Abstract
Maspin, an anti breast cancer protein, is produced in the normal mammary cells but not in malignant cells in breast cancer. We investigated the effect of aspirin induced increase of plasma nitric oxide (NO) on plasma maspin production in breast cancer patients. Fifteen breast cancer patients (35–65 years), who had not yet undergone any cancer therapy, and an equal number of age matched normal female volunteers participated in the study. They were asked not to take any medication for two weeks. All participants then ingested 150 mg of aspirin. Plasma NO and maspin levels were determined before and at 60 min after the ingestion of aspirin. It was found that the maspin level in plasma increased to 4.63±0.02 nM from the basal 0.95±0.012 nM (p < 0.001) with increase of plasma NO from 0.60±0.03 µM to 2.08±0.030 µM (p < 0.001) in breast cancer patients. In normal volunteers the basal maspin increased from 4.76±0.041 to 9.36±0.036 nM (p < 0.001) with increase of NO from 2.15±0.08 to 3.36±0.04 µM (p < 0.001) at the same period. These results indicated that the ingestion of aspirin might be beneficial for breast cancer through increased maspin production.
Nitric oxide (NO), also called “biologic messenger molecule” has been reported to possess various anticancer properties including its ability to induce apoptosis, differentiation and regression in neoplastic cells Citation[1–4] and to produce maspin in malignant breast cancer cells in tissue culture Citation[5]. Although the role of NO in maspin production in the setting of human breast cancer is not known, the protein has been reported to play a critically important role in the control/prevention of breast cancer in animal model Citation[6], Citation[7]. Also the production of the anti-breast cancer protein has been reported to decrease with the progression of the condition Citation[8], Citation[9]. Because of its potential therapeutic usefulness in human breast cancer, various strategies Citation[5], Citation[10], Citation[11] have been proposed to normalize maspin production in vivo in breast cancer. Unfortunately however, none of these proposed strategies has yet been reported to be successful in the normalization of maspin production in human breast cancer. Indeed some of the proposed strategies remain largely theoretical and might be too dangerous to try in human.
In this communication we describe a novel, simple and safe way which might be therapeutically useful to normalize impaired plasma maspin level in human breast cancer. This strategy was based on our report that the ingestion of low amounts of aspirin by normal volunteers resulted in increased plasma NO level Citation[12]. Furthermore this effect of aspirin on NO production was found to be independent of the well-known effect of the compound on the inhibition of prostaglandin synthesis. Since systemic increase of NO to physiologic level has been reported to improve various pathologic problems associated with various kinds of cancer in men Citation[4], we determined the effect of aspirin on plasma maspin level related to the increase of plasma NO level in breast cancer patients compared to appropriate controls.
Materials and methods
Chemicals
NG-nitro arginine methyl ester (NAME), and Goat anti-rabbit IgG-alkaline phosphatase were obtained from Sigma Chemical Co. Recombinant human maspin was a kind gift from Dr. Sally Twining of the Department of Biochemistry, Medical college of Wisconsin, Milwaukee, WI, USA. ELISA Maxisorp plates were obtained from Nunc, Denmark. All other chemicals used were of analytical grade.
Ethical clearance
Protocol used in the study was approved by the Internal Review Board, Sinha Institute of Medical Science and Technology, West Bengal. All participants were asked to sign an informed consent form.
Subject selection criteria
Female patients (n = 15) with operable breast cancer (previously diagnosed by biopsy) were randomly selected from the patients who were hospitalized with the condition. The selected patients were between the ages of 45–65 years (median 52 years). Nine patients were postmenopausal, six were premenopausal. These patients at presentation were staged as follows: T1=6, T2=5, T3=4, n0=2, N1–N2=13, stage I & II = 13, stage III = 2 all were M0. None of the selected patients, at presentation had diabetes mellitus or systemic hypertension, sever infection or life threatening cardiovascular condition. Furthermore none of them had undergone chemotherapeutic, radiation or surgical intervention, but were waiting for surgical resection for the condition.
An equal number of age and menopausal statuses matched female volunteers were also selected for the study. These volunteers were selected from general population that included housewives, nurse and health care professional, teachers, and secretaries at random. None had diabetes mellitus, hypertension, infection or sever cardiovascular condition. At presentation none took any medication at least for a month. None had ever taken any contraceptive medication.
To avoid personal bias and to minimize variations due to laboratory conditions the analyses of both NO and maspin were blinded by following the usual norms. To achieve this, after the samples were collected, they were randomly assigned a number, and the normal and the cancer patient's samples were mixed. The assayer (a technical staff) had no previous knowledge regarding the identity of the patients or of normal volunteers. The assayer also had no knowledge about the participants, who had ingested aspirin or not and the assigned numbers. Results were tabulated only after completion of experiments. The key to the assigned numbers of the participants was kept with the Registrar (a non professional person) of the institute for the safe keeping during the study.
Assay of Aspirin activated nitric oxide synthase (AANOS)
Aspirin activated nitric oxide synthase activity was determined by the formation of NO in reaction mixture by the conversion of oxyhemoglobin to methemoglobin Citation[13] as described before Citation[14]. The formation of NO was independently verified by chemiluminescence's method Citation[15]. Collected plasma sample was immediately used as far as practicable for the determination of plasma NO and maspin without putting the samples in the freezer.
Assay of plasma aspirin level
Plasma aspirin level was determined by FeCl3 method as described Citation[16].
Immunization
Polyclonal antibodies against r-human maspin were raised by repeated immunizations in rabbit as described Citation[17].
Development of ELISA for r-human maspin
Enzyme linked immunosorbant assay was performed as described before Citation[18]. Briefly, maspin was incubated with an equal vol of phosphate buffer saline (PBS) in the assay plate overnight at 4°C. The nonspecific binding was blocked by using 0.5% bovine serum albumin in the same buffer. The samples were next washed with PBS containing Tween-20 and subsequently incubated with diluted antibody in PBS (1:500) for 2 h, followed by washing with the same buffer containing Tween-20. The samples were next incubated with diluted goat anti rabbit IgG–alkaline phosphatase (1:2000) in PBS in the presence of p-nitrophenyl phosphate (1 mg/ml) in carbonate buffer containing 10 mM MgCl2. The development of the color was determined at 405 nm. The amount of maspin present in the plasma was determined by constructing a standard curve using different amounts of r-human maspin. The analytical precision of the recovery of added pmol quantities of maspin in plasma samples was found to be greater than 95%.
Statistical analysis
The significance (p) of the results was determined by Student's “t” test. The levels of aspirin and NO between normal and cancer patients was evaluated by one way analysis of variance.
ResultsEffect of ingestion of aspirin on plasma NO and maspin levels in normal females and in breast cancer patients
To determine the effects of oral ingestion of aspirin on the plasma NO and maspin level, 15 breast cancer patients and normal female volunteers, were asked to ingest one bolus dose of 150 mg aspirin as described in the Materials and methods. Both plasma NO, maspin and aspirin levels were determined before and after 60 min of the ingestion of the drug. It was found that after this period plasma aspirin level increased to 127±6.477 µM (mean±S.D) (ranges from 120.5 µM to133.4 µM, n = 15 in each group) in both groups. It was also found that the plasma NO level in breast cancer patients which was 0.60±0.03 µM (mean±SD) before the ingestion of aspirin increased to 2.08±0.03 µM after 60 min of taking aspirin (p < 0.001). More interestingly, it was also noted that the basal plasma maspin level, which was 0.95±0.01 nM in these breast cancer patients, increased to 4.63±0.02 nM (p < 0.001) after aspirin ingestion (). In normal female volunteers the basal plasma levels of NO, which was 2.15±0.08 µM before the ingestion of aspirin increased to 3.36±0.04 µM after the drug was ingested (p < 0.001). The plasma maspin level increased from the basal 4.76±0.04 nM to 9.36±0.036 nM after the compound was orally administered (p < 0.001). The plasma NO level in cancer and normal volunteers were found to be directly related to the increase of plasma aspirin level. As described above when 150 mg of aspirin was fed to these groups, the plasma aspirin level was found to be increased in the ranges of 120 to 134 µM, in each group with correlation coefficient (r2) = 0.9980 for normal and 0.84499 for breast cancer patient plasma respectively.
Figure 1. Effect of ingestion of aspirin on the plasma level of maspin and NO in normal female volunteers and in breast cancer patients. Each point represents (• = nitric oxide, ▴ = maspin), the plasma levels of NO and maspin in individual volunteer or breast cancer patients (n = 15, in each group).
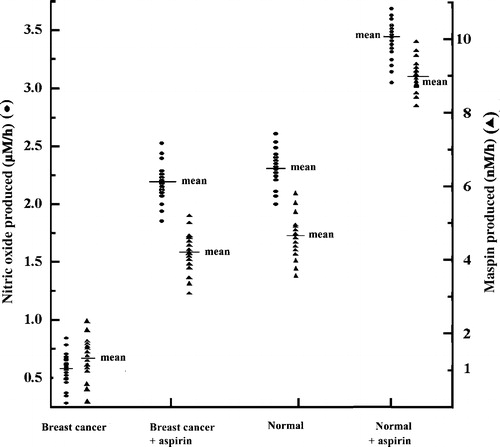
Interestingly, neither the menstrual phases of the patients or normal volunteers affect the effect of aspirin on the statuses of maspin or NO in plasma. This was also true for patients of different ages. Furthermore, marital status of these patients who took no contraceptive medication did not affect the plasma NO or maspin level.
Discussion
These results demonstrate that both plasma level of basal NO and maspin levels were significantly lower in breast cancer when compared to those in normal control (“p” values were < 0.001 in both the cases). The oral ingestion of bolus dose of 150 mg aspirin increased both NO and maspin levels in plasma in breast cancer to normal ranges in about 60 min. It was found that while the oral ingestion of aspirin increased the maspin level by two fold over the basal level in normal female volunteers, ingestion of similar amounts of aspirin (150 mg) by the patients with breast cancer resulted in nearly five fold increase of plasma maspin level over the basal control. Although the total amounts of plasma maspin production in breast cancer due to aspirin ingestion was almost half the amount of the protein present in plasma in normal volunteers, the restored amount of plasma level of maspin in breast cancer achieved through aspirin ingestion was 97% of the normal basal level (4.63 nM VS 4.76 nM). Since aspirin is chronically used for many years in the prevention of coronary artery disease Citation[19] with excellent safety records, it is perhaps be concluded that the chronic use of this compound could be safely used for the prevention and/or control of breast cancer through its ability to “correct” plasma maspin level through increased systemic NO synthesis, independent of cyclooxgenase Citation[12]. Although in this study 150 mg aspirin was consumed by the volunteers, as little as 15 mg aspirin ingestion increased the plasma maspin level by 38% in both breast cancer and normal subjects (unpublished) indicating a possible dose related increase of maspin production by the drug in vivo through NO. It should be mentioned here at this concentration of aspirin little or no inhibition of prostaglandin synthesis could be demonstrated Citation[12], indicating that low amounts of aspirin could be used for the optimal production of maspin in breast cancer patients without producing untoward effects which are created due to the systemic inhibition of prostaglandin synthesis. Furthermore, it was also found that the aspirin induced increase of plasma NO or maspin level was related neither positively nor negatively to the stage of the disease or to the statuses of age, marital status and menstrual phases of the participants.
Furthermore, as a corollary to the above results on the effect of aspirin on maspin production, it could be suggested that chronic users of aspirin might have lower frequency of breast cancer compared to control. Limited studies in this area indicated that the regular use of aspirin could result in the reduction of incidence of breast cancer Citation[20], Citation[21]. However, in these studies the amount of aspirin used was not titrated for the optimal systemic production of maspin in the participants.
Research described in this article was supported (in part) by Philip Morris USA Inc. and Philip Morris International.
References
- Jenkins DC, Charles LG, Thomsen LL, Moss LJ, Holmes LS, Baylis SA, et al. Roles of nitric oxide in tumor growth. Proc Natl Acad Sci USA 1995; 92: 4392–6
- Dong Z, Qi X, Xie K, Fidler IJ. Protein tyrosine kinase inhibitors decrease induction of nitric oxide synthase activity in lipopolysaccharides-responsive and lipopolysaccharides-nonresponsive murine macrophages. J Immunol 1993; 151: 2717–24
- Uchida T, Yamashita T, Araki A, Watanabe H, Sendo F. rIFN-gamma-activated rat neutrophils induce tumor cell apoptosis by nitric oxide. Int J Cancer 1997; 71: 231–6
- Sinha AK, Acharya K, Bhattacharya S, Patra SC, Guha M, Ray U, et al. Neutralization by “antineoplastin” of insulin-activated nitric oxide synthase antibody and its effects in cancer. J Cancer Res Clin Oncol 2002; 128: 659–68
- Khalkhali-Ellis Z, Hendrix MJ. Nitric oxide regulation of maspin expression in normal mammary epithelial and breast cancer cells. Am J Pathol 2003; 162: 1411–7
- Zou Z, Anisowicz A, Hendrix M, Thor A, Neveu M, Sheng S, et al. Maspin, a serpin with tumor – suppressing activity in human mammary epithelial cells. Science 1994; 263: 526–9
- Ngamkitidechakul C, Warejcka DJ, Burke JM, O'Brien WJ, Twining SS. Maspin: Synthesis by human cornea and regulation of in vitro stromal cell adhesion to extracellular matrix. J Biol Chem 2003; 278: 31796–806
- Maass N, Teffner M, Rosel F, Pawaresch R, Jonat W, Nagasaki K, et al. Decline in the expression of the serine proteinase inhibitor maspin is associated with tumor progression in ductal carcinomas of the breast. J Pathol 2001; 195: 321–6
- Hojo T, Akiyama Y, Nagasaki K, Maruyama K, Kikuchi K, Ikeda T, et al. Association of maspin expression with the malignancy grade and tumor vascularization in breast cancer tissues. Cancer Lett 2001; 171: 103–10
- Shi HY, Zhang W, Liang R, Abraham S, Kittrell FS, Medina D, et al. Blocking tumor growth, invasion, and metastasis by maspin in a syngeneic breast cancer model. Cancer Res 2001; 61: 6945–51
- Li Z, Shi HY, Zhang M. Targeted expression of maspin in tumor vasculatures induces endothelial cell apoptosis. Oncogene 2005; 24: 2008–19
- Chakraborty K, Khan GA, Banerjee P, Ray U, Sinha AK. Inhibition of human blood platelet aggregation and the stimulation of nitric oxide synthesis by aspirin. Platelets 2003; 14: 421–7
- Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: A dynamic activity of blood involved in vascular control. Nature 1996; 380: 221–6
- Bhattacharya S, Chakraborty Patra S, BasuRoy S, Khan NN, Sinha AK. Purification and properties of insulin-activated nitric oxide synthase from human erythrocyte membranes. Arch Physiol Biochem 2001; 109: 441–9
- Zafiriou OC, McFarland M. Determination of trace levels of nitric oxide in aqueous solution. Anal Chem 1980; 52: 1662–7
- Maruf A, Biswas MHU, Rahman MM, AlamBhuiyan MS, MostofaKamal M, Sadik G. Development of a spectrophotometric method for the determination of aspirin in blood sample. The Sciences 2001; 1: 61–2
- TlaskalovaHogenova H, Stepankova R. Development of antibody formation in germ free and conventionally reared rabbits: The role of intestinal lymphoid tissue in antibody formation to E. coli. Folia Biol (Praha) 1980; 26: 81–93
- Engvall E, Perlmann P. Enzyme-linked immunosorbant assay, ELISA. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol 1972; 109: 129–35
- Steering Committee of the Physicians’ Health study research group. Final report on the aspirin component of the aspirin component of the ongoing physicians health study. N Engl J Med 1989;32:129.
- Swede H, Mirand AL, Menezes RJ, Moysich KB. Association of regular aspirin use and breast cancer risk. Oncology 2005; 68: 40–7
- Jacobs EJ, Thun MJ, Connell CJ, Rodriguez C, Henley SJ, Feigelson HS, et al. Aspirin and other nonsteroidal anti-inflammatory drugs and breast cancer incidence in a large U.S. cohort. Cancer Epidemiol Biomarkers Prev 2005; 14: 261–4
