Abstract
Metaplastic carcinoma of the breast (MCB) is a rare form of cancer containing mixture of epithelial and mesenchymal elements in variable combinations. Few and conflicting clinical data are available in the literature addressing optimal treatment modalities, prognosis and outcome. A retrospective study was conducted to review all patients with MCB diagnosed and treated at King Faisal Specialist Hospital and Research Center between 1994–2004. The aim is to describe patient's clinicopathologic features and to analyze treatment results. Nineteen female patients were studied. The median age was 48 years (range, 14–58). The median tumor size was 9 cm (range, 3–18). Stage distribution was II in 8 patients, III in 9 and IV in 2. Nine cases were identified as purely epithelial and 10 (53%) as mixed epithelial and mesenchymal metaplasia. Hormone receptors were positive in only 2 patients. Modified radical mastectomy performed in 11 patients and 15 underwent axillary node dissection. Adjuvant chemotherapy was given to 9 patients and postoperative radiotherapy to 8. Twelve patients relapsed with median time of relapse of 12 months (range, 2–28). At a median follow-up of 21 months (range, 7–83), the 3-year event free survival (EFS) and overall survival for the patients diagnosed with loco-regional disease were 15% and 48% respectively. Tumor size correlated significantly with EFS. MCB is an aggressive form of breast cancer associated with poor outcome, high incidence of local recurrence and pulmonary metastases. The disease tends to be estrogen/progesterone receptor negative. Tumor size has an important impact on outcome. The best treatment approach is yet to be defined.
Metaplastic carcinoma of the breast (MCB) is a rare type of breast cancer accounting for < 1% of breast malignancies Citation[1–3]. The term metaplastic carcinoma was first introduced by Huvos et al. Citation[4]. Histologically, it is a poorly differentiated heterogeneous tumor containing ductal carcinoma cells admixed with areas of spindle, squamous, chondroid, or osseous elements Citation[1–3]. The wide range of microscopic appearance of MCB has resulted in variety of confusing classifications and designations including spindle cell carcinoma Citation[5], carcinosarcoma Citation[6], squamous cell carcinoma of ductal origin Citation[7], adenosquamous carcinoma Citation[8], carcinoma with pseudosarcomatous metaplasia Citation[9] and matrix producing carcinoma Citation[10]. The extent of metaplasia varies from microscopic foci to virtually complete replacement of the adenocarcinoma by the metaplastic elements Citation[1], Citation[11]. Regardless of the morphologic pattern, immunohistochemical and ultrastructural studies suggest that MCB are derived from multipotential undifferentiated cells Citation[8], Citation[12]. Some authors have proposed that myoepithelial cell might be the cell origin for these tumors Citation[3]. Because of the rarity of MCB, very few clinical studies are available that describe the clinical course, therapeutic approaches and prognostic factors. In an attempt to enhance our understanding of the natural history of this disease; we reviewed our experience on multi-disciplinary management of MCB cases treated over 10-year period at King Faisal Specialist Hospital and Research Center (KFSH & RC).
Patients and methods
We searched the records of surgical pathology and the Tumor Registry at our institution from 1994–2004. Institutional Review Board approval was obtained prior to data collection. We reviewed reports filed under metaplastic carcinoma of the breast as well as carcinosarcoma, sarcomatoid carcinoma, adenocarcinoma with squamous /sarcomatous metaplasia and spindle cell carcinoma to find any patients who may have been misfiled. The pathology slides were reviewed by one of the authors to confirm entry into the study. Cases were included if carcinoma was identified morphologically on hematoxylin and eosin stained slides and /or expressed epithelial differentiation by immunostains for cytokeratin. WHO classification system Citation[2] was applied to categorize the cases into purely epithelial or mixed epithelial and mesenchymal. Only patients who had adequate clinical data for treatment and follow-up were selected. Data were gathered with regard to age, menopausal status, duration of symptoms, tumor size, clinical stage, surgical treatment, adjuvant therapy and outcome. All patients were staged at the time of diagnosis with chest radiograph, bilateral mammography, whole body bone scan, and chest and abdominal computed tomography scans.
Event free survival (EFS) was calculated from the date of diagnosis till the date of relapse, death or last follow-up. Overall survival (OS) was calculated from the date of diagnosis to the date of death or last follow-up. EFS and OS were assessed for the non-metastatic cases and were estimated according to the Kaplan-Meier method. Univariate analysis was performed to assess if any prognostic variables conferred an improved survivorship. These included age, menopausal status, tumor size, disease stage, surgical procedure, nodal status and use of adjuvant therapy. Cumulative survival rates were compared by the log-rank test with p-values < 0.05 considered to be significant. SPSS statistical software version 10.0 (SPSS Inc, Chicago, IL) was used for the analysis.
Results
Clinical data
Nineteen patients with the diagnosis of MCB were identified. The main clinical features of the patients are depicted in and II. All patients were female. The median age at presentation was 48 years (range, 14–58). The most frequent presentation was a unilateral rapidly enlarging breast mass. One patient presented with inflammatory breast cancer. The duration of symptoms ranged from 1 to 24 months, with a median of 8 months. The median tumor size at diagnosis was 9 cm (range, 3–18). Nine patients (47%) had stage III disease and two (10%) had metastatic disease to the liver at presentation.
Table I. Characteristics of 19 patients with metaplastic carcinoma of the breast.
Pathological features
Five cases (25%) were classified as adenocarcinoma with spindle cell differentiation (SpCd) while another 5 as carcinosarcoma with malignant mesenchymal component. One patient had pure squamous metaplasia and 3 had adenosquamous variety. Five patients had heterologous tissues, 3 with chondroid and 2 with osseous metaplasia ( and II). Among the 15 patients known to have had an ALND, 8 (53%) were node positive. The histological subtype of the primary tumor in node positive patients was purely epithelial in 5 cases (2 adenosquamous, 2 adenocarcinoma with SpCd and 1 squamous) and mixed epithelial with chondroid metaplasia in 3. The median number of axillary lymph nodes (ALN) dissected was 12 (range, 2–24) and the median number of positive nodes was 2 (range, 1–9). The nodal metastases demonstrated only malignant epithelial elements. Estrogen/progesterone receptor (ER/PR) immunostaining was done in 17 cases and yielded negative results in 15 (88%). HER2/neu protein overexpression by immunohistochemistry was negative in 9 of 10 tumors examined.
Clinical course
The details of therapy and follow-up are shown in . All patients except one had surgical excision of their primary lesions. Eleven (58%) had modified radical mastectomy (MRM) and three had simple mastectomy. Wide local excision + axillary lymph node dissection (ALND) was performed in 4 patients. Induction chemotherapy prior to surgery was administered to 5 patients, 4 of whom received doxorubicin-based chemotherapy regimens. Two patients achieved clinical partial response and 3 had no response. Nine patients received adjuvant chemotherapy. The regimens used were doxorubicin-based in 7 patients and CMF in 1 and docetaxel in 1. The median number of administered cycles was 4 (range, 3–9). Adjuvant postoperative radiation therapy was given to 8 patients with doses ranged from 45–50 Gy. Two patients received tamoxifen in adjuvant setting. Twelve of the 17 patients (71%) who initially achieved complete remission relapsed with a median time of relapse of 12 months (range, 2–28). The sites of disease relapse are shown in . Local recurrences occurred in 7 patients (58%). No malignancy developed in the contra-lateral breast. The five patients with isolated local recurrences achieved second CR by surgery +/− radiation therapy. They had a median survival of 47 months (range, 18–65) from time of recurrence, and 2 of them died of their disease with subsequent metastatic spread. The other 7 patients received systemic therapy. The salvage chemotherapy regimens given were diverse: 2 patients treated with gemcitabine and cisplatin combination, 1 with paclitaxel and cisplatin, and 3 with doxorubicin based combination chemotherapy. One ER/PR positive patient was given letrozole on relapse and another HER2/neu positive patient was treated with herceptin/navelbine as third line therapy.
Table II. Therapy, response, outcome and survival for 19 patients with metaplastic carcinoma of the breast.
Table III. Patterns of first relapse in 12 patients
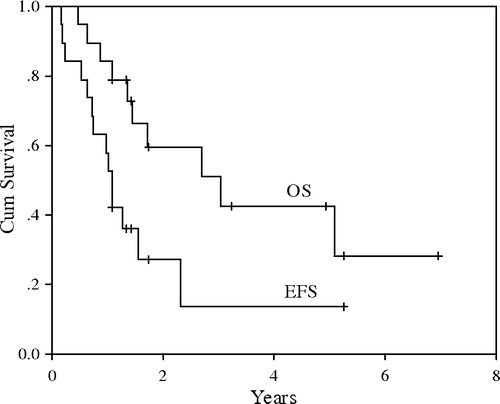
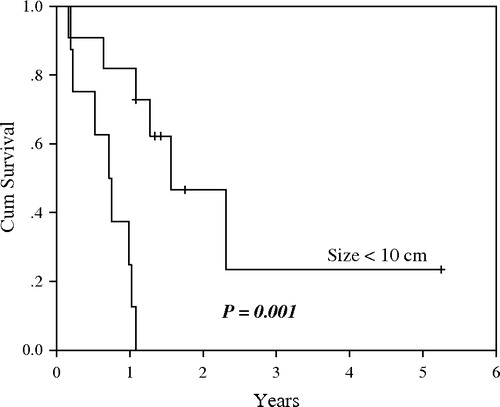
Overall, there was only one short-lived partial response to salvage doxorubicin-based regimens. The median survival after distant relapse was 8 months. For the 17 patients who followed-up for more than 6 months and had loco-regional disease at presentation, the median follow-up was 21 months (range, 7–83). Their 3-year EFS and OS were 15% and 48% respectively (). The log-rank test showed that tumor size was the only significant predictor of EFS (p = 0.001, ). OS measures were in favor of patients who received adjuvant systemic chemotherapy and those presented with tumor < 10 cm, however the differences did not reach statistical significance (p = 0.10 and 0.16 respectively). At the time of analysis, 10 patients (53%) have died of their disease and 9 are alive with no evidence of disease.
Discussion
Of the 3 160 new patients with breast carcinoma seen at KFSH & RC over a 10-year period, 19 (0.6%) had the histological metaplastic variant. This incidence is similar to that reported previously Citation[1], Citation[2]. Most of the series were published in journals of pathology, yet the classification of this disease is still confusing, and reflects opinions of expert pathologists rather than a consensus. The clinical features, treatment and outcome are equally diverse and are limited to small reports from few institutions including our own report. A literature review of five previously published clinical series Citation[13–17] is shown in . It has been reported that MCB is more likely to develop in women older than 50 year Citation[9], Citation[11], Citation[13], Citation[15]. The median age at diagnosis in our patients was 48 years, which is by far the lowest reported. The usual presentation is a breast mass, which tends to grow rapidly Citation[3], Citation[9]. The median tumor size was 9 cm compared to a maximum of 5 cm reported in many series Citation[10], Citation[11], Citation[13–17]. In contrast to others Citation[9], Citation[14], Citation[16] most of the cases in our review presented with advanced clinical stages. MCB has a low potential for lymph node metastasis. The reported incidence has been ranged from 0–50% Citation[5], Citation[9], Citation[11], Citation[13–19]. In our study, the rate was 53% and the median number of positive ALN was 2. This is still considered low if the tumor size and stage of the disease are taken into account and if historical comparison with adenocarcinoma of the breast is considered Citation[20]. In the 8 cases with pathological nodal involvement, malignant epithelial component was only seen. Many authors have reported this observation Citation[9], Citation[11], Citation[18]. One of the universal finding in all studies is the high rate of ER/PR negativity, in the range of 70–100% Citation[11], Citation[13–17]. This is not unexpected because these tumors typically have a high grade or poorly differentiated carcinomatous component Citation[21]. The absence of predominant glandular epithelial component in many cases might also explain the paucity of ER/PR expression Citation[1], Citation[7]. Consistent with the literature, 88% of the tumors examined in our study lacked ER/PR expression. Although data are limited in the literature, MCB rarely seems to overexpress HER2/neu oncoprotein. One of the 26 MCB cases reported by Barnes et al Citation[22] was HER2/neu positive. In our review HER2/neu over-expression was seen in only one (1/10) case with chondroid metaplasia.
Table IV. Literature review of metaplastic breast carcinoma clinical series.
To date there are no standard guidelines for the treatment of MCB. Modified radical or radical mastectomy was the surgical procedure commonly performed in most of the series Citation[13–17]. Because of the low risk of lymphatic spread, Caceres et al. Citation[23] suggested that wide local excision with cancer-free margins would be appropriate for local control. Unlike invasive carcinoma of the breast, ALN metastases in MCB do not correlate with clinical outcome Citation[6], Citation[9], Citation[11], Citation[14], Citation[15], Citation[19]. The higher incidence (53%) of nodal metastases and larger median tumor size seen in our patient cohort make us support MRM as optimal surgical treatment of choice. Some investigators have linked the risk of ALN metastases to the underlying histological subtype, being extremely low in spindle cell carcinoma Citation[5], Citation[11], Citation[19]. None of the 5 cases diagnosed as carcinosarcoma with malignant mesenchymal component in this review had clinical or pathological ALN involvement. Taking these observations into consideration, ALND might be spared in-patients with small size carcinosarcoma tumors.
Seven of our cases received doxorubicin or paclitaxel based chemotherapy as primary treatment, 2 for metastatic disease and 5 in induction setting. The overall response rate was 43% with no clinical complete remission noted. Experience with neo-adjuvant chemotherapy in MCB is extremely limited in the literature to evaluate its impact on the outcome. Adjuvant chemotherapy was administered to nine patients and two of them experienced distant relapse upon first disease recurrence. The 3-year OS for these patients was better than for those who did not receive adjuvant systemic therapy; however the difference was not statistically significant. In the study of Pitts et al. Citation[13], 7 of 34 received adjuvant chemotherapy and 4 of them remained disease free at a follow-up of 7–70 months. Rayson et al.Citation[15] found that 7 of the 9 cases that received adjuvant therapy relapsed indicating ineffectiveness of adjuvant chemotherapy in this disease. In the report of Choa et al. Citation[16], 5 of 6 patients who had adjuvant chemotherapy remained disease free at follow-up period ranged from 3–9 years. The OS analysis in their study was in favor of patients who never received chemotherapy; however the difference was not statistically significant. On the other hand, Gutman et al. Citation[14] noted significant OS and DFS improvements only for patients with stage I/II treated with mastectomy and adjuvant therapy. In all these studies including ours, the numbers receiving adjuvant chemotherapy remains small to reach firm recommendations regarding its use and warrants multicentric trials to examine its influence on survival. Several authors Citation[14], Citation[24] found no survival advantage for patients treated with chemotherapy or hormonal therapy for metastatic disease. Rayson et al. Citation[15] found only one response in seven patients treated with salvage chemotherapy (doxorubicin-based), and reported median survival of 8 months from detection of disease relapse. Our results were similar. The median survival, after distant relapse, was short (8 months) and only one of 6 patients had short-lived partial response to doxorubicin-based therapy. Our experience with newer agents including gemcitabine, taxanes, navelbine and herceptin was not encouraging. The disease seems to be refractory in the metastatic setting to the current chemotherapeutic drugs available but the small number of cases treated makes it difficult to draw satisfactory conclusions. These data also suggests that patients with metastatic MCB should be considered for investigational phase II trials. In view that most of our patients were ER/PR negative, our experience with hormonal therapy is limited. Rayson et al Citation[15] found no response in 4 ER/PR positive patients treated with tamoxifen at time of relapse. Seven (41%) of our patient cohort experienced local relapse and 9 (53%) developed pulmonary metastases during their clinical course. Noticeable tendencies for local failure and pulmonary metastases were universal features to MCB in many reports Citation[13–15], Citation[24], Citation[25], suggesting that the clinical behavior is similar to sarcomas. The high incidence of local failure in our study could also be attributed to the large tumor size and the higher tumor stages at presentation. Rosen and Ernsberger Citation[8] advised the routine use of adjuvant radiation therapy in MCB patients. It has been shown that the only patients who had survival advantage from salvage treatment were those with isolated local recurrences that could be treated with local therapy Citation[14], Citation[15], Citation[26]. Five cases with local recurrence in our series were successfully salvaged by surgery +/− radiotherapy and had a median survival of 47 months. The overall survival across the studies at 3–5 years ranged from 39–72% Citation[13–17]. Our low survival rate can be contributed to certain unique patient's characteristics: larger tumor size, higher stages, higher rate of nodal involvement and younger age group. MCB has been described to have poor outcome as compared to adenocarcinoma Citation[9], Citation[11], Citation[14], Citation[15], Citation[24]. Conversely, few others reported favorable prognosis Citation[16], Citation[17]. The tumor size at presentation best correlated with outcome Citation[7], Citation[9], Citation[11], Citation[16], Citation[26]. In this review, the tumor size had an impact on EFS but not on OS, possibly because of successful salvage treatment for local recurrences. Clinical stage I/II, age > 50 years and absence of nodal metastases at presentation were favorable prognostic factors found in few series Citation[9], Citation[14], Citation[16]. The presence of a mesenchymal metaplastic element in carcinoma of the breast is a poor prognostic factor Citation[6], Citation[14], Citation[24], Citation[26]. However, several authors showed that the differences in survival among the various subgroups of MCB are minor Citation[11], Citation[18] and Pitts et al. Citation[13] advised that sub-classification of MCB is of greater pathologic than clinical interest. The type of metaplasia in our report did not affect the survival but the low number of cases makes firm conclusions difficult.
In conclusion, MCB is a rare form of breast cancer. The results of the present study demonstrate that MCB is an aggressive disease and has poor prognosis. Pathological classification of this disease needs to be unified. The clinical behavior is as diverse as the histology. The majority of these tumors are receptor negative. The lymph nodes are involved mainly by carcinomatous elements. The disease tends to recur locally and frequently metastasizes to the lung. The small number of patients in most of the studies makes solid conclusions as regards to the optimal treatment difficult. The prognosis reported in the literature is quite variable and the tumor size at diagnosis is the single most important prognostic factor. Multi institutional prospective trials after consensus on pathology are needed to advance our knowledge in understanding and managing this uncommon disease. The search for biological prognostic factors and innovative therapies are required to improve the outcome of this disease.
References
- Rosen PP. Carcinoma with metaplasia. Breast Pathology, PP Rosen. Lippincott-Raven, Philadelphia 1997; 375–95
- Ellis IO, Cornelisse CJ, Schnitt SJ, Sasco AJ, Sastre-Garau X, Kaaks R, Sasco AJ, Sastre-Garau X, Kaaks R, . Tumors of the breast. Invasive breast carcinoma. Pathology and Genetics of Tumors of the Breast and Female Genital Organs, FA Tavassoli, P Devilee, et al. IARC Press, Lyon 2003; 37–41
- Tavassoli FA. Classification of metaplastic carcinomas of the breast. Pathol Annu 1992; 27: 89–119
- Huvos AG, Lucas JC, Foote FW. Metaplastic breast carcinoma: rare form of mammary cancer. NY State J Med 1973; 73: 1078–82
- Gersell DJ, Katzenstein AA. Spindle cell carcinoma of the breast. Hum Pathol 1981; 12: 550–60
- Paterson DA. Carcinosarcoma and metaplastic carcinomas. The breast 1992; 1: 136–7
- Eggers JW, Chesney TM. Squamous cell carcinoma of the breast: A clinicopathologic analysis of eight cases and review of the literature. Hum Pathol 1984; 15: 526–31
- Rosen PP, Ernsberger D. Low grade adenosquamous carcinoma. A variant of metaplastic mammary carcinoma. Am J Surg Pathol 1987; 115: 351–8
- Kaufman MW, Marti JR, Gallager HS, Hoehn JL. Carcinoma of the breast with pseudosarcomatous metaplasia. Cancer 1984; 53: 1908–17
- Wargotz ES, Norris HJ. Metaplastic carcinoma of the breast: I. Matrix-producing carcinoma. Hum Pathol 1989; 20: 628–35
- Oberman HA. Metaplastic carcinoma of the breast. A clinicopathologic study of 29 patients. Am J Sur Pathol 1987; 11: 918–29
- Kline TS, Kline IK. Metaplastic carcinoma of the breast- A diagnosis by aspiration biopsy cytology: report of two cases and literature review. Diagn Cytopathol 1990; 6: 63–7
- Pitts WC, Rojas VA, Gaffey MJ, Rouse RV, Esteban J, Frierson HF, et al. Carcinomas with metaplasia and sarcomas of the breast. Am J Clin Pathol 1991; 95: 623–32
- Gutman H, Pollock RE, Janjan NA, Johnston DA. Biologic distinctions and therapeutic implications of sarcomatoid metaplasia of epithelial carcinoma of the breast. J Am Coll Surg 1995; 180: 193–9
- Rayson D, Adjei AA, Suman VJ, Wold LE, Ingle JN. Metaplastic breast cancer: Prognosis and response to systemic therapy. Ann Oncol 1999; 10: 413–9
- Choa TC, Wang CS, Chen SC, Chen MF. Metaplastic carcinoma of the breast. J Surg Oncol 1999; 71: 220–5
- Neumann SP, Sibilot DM, Salomon AV, Jouve M, Laurence V, Des Guetz G, et al. Metaplastic breast cancer: clinical presentation and outcome. Proc Am Soc Clin Oncol 2002; 21: 44b
- Kurian KM, Al-Nafussi A. Sarcomatoid / metaplastic carcinoma of the breast: a clinicopathological study of 12 cases. Histopathology 2002; 40: 58–64
- Bauer TW, Rostock RA, Eggleston JC, Baral E. Spindle cell carcinoma of the breast: Four cases and review of the literature. Hun Pathol 1984; 15: 147–52
- Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status and survival in 24,740 breast cancer cases. Cancer 1969; 63: 181–7
- Chhieng C, Cranor M, Lesser ME, Rosen PP. Metaplastic carcinoma of the breast with osteocartilaginous heterologous elements. Am J Surg Pathol 1998; 22: 188–94
- Barnes PJ, Boutilier R, Chiasson D, Rayson D. Metaplastic breast carcinoma: clinical-pathologic characteristics and HER2/neu expression. Breast Cancer Res Treat 2005; 91(2)173–8
- Caceres M, Shih J, Eckert M, Gardner R. Metaplastic carcinoma in an Ectopic Breast. South Med J 2002; 95: 462–6
- Wargotz ES, Norris HJ. Metaplastic carcinoma of the breast: III. Carcinosarcoma. Cancer 1989; 64: 1490–9
- Gobbi H, Simpson JF, Borowsky A, Jensen RA, Page DL. Metaplastic breast tumors with a dominant fibromatosis-like phenotype have a high risk of local recurrence. Cancer 1999; 85: 2170–82
- Wargotz ES, Deos PH, Norris HJ. Metaplastic carcinoma of the breast: II. Spindle cell carcinoma. Hum Pathol 1989; 20: 732–40