Abstract
We aim to investigate the incidence, patterns and timing of brain metastases in advanced breast cancer patients who have previously received trastuzumab. Eighty-seven patients who had received trastuzumab for advanced breast cancer from November 1999 to September 2003 at the Royal Marsden Hospital were assessed. With a median follow-up period of 11 months from commencing trastuzumab, 23 patients developed brain metastases (30% at 1 year; 95% CI 58–82%). Among 57 patients who had clinical benefits on trastuzumab, 12 (21%) patients developed first disease progression in brain with 75% of them had isolated CNS progression. Moreover, among patients who received trastuzumab as first line treatment, isolated brain metastases were the initial site of progression in 17% patients. Nearly all patients developed parenchymal brain disease. This study shows brain metastases are common phenomenon in HER2 positive advanced breast cancer patients receiving trastuzumab and also may implicate the brain as a sanctuary site for early relapse in this patient cohort.
HER2 is a member of the erb-B epidermal growth factor receptor tyrosine kinase family. It is over-expressed in 20–30% of human breast cancers Citation[1]. Clinically, its over-expression is an independent adverse prognostic factor and is associated with an aggressive clinical course and poor survival in breast cancer patients Citation[2].
Trastuzumab is a humanised monoclonal antibody that binds with high affinity to the extra-cellular domain of HER2. It is active and well tolerated as a single agent in patients with HER2 positive (+) metastatic breast cancer with a response rate of 11–26% Citation[3]. Pivotal phase III trial has demonstrated that its use in combination with chemotherapy results in a significant improvement in survival, time to progression, and response Citation[4]. Moreover, trastuzumab in combination with the vinorelbine or platinum salts augments response rates Citation[5], Citation[6].
Historically, the incidence of clinically apparent central nervous system (CNS) metastases in patients with breast cancer is 10–20%. The median time from diagnosis of breast cancer to CNS metastases was 33 months with 5 months median survival time once diagnosed with cerebral involvement Citation[7]. Brain metastases are usually associated with negative estrogen and progesterone receptor status, young age and the presence of visceral metastases in historical cohorts Citation[8].
HER2-expressing tumours may have a predilection for brain metastases. Kallioniemi and colleagues demonstrated that HER2 +ve disease is associated with a different pattern of metastatic spread and those with over-expression of HER2 gene metastasized three times more often (p = 0.0002) to brain Citation[9]. Furthermore, HER2 over-expression was shown to be a predictive factor for CNS metastases in advanced breast cancer Citation[10]. The CNS may represent a sanctuary site at risk for disease relapse due to the presence of the blood brain barrier (BBB), which prevents CNS penetration of molecules with molecular weight greater than 200 daltons. Monoclonal antibodies such as trastuzumab with a molecular weight of 145 000 daltons may not therefore cross the BBB, as evidenced by low CSF levels Citation[11].
Bendell et al. Citation[12] reviewed a cohort of 122 patients with metastatic breast cancer who received trastuzumab and reported a 34% incidence of brain metastases. However, this study did not accurately determine HER2 status and assumed that patients had HER2 positive metatstatic breast cancer if they received trastuzumab. Similar results were reported by Clayton et al. Citation[13] demonstrating the development of cerebral metastases in 25% of patients treated with trastuzumab. Other studies Citation[14] have reported similar findings. In contrast, recent studies by Lower et al. Citation[15] and Lai et al. Citation[16] did not find a higher incidence of brain metastases in metastatic breast cancer patients who had previously received trastuzumab.
This issue is important as there is likely to be a substantial increase in the number of patients receiving trastuzumab in the neo-adjuvant and adjuvant setting in the near future. If the CNS is a sanctuary site of disease and early relapse in patients with HER2 +ve disease despite trastuzumab therapy, additional therapeutic strategies should be considered in this patient group.
We therefore aim to investigate the incidence, patterns and timing of brain metastases in patients with advanced breast cancer who have previously received trastuzumab.
Patients and methods
Ninety-four patients who had received trastuzumab for treatment of advanced breast cancer (defined as locally unresectable recurrence or metastatic disease) at the Royal Marsden Hospital (RMH), NHS Trust from November, 1999 to September, 2003 were identified retrospectively from pharmacy records. Seven patients who developed brain metastases before the start of trastuzumab were excluded leaving 87 patients for analysis.
Diagnosis of advanced breast cancer was made by both clinical examination and radiological staging. Biopsy of the metastatic sites was performed only in the event of diagnostic uncertainty. All patients with either locally unresectable recurrence or metastatic disease were tested for HER2 during the study period if the tissues were available. Herceptest was used throughout the whole study period for immunohistochemical staining of HER2. HER2 positivity was defined as either HER2 3 + ve by immunohistochemical staining or HER2 2 + ve and FISH +ve. Computerized tomography (CT) scan and /or magnetic resonance imaging (MRI) scan was used to diagnose brain metastases.
Information collected retrospectively from the computerised hospital information system and from the patients notes, included demographic data, details of primary tumour including nodal and ER status, date of recurrence and treatments for advanced disease. The sites of disease at the start of trastuzumab, the duration of treatment response, site of progression and last follow-up were also recorded.
All patients received trastuzumab at 4 mg/kg as a loading dose and then 2 mg/kg weekly or 8 mg/kg loading dose followed by 6 mg/kg three-weekly thereafter until disease progression. Response to trastuzumab was determined by both clinical examination and radiological findings. Response was categorized as complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD) according to the UICC criteria. Patients were classified as deriving clinical benefit on trastuzumab if they showed CR, PR or SD.
Statistical Analysis
CNS metastases free survival was measured from the start of trastuzumab until the diagnosis of metastases. Survival curves were calculated by the method of Kaplan-Meier. Prognostic factors [including age and nodal status at primary presentation, ER status, adjuvant therapy, line of trastuzumab treatment, type of treatment (single agent or in combination with chemotherapy) and sites of disease at time of trastuzumab treatment] for the development of CNS metastases were investigated in a univariate analysis and differences between patients with and without CNS metastases were assessed by means of the log rank test. The proportional hazards model was used to calculate the relative risk (RR) of developing CNS metastases.
Results
A) Demographics
details the demographic data of the 87 patients. The median age at the time of presentation of the primary breast cancer was 49 (range 26–90). The majority of patients had underlying invasive ductal carcinoma (n = 80). Forty-one (47%) patients had estrogen-receptor (ER) positive breast cancer. Most patients had node-positive disease (n = 54) at primary presentation. Nodal status could not be evaluated in 20 patients; 13 of these patients received neo-adjuvant treatment and axillary surgery was not done in seven patients. Most patients received adjuvant or neo-adjuvant chemotherapy, in particular anthracycline-based regimes (n = 48). At the time of starting trastuzumab, 62 patients (71%) had visceral (liver or lung) involvement. Sixty-seven patients had weekly Herceptin, 18 had three weekly Herceptin and the schedule was not known in two patients. The median duration of trastuzumab treatment in our cohort was 31 weeks (Intequartile range 9–44 weeks and range 1–137 weeks).
Table I. Demographics of patients.
B) Response and incidence of brain metastases
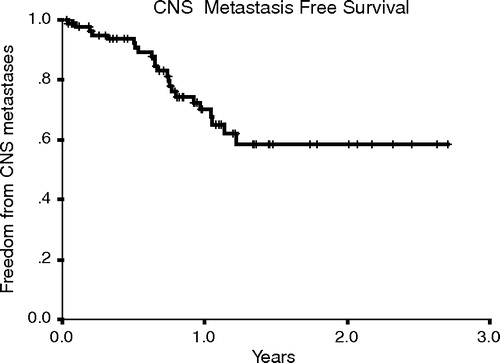
Four patients achieved CR, 31 patients achieved PR and 22 patients had stable disease. However, 27 patients progressed, while three patients could not be evaluated for response. 23 patients developed brain metastases and the actuarial incidence of brain metastases was 30% (95% CI 58–82%) one year after the start of trastuzumab. Brain metastases were the first site of progression in 12 (21%) of the 57 patients who derived clinical benefit on trastuzumab, with nine patients (75%) of them developing isolated CNS progression. In contrast, brain metastases were the initial site of progression in four (15%) of the 27 patients who did not derive clinical benefit on trastuzumab with only two patients developing isolated CNS progression.
Nearly all patients developed parenchymal brain disease with the exception of one patient with isolated leptomeningeal involvement. They all presented with neurological symptoms and diagnosis was confirmed by CT, MRI scans or both. In most cases, brain metastases developed within one year of trastuzumab treatment (). The median time for development of brain metastases was 36 months from primary diagnosis and 17 months from first recurrence.
All patients received corticosteroids for brain metastases, 19 underwent whole brain radiotherapy, one received gamma-knife treatment followed by radiotherapy, and two patients died before the intervention of any treatment. One patient refused cranial irradiation and was treated with corticosteroids only.
C) First site(s) of progression in patients who received first line trastuzumab based therapy
Forty-three patients received trastuzumab as first line treatment for their advanced breast cancer. Twelve patients received it as a single agent and 31 patients received it together with chemotherapy. Thirty-five patients derived clinical benefit on trastuzumab therapy (2 CR, 21 PR and 12 SD). Of these 35 patients, isolated CNS metastases was the initial site of relapse in six (17%) patients.
D) Predictive factors for CNS metastases
analyses the potential risk factors for CNS involvement. No statistically significant factor was found to be predictive of CNS disease. In particular, ER status (p = 0.9) and the use of trastuzumab as a single agent or in combination with chemotherapy (p = 0.9) did not influence the likelihood of CNS metastases. There was a trend towards a higher incidence of brain metastases in patients with visceral disease at the time of trastuzumab treatment (relative risk 2.3 with p = 0.09).
Table II. Prognostic factors for CNS metastases.
E) Impact of brain metastases on survival
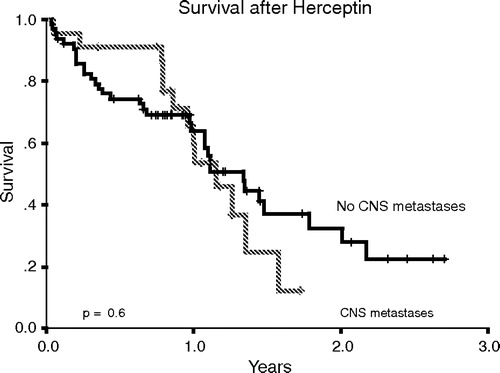
The median survival of all patients in our cohort was 14 months from the start of trastuzumab. At the time of analysis, 13 (57%) of the 23 patients with CNS metastases had died. Ten of 13 (77%) patients died due to progression of CNS metastases. The median survival from the diagnosis of CNS metastases was only four months. shows the survival of the patients with CNS metastases compared to those without metastases at the time of analysis.
Discussion
Our results show brain metastases are common phenomenon among advanced breast cancer patients who received trastuzumab based treatment. This finding is in agreement with results from other centres Citation[12], Citation[13]. We further demonstrate that patients deriving clinical benefit with trastuzumab or on first line trastuzumab therapy are at risk of suffering from isolated CNS relapse. These findings may suggest that whilst trastuzumab achieves good disease control at other sites, brain is a sanctuary site for early relapse in patients on this treatment.
Our study found a higher incidence of parenchymal CNS involvement than leptomeningeal disease in these patients, in agreement with other studies Citation[12–14] This could be attributed to the fact that brain parenchymal cells secrete the neuregulin family of growth factors Citation[17], that influence metastatic-related properties in vitro Citation[18] and have a high affinity for the other family members of HER family with which HER2 may form dimers Citation[19].
This raises the question of the mechanism of CNS progression. There are at least two possible explanations. The first is that HER2 over-expression is itself responsible for cerebral tropism. Evidence suggests that HER2 up-regulates genes involved in breast cancer metastases as for example S100A4 which promotes medulloblastoma cell invasion in vitro. Both the invasive and downstream transcriptional effects of HER2 can be overcome by tyrosine kinase inhibition Citation[20]. From a clinical perspective, inhibition of HER2 signalling with trastuzumab may be expected to alter the metastatic properties of the tumour. Alternatively, expression of other genes amplified within the HER2 amplicon at 17q12 may be involved in the metastatic process and possibly implicated in cerebral tropism. Genes such as Grb 7 (an SH2 domain receptor tyrosine kinase) are co-amplified within the HER2 amplicon Citation[21]. Evidence suggests that Grb 7 plays a role in migration and invasion Citation[22]. If genes co-amplified within the HER2 locus, apart from HER2 itself, contribute to the biological properties of the tumour in certain patients, one would expect trastuzumab therapy to have less clinical benefit in halting visceral disease progression.
There is a trend towards a higher incidence of brain metastases in patients with visceral disease at the time of trastuzumab treatment (p = 0.09). This is in keeping with previously reported data Citation[12]. However, estrogen receptor status of the tumour is not found to be a significant prognostic factor in our series. Notably, the median survival of patients with brain metastases in our cohort is poor and similar to patients in historical cohort (4 versus 5 months, respectively).
CNS prophylaxis has been proven to be a successful strategy in management of patients with acute lymphoblastic leukaemia (ALL) and small cell lung cancer (SCLC) Citation[23]. This strategy decreases the incidence of brain metastases and also prolongs survival Citation[24]. In this study, we found that CNS progression was the main cause of death in advanced breast cancer patients who had received trastuzumab and developed CNS metastases. This group of patients might therefore benefit from prophylactic measures to prevent the occurrence of CNS metastases. However, our study is a descriptive one without proper comparison group. Hence, the results from our study are just hypothesis generating rather than conclusive. Therefore, we suggest the need for randomised clinical trials to address the value of prophylactic cranial radiotherapy in patients with visceral metastatic disease at the time of initiation of trastuzumab therapy.
It is equally important to have vigilant surveillance in asymptomatic patients. Radiological examinations should be employed promptly to exclude brain metastases in patients presenting with suspicious neurological symptoms.
Conflict of interest
All the contributing authors do not have any conflicts of interest that may inappropriately bias this article
References
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: Correlation of relapse and survival with amplification of the HER2/neu oncogene. Science 1987; 235(4785)177–82
- Sjogren S, Inganas M, Lindgren A, Holmberg L, Bergh J. Prognostic and predictive value of c-erbB-2 overexpression in primary breast cancer, alone and in combination with other prognostic markers. J Clin Oncol 1998; 16(2)462–9
- Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 2002; 20(3)719–26
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344(11)783–92
- Pegram MD, Pienkowski T, Northfelt DW, Eiermann W, Patel R, Fumoleau P, et al. Results of two open-label, multicenter phase II studies of docetaxel, platinum salts, and trastuzumab in HER2-positive advanced breast cancer. J Natl Cancer Inst 2004; 96(10)759–69
- Burstein HJ, Kuter I, Campos SM, Gelman RS, Tribou L, Parker LM, et al. Clinical activity of trastuzumab and vinorelbine in women with HER2-overexpressing metastatic breast cancer. J Clin Oncol 2001; 19: 2722–30
- Fokstuen T, Wilking N, Rutqvist LE, Wolke J, Liedberg A, Signomklao T, et al. Radiation therapy in the management of brain metastases from breast cancer. Breast Cancer Res Treat 2000; 62(3)211–6
- Sparrow GE, Rubens RD. Brain metastases from breast cancer: Clinical course, prognosis and influence of treatment. Clin Oncol 1981; 7(4)291–301
- Kallioniemi OP, Holli K, Visakorpi T, Koivula T, Helin HH, Isola JJ. Association of c-erbB-2 protein over-expression with high rate of cell proliferation, increased risk of visceral metastasis and poor long-term survival in breast cancer. Int J Cancer 1991; 49(5)650–5
- Miller KD, Weathers T, Haney LG, Timmerman R, Dickler M, Shen J, et al. Occult central nervous system involvement in patients with metastatic breast cancer: Prevalence, predictive factors and impact on overall survival. Ann Oncol 2003; 14(7)1072–7
- Pestalozzi BC, Brignoli S. Trastuzumab in CSF. J Clin Oncol 2000; 18(11)2349–51
- Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, Kuter I, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 2003; 97(12)2972–7
- Clayton AJ, Danson S, Jolly S, Ryder WD, Burt PA, Stewart AL, et al. Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer 2004; 91(4)639–43
- Shmueli E, Wigler N, Inbar M. Central nervous system progression among patients with metastatic breast cancer responding to trastuzumab treatment. Eur J Cancer 2004; 40(3)379–82
- Lower EE, Drosick DR, Blau R, Brennan L, Danneman W, Hawley DK. Increased rate of brain metastasis with trastuzumab therapy not associated with impaired survival. Clin Breast Cancer 2003; 4(2)114–9
- Lai R, Dang CT, Malkin MG, Abrey LE. The risk of central nervous system metastases after trastuzumab therapy in patients with breast carcinoma. Cancer 2004; 101(4)810–6
- Law AJ, Shannon Weickert C, Hyde TM, Kleinman JE, Harrison PJ. Neuregulin-1 (NRG-1) mRNA and protein in the adult human brain. Neuroscience 2004; 127(1)125–36
- Yao J, Xiong S, Klos K, Nguyen N, Grijalva R, Li P, et al. Multiple signaling pathways involved in activation of matrix metalloproteinase-9 (MMP-9) by heregulin-beta1 in human breast cancer cells. Oncogene 2001; 20(56)8066–74
- Sliwkowski MX, Schaefer G, Akita RW, Lofgren JA, Fitzpatrick VD, Nuijens A, et al. Coexpression of erbB2 and erbB3 proteins reconstitutes a high affinity receptor for heregulin. J Biol Chem 1994; 269(20)14661–5
- Hernan R, Fasheh R, Calabrese C, Frank AJ, Maclean KH, Allard D, et al. ERBB2 up-regulates S100A4 and several other prometastatic genes in medulloblastoma. Cancer Res 2003; 63(1)140–8
- Kauraniemi P, Barlund M, Monni O, Kallioniemi A. New amplified and highly expressed genes discovered in the ERBB2 amplicon in breast cancer by cDNA microarrays. Cancer Res 2001; 61(22)8235–40
- Han DC, Guan JL. Association of focal adhesion kinase with Grb7 and its role in cell migration. J Biol Chem 1999; 274(34)24425–30
- Omura GA, Moffitt S, Vogler WR, Salter MM. Combination chemotherapy of adult acute lymphoblastic leukemia with randomized central nervous system prophylaxis. Blood 1980; 55(2)199–204
- Auperin A, Arriagada R, Pignon JP, Le Pechoux C, Gregor A, Stephens RJ, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Eng J Med 1999; 341: 476–84