Abstract
To assess activity and toxicity of gemcitabine treatment in heavily pretreated epithelial ovarian cancer (EOC) patients and compare the outcome between platinum-sensitive and platinum-resistant patients. We conducted a retrospective analysis of 43 women with EOC treated with gemcitabine on Days 1, 8 and 15 every 28 days. Response was evaluated by physical examination and serial CA 125 measurements. The patients (median age 62 years, range 29–87) were previously exposed to a median of 3 (2–8) chemotherapy regimens. A median of 3.5 (1–14) gemcitabine cycles were administered. Eleven (25.6%) patients showed partial response, 19 (44.2%) had stable disease and 13 (30.2%) progressed. Among 22 platinum-sensitive and 21 platinum-resistant patients, the response rate was 45.5% and 4.7% respectively (p = 0.001), and the median time to progression was 5.0 and 2.8 months, respectively (p = 0.0006). The respective median survival was 16.5 and 6.3 months (p = 0.0001). Grade III–IV hematological toxicities included anemia in four (9.3%) patients, thrombocytopenia in four (9.3%) and leucopenia in two (4.7%). The main non-hematological toxicities were grade III fatigue in two patients (4.7%) and nausea and vomiting in two (4.7%). Single agent gemcitabine is an attractive option for heavily pretreated EOC patients. The significant difference between platinum-sensitive and resistant patients’ warrants further investigation.
Ovarian cancer is the fifth most common cause of death from cancer in women and the leading cause of gynecologic cancer deaths in western countries Citation[1]. Post-operative adjuvant platinum and paclitaxel combination therapy has become the primary standard of treatment since 1996 Citation[2], Citation[3], leading to a median survival of 38 months Citation[2] with a 5-year survival rate of almost 50% Citation[1]. Most patients experience relapse after this primary therapy and the disease-free interval appears to be significant in predicting response to subsequent chemotherapy. Patients who experience recurrence after >6 months (platinum sensitive) have a better prognosis Citation[4] and 50–60% response rate (RR) to second-line chemotherapy Citation[5], whereas patients with a disease-free interval of ≤6 months (platinum resistant) or those with refractory disease will have lower RR of 20–30% and 10% respectively Citation[5]. Since most patients with recurrent ovarian cancer will succumb to their disease, the priority of second-line chemotherapy is palliative, i.e., to postpone symptomatic disease progression while maintaining the best possible quality of life.
Gemcitabine is a deoxycytidine nucleotide analogue of cytosine arabinoside Citation[6]. The active metabolites of gemcitabine, gemcitabine diphosphate and gemcitabine triphosphate block ribonucleotide reductase, causing lower levels of native deoxycytidine and inhibition of DNA replication and repair Citation[7]. Difluoro-deoxycytidine triphosphate competitively inhibits DNA polymerase and terminates DNA-chain elongation Citation[8]. Gemcitabine has been studied as single agent chemotherapy for recurrent ovarian cancer, demonstrating activity with a response rate of 11–29% Citation[9–19]. Some of these studies Citation[10], Citation[11], Citation[13], Citation[17], Citation[19] indicated that gemcitabine monotherapy is also active in platinum-resistant and refractory patients with response rates of 17% and 16% respectively Citation[17], Citation[19] and a 36% disease stabilization. However, some of the women included in these studies were actually sensitive to the initial platinum treatment but experienced secondary resistance Citation[20]. Moreover, no comparison was done between platinum resistant and platinum-sensitive patients.
In the present study, we report our experience with single-agent gemcitabine in heavily pretreated patients with epithelial ovarian cancer (EOC) and compare the outcome between those that were sensitive and those that were resistant to the initial platinum treatment.
Patients and methods
We conducted a retrospective chart analysis of all patients with persistent or recurrent EOC treated with single-agent gemcitabine between 1998–2005 at the Department of Oncology, Tel Aviv Sourasky Medical Center, Tel Aviv and at the Gynecologic Oncology Division, Wolfson Medical Center, Holon, Israel, after institutional review board approval.
Most women had been heavily pretreated by other chemotherapeutic agents (). Patients were defined as being “platinum resistant” if they had progressed within six months of their first-line platinum-based treatment and “platinum sensitive” if they progressed after more than six months.
Table I. Selected patient characteristics (n = 43).
Treatment plan
Single-agent gemcitabine at an initial dose of 1000 mg/m2 was administered as 1-hour infusion on Days 1, 8 and 15 of a 28-day cycle. Patients underwent clinical assessment and measurement of their serum CA-125 levels at the beginning of each new course of therapy. Treatment was discontinued if there was evidence of disease progression or by patient request. Duration of treatment was dependent upon response. All patients were assessable for toxicity and patients who received at least two courses of chemotherapy were assessable for response.
Evaluation of response
Since most patients had been heavily pretreated, the treatment goals were palliation and the delay of disease progression. In most cases if CA-125 was informative, we used it as a response tool, otherwise clinical evaluation and imaging studies were done.
A complete response (CR) was defined as the complete disappearance of all measurable disease. A partial response (PR) was defined as a ≥50% reduction in the measurement of each palpable lesion. Disease progression (PD) was defined as a ≥25% increase in the measurement of any lesion documented within eight weeks from start of treatment or the appearance of any new lesion. Disease evaluable by CA-125 was assessed using the Rustin criteria Citation[21–23]: response was defined as a 50% decrease in serum CA-125 level over two samples or a serial decrease of 75% in serum CA-125 over three samples. The final sample had to be taken at least four weeks after the previous one. Disease progression was defined as serial increase in serum CA-125 level during treatment or any clinical sign of progression. Stable disease (SD) was considered if any of the above criteria were not met.
Time-to-progression (TTP) was defined as the time between the first day of gemcitabine treatment to the time of the first sign of disease progression. Overall survival (OS) was calculated from the first day of gemcitabine treatment to the time of death. Adverse events were evaluated according to common toxicity criteria.
Statistics
TTP and survival were analyzed by the Kaplan-Meier method and comparisons by the log-rank test. Platinum-sensitive and -resistant patients were compared using the χ2 test. All tests were two-sided and considered significant at p < 0.05.
Results
A total of 43 patients were treated with gemcitabine for recurrent or persistent EOC. The cohort's median age was 62 years (range 29–87) and the median Eastern Oncology Cooperative Group (ECOG) performance status was 1 (range 0–2). Additional selected characteristics are outlined in . The great majority of patients had FIGO stage III–IV (93%) disease, serous adenocarcinoma (79.1%) and serum CA-125 levels ≥100 µ/ml (67.4%). The median number of prior chemotherapy regimens was 3 (range 2–8). Twenty-two (51.2%) women had platinum-sensitive disease and 21 (48.8%) were platinum resistant.
A total of 171 chemotherapy cycles were administered with a median of 3.46 (range 1–13) per patient. All 43 patients were evaluable for toxicity. The adverse effects of the treatment are listed in . Grade III and IV hematological toxicities were anemia in four (9.3%) patients, thrombocytopenia in four (9.3%) patients and leukopenia in two (4.6%) patients. None developed neutropenic fever. Non-hematological grade III-IV side effects were recorded in eight (18.6%) patients: fatigue in two (4.6%), nausea and vomiting in two (4.6%), edema in two (4.6%), shortness of breath in one (2.3%) and stomatitis in one (2.3%) patient. Six patients discontinued treatment due to side effects: five due to grade II and III fatigue and one due to grade III shortness of breath after five consecutive cycles. Dose reduction by 20% was needed in four patients with thrombocytopenia and in two women with grade III fatigue.
Table II. Toxicity (n = 43 patients).
Four patients required hospitalization: one for abdominal pain, one for pulmonary conditions (shortness of breath, pneumonia) and two for fatigue. One of the fatigued patients also suffered from severe stomatitis. Fifteen blood transfusions were administered to five (11.6%) patients.
Of the 43 patients, 34 (79.1%) received ≥2 chemotherapy cycles. Ten patients received less than 2 complete cycles: six because they declined continuation of treatment and four due to progressive disease and deterioration.
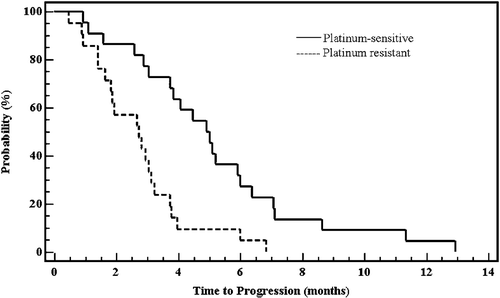
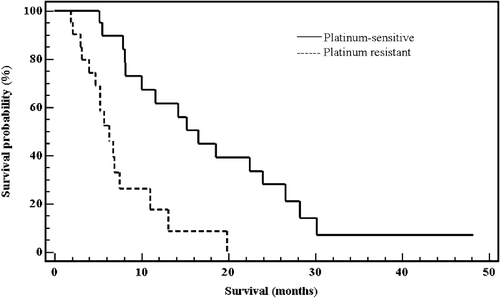
The response rate, TTP and survival according to platinum sensitivity are presented in . There were no complete responders, eleven patients had partial response (25.6%; 95% confidence interval: 0.13–0.41), 19 SD (44.2%; 95% confidence interval: 0.29–0.6) and 13 (30.2%; 95% confidence interval: 0.17–0.46) experienced PD. Platinum-sensitive patients had significantly higher rate of PR and lower rate of PD compared to platinum-resistant patients (45.5% vs 4.8% and 9% vs 52.4% respectively (p = 0.001)). Disease stabilization was similar in both groups. The median TTP was 3.73 months (range: 0.47–12.93 months; 95% confidence interval: 2.8–4.66; SE±0.47). The TTP was significantly longer in platinum-sensitive compared to the platinum-resistant patients: 5.0 months vs 2.8 months (p = 0.0006; 95% CI: 0.12–0.57) (). The overall median survival was 10 months (range: 0.93–29; 95% CI: 5.93–14.07; SE±2.07). Median survival time was also significantly longer in platinum-sensitive patients: 16.5 months vs 6.3 months (p = 0.0001; 95% CI: 0.07–0.41) ().
Table III. Response rate, median TTP and median survival of all patients and according to platinum sensitivity (n = 43).
Discussion
Our results indicate a clinically meaningful benefit of single agent gemcitabine in heavily pretreated EOC patients with a 25.6% PR, 44.2% SD rate and a median TTP of 3.73 months. Previous studies reported a 15–20% response rate to gemcitabine monotherapy in recurrent ovarian cancer Citation[9–19]. Our response rates in this heavily pretreated population can be explained by the implementation of the Rustin criteria Citation[21], Citation[22] without other objective measurements. Regretfully, the absence of consecutive evaluation by imaging modalities in our retrospective study precludes us from reporting objective measurable responses.
Heavily pretreated EOC patients who are not on prospective clinical trials are usually evaluated by physical examination and serial CA-125 measurements. Decisions regarding treatment continuation or discontinuation are usually made according to these parameters and not by expensive imaging studies. The Rustin CA-125 response criteria were recently introduced as a tool to assess response in ovarian cancer trials Citation[21–24]. Subsequently, Bridgewater et al. Citation[25] showed that these CA-125 criteria give response rates that are similar to the ones obtained by the standard WHO criteria [26]. Their study Citation[25] also showed that patients who had stable disease according to standard imaging assessment and response according to CA-125 criteria had statistically significant better progression-free survival (PFS) compared to patients who did not respond according to those criteria (10.6 vs 4.8 months, p < 0.0001), suggesting that serial CA-125 measurements are effective and sufficient for response evaluation. Using the CA-125 evaluation resulted in a false positive rate of 2.9% and a false negative rate of 21%. However, the standard criteria are also liable to error [27] and to high interobserver variability [28,29]. Therefore, adopting the Rustin CA-125 response criteria can be used as a substitute for the WHO criteria for the purpose of evaluating response to treatment in heavily pretreated ovarian cancer patients.
Many drugs have been tested as salvage therapy in ovarian cancer, and most of them achieve better response rates in platinum-sensitive disease [30,31]. A recent phase III study [32] compared carboplatin plus gemcitabine vs carboplatin alone for recurrent platinum sensitive EOC. Patients receiving the combination chemotherapy had significantly higher response rate of 47.2% vs 30.9% (p = 0.0016) and prolonged progression-free survival (PFS) of 8.6 months vs 5.8 months (p = 0.0032) compared to patients treated with carboplatin alone. This study also showed improved quality of life in patients receiving the combination chemotherapy and faster palliation of abdominal symptoms. In platinum resistant patients less favorable results were obtained. Preclinical studies have shown that gemcitabine-based combination chemotherapy increases the cytotoxic action of the treatment and potentially overcomes drug resistance [33–36]. Rose et al. [37] reported that gemcitabine combined with cisplatin can reverse platinum resistance and results in a 42.2% response rate. Garcia et al. [38] further showed that the combination of gemcitabine with weekly palitaxel achieved a 40% response rate and a stable disease of 37% in patients with platinum and paclitaxel-resistant ovarian cancer. Recently, Brewer et al. [39] conducted a phase II study evaluating cisplatinum plus gemcitabine in platinum refractory EOC and found an overall response rate of 15.8% with a median TTP of six months in this poor prognostic group. Previous studies that evaluated gemcitabine monotherapy in platinum and paclitaxel refractory Citation[19] and resistant Citation[17] patients showed similar results with partial response rates of 16 and 17%, respectively.
To the best of our knowledge, our report is the first to compare gemcitabine treatment results in heavily pretreated platinum-sensitive and platinum-resistant patients. A significantly higher response rate (45.5% vs 4.8% (p = 0.001)) was achieved in platinum sensitive compared to platin resistant patients as well as prolonged TTP (5 vs 2.8 months (p = 0.0006)) and median survival (16.5 months vs 6.3 months (p = 0.0001)). The significant difference in response and survival can probably be related to different tumor biology and aggressiveness and to the greater probability of platinum-sensitive patients to respond to any type of additional therapy Citation[4]. The very low response rate documented in our platinum-resistant group can be explained by the fact that we included patients who experienced recurrence < six months after the completion of first-line chemotherapy whereas previous reports Citation[10], Citation[11], Citation[13], Citation[17], Citation[19] included also patients that were primarily platinum-sensitive, but experienced secondary resistance to platinum or paclitaxel. Heavily pretreated patients who have primary platinum-resistant or refractory disease should expect stabilization of disease under gemcitabine monotherapy rather than actual response and should be accordingly informed.
In the present study, gemcitabine was administered after a median of three previous chemotherapy regimens. Nevertheless, it was well tolerated, with grade III fatigue as the main reason for discontinuing treatment. Hematological grade III and IV toxicity was not frequently encountered, no neutropenic fever was observed and dose reduction was rarely needed.
In conclusion, the favorable response rate, TTP and median survival and the relatively low toxicity make gemcitabine monotherapy an attractive option in heavily pretreated platinum-sensitive EOC patients. Our results suggest that administration of gemcitabine to platinum-resistant patients might be of little value. However, due to the small number and heterogeneity of our patient group in this retrospective study, the statistically significant differences between platinum-sensitive and resistant patients warrant validation by prospective, randomized trials.
Esther Eshkol, M.A., is thanked for editorial assistance.
References
- Jemal A, Murray T, Samuels A, et al. Cancer statistics, 2003. CA Cancer J Clin 2003; 53: 5–26
- McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 1996; 334: 1–6
- Markman M, Bookman MA. Second-line treatment of ovarian cancer. The Oncologist 2000; 5: 26–35
- Heinemann V, Xu YZ, Chubb S, et al. Cellular elimination of 2′×2′ difluoredeoxycytidine 5′ triphosphate: A mechanism of self-potentiation. Cancer Res 1992; 52: 533–9
- Carmichael J. The role of gemcitabine in the treatment of other tumors. Br J Cancer 1998; 78(Supp 3)7821–5
- Lund B, Hansen OP, Theilade K, Hansen M, Neijt JP. Phase II study of gemcitabine (2′,2′-difluorodeoxycytidine) in previously treated ovarian cancer patients. J Natl Cancer Inst 1994; 86: 1530–3
- Shapiro JD, Millward MJ, Rischin D, et al. Activity of gemcitabine in patients with advanced ovarian cancer: Responses seen following platinum and paclitaxel. Gynecol Oncol 1996; 63: 89–93
- Friedlander M, Milward MJ, Bell D, et al. A phase II study of gemcitabine in platinum pre-treated patients with advanced epithelial ovarian cancer. Ann Oncol 1998; 9: 1343–5
- Von Minckwitz G, Bauknecht T, Visseren-Grul CM, Neijt JP. Phase II study of gemcitabine in ovarian cancer. Ann Oncol 1999; 10: 853–5
- Silver DF, Piver MS. Gemcitabine salvage chemotherapy for patients with gynecologic malignancies of the ovary, fallopian tube, and peritoneum. Am J Clin Oncol 1999; 22: 450–2
- D'Agostino G, Amant F, Berteloot P, Scambia G, Vergote I. Phase II study of gemcitabine in recurrent platinum-and paclitaxel-resistant ovarian cancer. Gynecol Oncol 2003; 88: 266–9
- Fowler WC, Jr, Van Le L. Gemcitabine as a single-agent treatment for ovarian cancer. Gynecol Oncol 2003; 90: S21–3
- Markman M, Webster K, Zanotti K, Kulp B, Peterson G, Belinson J. Phase 2 trial of single-agent gemcitabine in platinum-paclitaxel refractory ovarian cancer. Gynecol Oncol 2003; 90: 593–6
- Rustin G, Nelstrop A, McLean P, et al. Defining response of ovarian carcinoma to initial chemotherapy according to serum CA 125. J Clin Oncol 1996; 14: 1545–51
- Rustin GJ. Use of CA-125 to assess response to new agents in ovarian cancer trials. J Clin Oncol 2003; 21(Supp 10)187s–193
- Bridgewater JA, Nelstrop AE, Rustin GJ, Gore ME, McGuire WP, Hoskins WJ. Comparison of standard and CA-125 response criteria in patients with epithelial ovarian cancer treated with platinum or paclitaxel. J Clin Oncol 1999; 17: 501–8
- Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer 1981; 47: 207–14
- Gwyther S, Bolis G, Gore M, et al. Experience with independent radiological review during a topotecan trial in ovarian cancer. Ann Oncol 1997; 8: 463–8
- Thiesse P, Ollivier L, di Stefano-Louineau D, et al. Response rate accuracy in oncology trials: Reasons for interobserver variability. J Clin Oncol 1997; 15: 3507–14
- ten Bokkel Huinink W, Gore M, Carmichael J, et al. Topotecan versus paclitaxel for the treatment of recurrent epithelial ovarian cancer. J Clin Oncol 1997; 15: 2183–93
- Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ. Recurrent epithelial ovarian carcinoma: A randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol 2001; 19: 3312–22
- Pfisterer J, Vergote I, Du Bois A, et al. Combination therapy with gemcitabine and carboplatin in recurrent ovarian. Int J Gynecol Cancer 2005; 15(Suppl 1)36–41
- Sehouli J. Review of gemcitabine-based combinations for platinum resistant ovarian cancer. Int J Gynecol Cancer 2005; 15(Suppl 1)23–30
- Rose PG, Mossbruger K, Fusco N, et al. Gemcitabine reverses cisplatin resistance: Demonstration of activity in platinum- and multidrug-resistant ovarian and peritoneal carcinoma. Gynecol Oncol 2003; 88: 17–21
- Garcia AA, O'Meara A, Bahador A, et al. Phase II of gemcitabine and weekly paclitaxel in recurrent platinum-resistant ovarian cancer. Gynecol Oncol 2004; 93: 493–8