 patients because of death in nine cases, enucleation in fourteen cases, inclusion in 2000 and follow-up until the end of 2004 in five cases, and lost to follow-up in one case. For the 26 patients followed longer than 5 years two had died, six had been enucleated, one had emigrated, and four had been lost to follow-up.
Visual acuities better than counting fingers were preoperatively best corrected in 90% (46/51), uncorrected in 4% (2/51), and unknown in 6% (3/51) of cases and postoperatively best corrected in 79% (418/529), uncorrected in 10% (53/529), and unknown in 11% (58/529) of cases.
A scatter plot of preoperative versus last postoperative visual acuity is illustrated in .
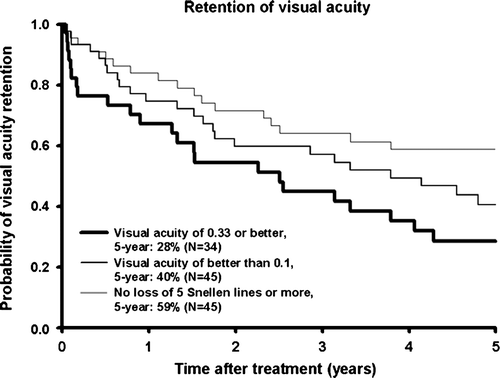
Figure 1. Presentation of the preoperative and last measured postoperative visual acuity after 106Ru/Rh brachytherapy. Eyes enucleated were set at no light perception. Visual acuity is shown in Snellen fractions except CF: counting fingers, HM: hand movements, +LP: light perception, -LP: no light perception.
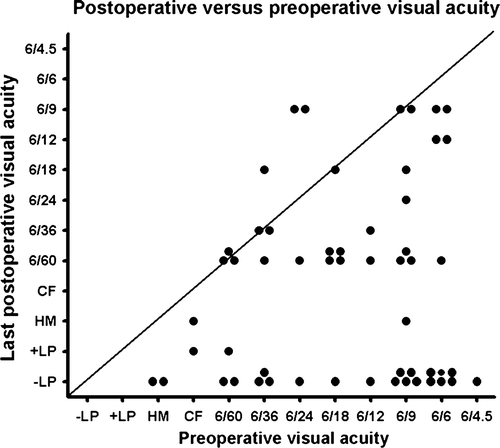
The median preoperative and last postoperative visual acuity was 0.5 and 0.1, respectively. The median change in visual acuity was a loss of three Snellen lines (range: loss of eleven to gain of three lines). Thirty-three patients had impaired, 20 equal, and 2 improved visual acuity at the end of follow-up. The 5-year probability for not losing at least 5 Snellen lines was 59% (n = 45) (). The 5-year probability for retaining visual acuity of 0.33 or better if 0.33 or better preoperatively was 28% (n = 34) and for retaining visual acuity of better than 0.1 if better than 0.1 preoperatively was 40% (n = 45) (). The prognostic factors for visual outcome (losing at least 5 Snellen lines or losing 0.33 or 0.1 visual acuity) evaluated by univariate Cox proportional hazards analysis are shown in . Tumour height and largest basal diameter and local recurrence were significant risk factors for visual acuity loss.
Table I. Risk of vision loss for different parameters evaluated by univariate Cox proportional hazards analysis. Calculations were made for those eyes that had the possibility for a specific vision loss.
Local tumour control
Information on local tumour control was known for 47 patients within one year and for eight patients more than one year before the end of follow-up, enucleation for complication, or death. The median follow-up time was 4.4 years (0.2–12.9 years).
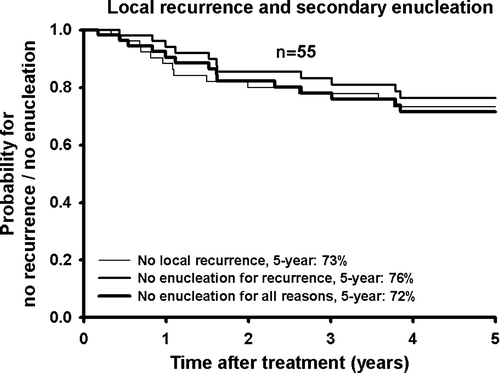
Local recurrence was found in fifteen patients, fourteen of which were treated by enucleation and one left untreated because of metastatic disease. The 5-year probability for no local recurrence was 73% (n = 55) (). The relation between local recurrence and tumour largest basal diameter and height is shown in . Local recurrence was especially seen for large but also for some small tumours. Prognostic factors for local recurrence are shown in . A significantly higher risk of local recurrence was seen for tumours located anteriorly, with large basal diameter, and higher than 8 mm.
Figure 4. Relation between local tumour control and tumour largest basal diameter and height after 106Ru/Rh brachytherapy. For one tumour no information on largest basal diameter was available and for another no information on height.
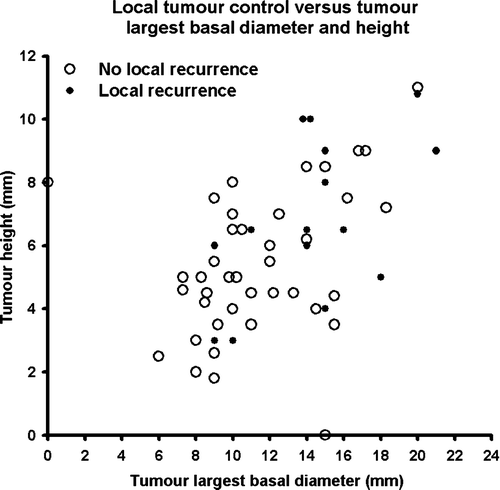
Table II. Risk of local recurrence and enucleation for reasons for different parameters evaluated by univariate Cox regression analysis.
Eye preservation
Information on eye preservation was known for 47 patients within one year and for eight patients more than one year before the end of follow-up or death. The median follow-up time was 4.6 years (0.2–12.9 years).
Enucleation was performed because of local recurrence (n = 14; 25% of all eyes/70% of all enucleations), neovascular glaucoma (n = 4; 7%/20%), phthisic eye (n = 1; 2%/5%), and on patient′s request despite local tumour control (n = 1; 2%/5%). The 5-year eye preservation probability considering only local recurrences leading to enucleation was 76% and including enucleations for secondary complications and patient′s request the probability was 72% (n = 55) (). The risk of enucleation was significantly higher for tumours located anteriorly and with large basal diameter and height ().
Discussion
The study cohort consists of a small consecutive series of patients evaluated in a historical follow-up design. With the clinical and paraclinical diagnostic modalities available throughout the study period, the diagnostic accuracy in our series can be regarded as high Citation[12].
Risk estimates for the different outcomes were evaluated by univariate analyses only, as the material was too small for multivariate analysis, which could have reduced potential confounding.
Visual acuity
The visual acuity stated in the medical records might have been better if thorough optical refraction had been done for all patients under standardised conditions, however, for evaluation of change in visual acuity this might be of minor importance.
Vision loss caused by non-radiation-related diseases such as age-related macular degeneration or senile cataract was not accounted for. The presented vision loss might therefore be an overestimation of radiation side effects.
The visual acuity decreased over time, leaving a relatively large number of patients with poor but some vision years later. The median last postoperative visual acuity was 0.1, which was in accordance with a Swedish series Citation[13]. The median decrease in visual acuity was three lines through a median follow-up time of 4.6 years. In the Collaborative Ocular Melanoma Study (COMS) using iodine 125 brachytherapy for medium-sized tumours, an average loss of two lines per year was found in the first three years Citation[14].
For every ten eyes, three eyes retained visual acuity of 0.33 or better and four retained visual acuity of better than 0.1 five years after treatment. A large Swedish ruthenium series showed a 5-year probability for retaining visual acuity of 0.5 or better of 31% and better than 0.1 of 49% Citation[15], and another large series of radiation brachytherapy (iodine in 59%, cobalt in 27%, and iridium in 9%, ruthenium in 5% of patients) showed a 5-year probability for retaining visual acuity better than 0.1 of 66%, which was much better than in our study, and a 5-year probability for not losing 5 Snellen lines or more of 67% compared to our 59% Citation[16]. However, the tumours in these two series were smaller than in our material, which could partly explain their better results. For tumours higher than 8 mm treated by iodine, cobalt, iridium, or ruthenium, a 5-year probability for retaining vision better than 0.1 of 43% has been found Citation[17]. Lommatzsch found for ruthenium brachytherapy a 10-year probability for visual acuity of 0.1 or better of 37% Citation[18]. In the COMS the 3-year probability for retaining vision of better than 0.1 was 57% and for not losing 6 lines 51% Citation[14]. As no international consensus exists on how to report visual outcome it is difficult to compare studies, however, the tendency towards vision loss through the years is evident.
Visual outcome seems better after 106Ru/rh compared to 125I Citation[19] and proton radiation Citation[20]. 106Ru and proton radiation have a more marked fall-off in radiation dose and might be a better choice in tumours located near the optic disc Citation[19], Citation[21]. Alternatively, visual function can often be retained by local tumour resection in carefully selected patients as described by Damato et al. Citation[22], Citation[23].
The reasons for visual acuity loss after radiation brachytherapy are retinopathy, maculopathy, optic atrophy, cataract, and neovascular glaucoma Citation[24–27] . High tumour dose rate in radiation brachytherapy has shown increased risk of retinopathy and poor vision Citation[24], Citation[28] . The visual outcome after brachytherapy for juxtapapillary or macular choroidal melanomas is especially poor Citation[29–32]. Brachytherapy for ciliary body melanomas has an increased risk of anterior segment complications, i.e. especially cataract and neovascular glaucoma, whereas the risk of posterior segment complications, i.e. maculopathy and optic atrophy, is lower Citation[33].
In this study, the prognostic factors for visual acuity loss were in univariate analysis tumour height, largest basal tumour diameter, and local recurrence. The eyes with local recurrence were almost all enucleated with postoperative vision set at no light perception. In the literature, the risk factors for visual acuity loss are higher age, poor initial visual acuity, increasing tumour height, proximity to fovea or optic disc, anterior tumour margin posterior to the equator, presence of subretinal fluid, and tumour recurrence Citation[14–16], Citation[18], Citation[19], Citation[24], Citation[32], Citation[34], Citation[35] .
Local tumour control and eye preservation
The 5-year probability for no local recurrence was 73% compared to 59% Citation[36], 78% Citation[37], and 98% Citation[38] in other Ruthenium series. The long-term results after 106Ru/Rh treatment have shown a 10-year probability for no recurrence of 76% Citation[37] and a 15-year probability of 63% Citation[18]. In the COMS using iodine 125 brachytherapy for medium-sized tumours, the 5-year probability for no local recurrence was 90% Citation[39]. Local recurrence occurred more frequently in tumours with large height, especially higher than 8 mm, large basal diameter, and anterior location, which was in accordance with other studies Citation[17], Citation[18], Citation[20], Citation[26], Citation[36–38], Citation[40]. Tumour location close to the optic disc or fovea might pose a problem in plaque positioning and increase the risk of local recurrence, although this was not the case in this study. Increased risk of local recurrence has been found for lower radiation dose to tumour apex Citation[41–43] and lower dose rate to the apex Citation[43]. By routine, the radiation dose was 100 Gy in the design depth of the plaque at 6 mm including the sclera. Many relatively high tumours (median height was 5.8 mm) had been treated, which could partly explain the tendency towards more recurrences than in other studies.
An increased risk for local recurrence has been found for 106Ru/Rh compared to 125I or proton radiation Citation[17], Citation[20] and for 125I brachytherapy compared to proton or helium ion radiation Citation[44], Citation[45]. Local tumour control has been found high after proton and helium ion radiation, which can deliver a high dose to a well-defined area Citation[44–47]. However, the charged particle radiation results in a high risk of anterior segment complications, i.e. cataract, iridocyclitis and especially neovascular glaucoma, compared to 106Ru/Rh and 125I brachytherapy Citation[6], Citation[20], Citation[46], Citation[48]. Local tumour control can also in selected small pigmented choroidal melanomas be obtained by transpupillary thermotherapy alone Citation[49] or in combination with e.g. brachytherapy Citation[49–52]. Finally, local tumour control has been achieved by transscleral local resection Citation[23] and by stereotactic external photon beam radiation, which is a relatively new treatment modality for uveal melanoma Citation[53].
The 5-year probability for eye preservation was 72% compared to 82% in a French Citation[37], 83% in a Swedish Citation[15], and 85% in a Finish series Citation[27]. The tumours were larger than in the French and Swedish but similar to the Finish series. The 5-year eye preservation for tumours higher than 8 mm treated by various isotopes has been found to be 76% Citation[17] and in the COMS (iodine 125) treating medium-sized tumours by iodine 125 it was 88% Citation[39]. The long-term results after 106Ru/Rh have shown a 10-year eye preservation of 78% Citation[15] and 81% Citation[37] and a 15-year eye preservation of 66% Citation[18]. The main reasons for enucleation after radiation brachytherapy are local tumour recurrence in 51% to 75% Citation[15], Citation[31], Citation[54] and neovascular glaucoma in 17% to 31% Citation[13], Citation[31], Citation[54]. The reasons for enucleation in these studies are comparable to this study. Other reasons are patient's request, scleral necrosis, retinal detachments, vitreous hemorrhage, or optic atrophy Citation[13], Citation[31], Citation[54].
Conclusion
In this consecutive series, patients retained some useful vision in the first postoperative years and a few even got better vision. However, the long-term visual outcome is poor with a continuing visual acuity loss over time and a large number of patients became blind or lost reading ability after 5 years, either because of radiation complications or secondary enucleation. Most patients had no local recurrence and retained the eye 5 years after treatment. 106Ru/Rh brachytherapy can be regarded as a good treatment option for small and medium-sized tumours but not for large tumours. In a general sense, the results of this series were comparable to other studies of 106Ru/Rh brachytherapy treated patients.
References
- Isager P, Østerlind A, Engholm G, Heegaard S, Lindegaard J, Overgaard J, et al. Malignant melanoma in the ocular region in Denmark 1943–97. Incidence and validation study. Ophthal Epidemiol 2005; 12: 223–32
- The Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: Initial mortality findings. COMS Report No. 18. Arch Ophthalmol 2001; 119: 969–82
- Augsburger JJ, Schneider S, Freire J, Brady LW. Survival following enucleation versus plaque radiotherapy in statistically matched subgroups of patients with choroidal melanomas: Results in patients treated between 1980 and 1987. Graefes Arch Clin Exp Ophthalmol 1999; 237: 558–67
- Courdi A, Caujolle JP, Grange JD, Diallo-Rosier L, Sahel J, Bacin F, et al. Results of proton therapy of uveal melanomas treated in Nice. Int J Radiat Oncol Biol Phys 1999; 45: 5–11
- Seddon JM, Gragoudas ES, Egan KM, Glynn RJ, Howard S, Fante RG, et al. Relative survival rates after alternative therapies for uveal melanoma. Ophthalmology 1990; 97: 769–77
- Castro JR, Char DH, Petti PL, Daftari IK, Quivey JM, Singh RP, et al. 15 years experience with helium ion radiotherapy for uveal melanoma. Int J Radiat Oncol Biol Phys 1997; 39: 989–96
- Foulds WS, Damato BE, Burton RL. Local resection versus enucleation in the management of choroidal melanoma. Eye 1987; 1(Pt 6)676–9
- Shields JA, Shields CL, Shah P, DePotter P. Current alternatives in the management of posterior uvea melanomas. Trans Pa Acad Ophthalmol Otolaryngol 1990; 42: 938–44
- Shields JA, Shields CL. Current management of posterior uveal melanoma. Mayo Clin Proc 1993; 68: 1196–200
- Damato BE. An approach to the management of patients with uveal melanoma. Eye 1993; 7(Pt 3)388–97
- Union Internationale Contre le Cancer. 1997. TNM Classification of Malignant Tumours 5th edition.
- The Collaborative Ocular Melanoma Study. Histopathologic characteristics of uveal melanomas in eyes enucleated from the Collaborative Ocular Melanoma Study. COMS report no. 6. Am J Ophthalmol 1998;125:745–66.
- Seregard S, af Trampe E, Lax I, Kock E, Lundell G. Results following episcleral ruthenium plaque radiotherapy for posterior uveal melanoma. The Swedish experience. Acta Ophthalmol Scand 1997; 75: 11–6
- Collaborative Ocular Melanoma Study. Collaborative Ocular Melanoma Study (COMS) randomized trial of I-125 brachytherapy for medium choroidal melanoma. I. Visual acuity after 3 years COMS report no. 16. Ophthalmology 2001; 108: 348–66
- Bergman L, Nilsson B, Lundell G, Lundell M, Seregard S. Ruthenium brachytherapy for uveal melanoma, 1979–2003: Survival and functional outcomes in the Swedish population. Ophthalmology 2005; 112: 834–40
- Shields CL, Shields JA, Cater J, Gündüz K, Miyamoto C, Micaily B, et al. Plaque radiotherapy for uveal melanoma: Long-term visual outcome in 1106 consecutive patients. Arch Ophthalmol 2000; 118: 1219–28
- Shields CL, Naseripour M, Cater J, Shields JA, Demirci H, Youseff A, et al. Plaque radiotherapy for large posterior uveal melanomas (>or = 8-mm thick) in 354 consecutive patients. Ophthalmology 2002; 109: 1838–49
- Lommatzsch PK, Werschnik C, Schuster E. Long-term follow-up of Ru-106/Rh-106 brachytherapy for posterior uveal melanoma. Graefes Arch Clin Exp Ophthalmol 2000; 238: 129–37
- Bornfeld N, Chauvel P, Sauerwein W, Friedrichs W, Tiburtius T, Wessing A, et al. Metastatic disease, eye retention and visual function in conservative treatment of uveal melanoma. Front Radiat Ther Oncol 1997; 30: 97–110
- Wilson MW, Hungerford JL. Comparison of episcleral plaque and proton beam radiation therapy for the treatment of choroidal melanoma. Ophthalmology 1999; 106: 1579–87
- Lommatzsch PK, Lommatzsch R. Treatment of juxtapapillary melanomas. Br J Ophthalmol 1991; 75: 715–7
- Damato BE, Paul J, Foulds WS. Predictive factors of visual outcome after local resection of choroidal melanoma. Br J Ophthalmol 1993; 77: 616–23
- Damato BE, Paul J, Foulds WS. Risk factors for residual and recurrent uveal melanoma after trans-scleral local resection. Br J Ophthalmol 1996; 80: 102–8
- Gündüz K, Shields CL, Shields JA, Cater J, Freire JE, Brady LW. Radiation retinopathy following plaque radiotherapy for posterior uveal melanoma. Arch Ophthalmol 1999; 117: 609–14
- Fontanesi J, Meyer D, Xu S, Tai D. Treatment of choroidal melanoma with I-125 plaque. Int J Radiat Oncol Biol Phys 1993; 26: 619–23
- Lommatzsch PK. Results after beta-irradiation (106Ru/106Rh) of choroidal melanomas: 20 years' experience. Br J Ophthalmol 1986; 70: 844–51
- Summanen P, Immonen I, Kivela T, Tommila P, Heikkonen J, Tarkkanen A. Radiation related complications after ruthenium plaque radiotherapy of uveal melanoma. Br J Ophthalmol 1996; 80: 732–9
- Jones R, Gore E, Mieler W, Murray K, Gillin M, Albano K, et al. Posttreatment visual acuity in patients treated with episcleral plaque therapy for choroidal melanomas: Dose and dose rate effects. Int J Radiat Oncol Biol Phys 2002; 52: 989–95
- De Potter P, Shields CL, Shields JA, Cater JR, Brady LW. Plaque radiotherapy for juxtapapillary choroidal melanoma. Visual acuity and survival outcome. Arch Ophthalmol 1996; 114: 1357–65
- Lommatzsch PK, Alberti W, Lommatzsch R, Rohrwacher F. Radiation effects on the optic nerve observed after brachytherapy of choroidal melanomas with 106Ru/106Rh plaques. Graefes Arch Clin Exp Ophthalmol 1994; 232: 482–7
- Gündüz K, Shields CL, Shields JA, Cater J, Freire JE, Brady LW. Radiation complications and tumor control after plaque radiotherapy of choroidal melanoma with macular involvement. Am J Ophthalmol 1999; 127: 579–89
- Gragoudas ES, Li W, Lane AM, Munzenrider J, Egan KM. Risk factors for radiation maculopathy and papillopathy after intraocular irradiation. Ophthalmology 1999; 106: 1571–7
- Gündüz K, Shields CL, Shields JA, Cater J, Freire JE, Brady LW. Plaque radiotherapy of uveal melanoma with predominant ciliary body involvement. Arch Ophthalmol 1999; 117: 170–7
- Char DH, Kroll S, Quivey JM, Castro J. Long term visual outcome of radiated uveal melanomas in eyes eligible for randomisation to enucleation versus brachytherapy. Br J Ophthalmol 1996; 80: 117–24
- Summanen P, Immonen I, Kivela T, Tommila P, Heikkonen J, Tarkkanen A. Visual outcome of eyes with malignant melanoma of the uvea after ruthenium plaque radiotherapy. Ophthalmic Surgery and Lasers 1995; 26: 449–60
- Summanen P, Immonen I, Heikkonen J, Tommila P, Laatikainen L, Tarkkanen A. Survival of patients and metastatic and local recurrent tumor growth in malignant melanoma of the uvea after ruthenium plaque radiotherapy. Ophthalmic Surgery 1993; 24: 82–90
- Rouberol F, Roy P, Kodjikian L, Gerard JP, Jean-Louis B, Grange JD. Survival, anatomic, and functional long-term results in choroidal and ciliary body melanoma after ruthenium brachytherapy (15 years' experience with beta-rays). Am J Ophthalmol 2004; 137: 893–900
- Damato, B, Patel, I, Campbell, IR, Mayles, HM, Errington, RD. Local tumor control after (106)Ru brachytherapy of choroidal melanoma. Int J Radiat Oncol Biol Phys 2005.
- Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: IV. Local treatment failure and enucleation in the first 5 years after brachytherapy. COMS report no. 19. Ophthalmology 2002; 109: 2197–206
- Kleineidam M, Guthoff R, Bentzen SM. Rates of local control, metastasis, and overall survival in patients with posterior uveal melanomas treated with ruthenium-106 plaques. Radiother Oncol 1993; 28: 148–56
- Muller RP, Busse H, Potter R, Kroll P, Haverkamp U. Results of high dose 106-ruthenium irradiation of choroidal melanomas. Int J Radiat Oncol Biol Phys 1986; 12: 1749–55
- Tjho-Heslinga RE, Davelaar J, Kemme HM, de Vroome H, Oosterhuis JA, Bleeker JC, et al. Results of ruthenium irradiation of uveal melanomas: The Dutch experience. Radiother Oncol 1999; 53: 133–7
- Quivey JM, Augsburger J, Snelling L, Brady LW. 125I plaque therapy for uveal melanoma. Analysis of the impact of time and dose factors on local control. Cancer 1996; 77: 2356–62
- Char DH, Quivey JM, Castro JR, Kroll S, Phillips T. Helium ions versus iodine 125 brachytherapy in the management of uveal melanoma. A prospective, randomized, dynamically balanced trial. Ophthalmology 1993; 100: 1547–54
- Char DH, Kroll S, Phillips TL, Quivey JM. Late radiation failures after iodine 125 brachytherapy for uveal melanoma compared with charged-particle (proton or helium ion) therapy. Ophthalmology 2002; 109: 1850–4
- Munzenrider JE, Gragoudas ES, Seddon JM, Sisterson J, McNulty P, Birnbaum S, et al. Conservative treatment of uveal melanoma: Probability of eye retention after proton treatment. Int J Radiat Oncol Biol Phys 1988; 15: 553–8
- Gragoudas E, Li W, Goitein M, Lane AM, Munzenrider JE, Egan KM. Evidence-based estimates of outcome in patients irradiated for intraocular melanoma. Arch Ophthalmol 2002; 120: 1665–71
- Gragoudas ES, Egan KM, Walsh SM, Regan S, Munzenrider JE, Taratuta V. Lens changes after proton beam irradiation for uveal melanoma. Am J Ophthalmol 1995; 119: 157–64
- Shields CL, Shields JA, Perez N, Singh AD, Cater J. Primary transpupillary thermotherapy for small choroidal melanoma in 256 consecutive cases: Outcomes and limitations. Ophthalmology 2002; 109: 225–34
- Seregard S, Landau I. Transpupillary thermotherapy as an adjunct to ruthenium plaque radiotherapy for choroidal melanoma. Acta Ophthalmol Scand 2001; 79: 19–22
- Shields CL, Cater J, Shields JA, Chao A, Krema H, Materin M, et al. Combined plaque radiotherapy and transpupillary thermotherapy for choroidal melanoma: Tumor control and treatment complications in 270 consecutive patients. Arch Ophthalmol 2002; 120: 933–40
- Journee-de Korver JG, Keunen JE. Thermotherapy in the management of choroidal melanoma. Prog Retin Eye Res 2002; 21: 303–17
- Dieckmann K, Georg D, Zehetmayer M, Bogner J, Georgopoulos M, Potter R. LINAC based stereotactic radiotherapy of uveal melanoma: 4 years clinical experience. Radiother Oncol 2003; 67: 199–206
- Shields CL, Shields JA, Karlsson U, Markoe AM, Brady LW. Reasons for enucleation after plaque radiotherapy for posterior uveal melanoma. Clinical findings. Ophthalmology 1989; 96: 919–23