Abstract
Between 1975 and 2005, we treated 52 newly diagnosed germinoma patients. Until 1991, patients with pure germinomas or germinomas with syncytiotrophoblastic giant cells (STGCs) received whole-brain radiotherapy only. Of the 52 patients, 30 were treated with a reduced radiation volume and combined chemotherapy; seven of these received local irradiation with 24 Gy, two received whole-brain (30 Gy) plus local irradiation (20 Gy), 16 received extended local irradiation delivered to the whole ventricles (30 Gy) plus local (20 Gy) irradiation, and five received extended local irradiation (24 Gy). Of the 30 patients treated with a reduced radiation volume and combined chemotherapy, four experienced tumor recurrence; three patients had been treated with 24 Gy of local radiotherapy and one had received extended local (30 Gy) plus local (20 Gy) irradiation in addition to chemotherapy. In these patients, the delivered radiotherapy was inadequate and the origin of the recurrent tumors was outside the radiation field. None of the patients who had received at least 24 Gy of whole ventricle radiotherapy combined with chemotherapy experienced tumor recurrence. In combination with chemotherapy, the delivery of irradiation covering the ventricles effectively reduced the incidence of tumor recurrence in patients with germinomas or germinomas with STGCs.
Prior to 1990, radiotherapy was considered the most effective treatment in patients with germinomas including children who usually received around 50 Gy of whole-brain irradiation Citation[1–4]. The overall survival rate following radiotherapy alone exceeded 90% Citation[3], Citation[5]. Concerns regarding radiation-induced complications such as neurocognitive dysfunction and neuro-endocrine disorders have led to reductions in the radiation field and dose Citation[6–8]. However, as the delivery of local radiotherapy alone resulted in high tumor recurrence rates, several investigators stressed that combined chemotherapy was required in patient with germinomas Citation[9]. According to a report of The First International Central Nervous System Germ Cell Tumor Study Group Citation[10], 22 of 45 germinoma patients treated with chemotherapy but no radiotherapy suffered tumor recurrence. Therefore, chemotherapy alone is now considered inadequate for the achievement of a complete and curable response and most germinoma patients undergo combined chemo- and radiotherapy Citation[11], Citation[12].
Until 1991, germinoma patients treated at our institution (n = 22) underwent only whole-brain irradiation. However, as the quality of life of several of these patients was unsatisfactory due to cognitive, neuropsychologic and/or neuro-endocrine sequelae, we began to reduce the radiation volume in 1992 and added chemotherapy. We employed two radiotherapeutic strategies; one used local irradiation, the other involved extended local irradiation covering the lateral ventricles, third ventricle, pineal, and neurohypophyseal region, and the upper third of the fourth ventricle. In this retrospective study we compared the clinical outcomes of these two strategies.
Patients and methods
Between 1975 and 2005, we treated 52 patients with newly diagnosed germinomas with and without syncytiotrophoblastic giant cells (STGCs) at Kumamoto University. Patients seen before 1992 (n = 22) received only whole-brain radiotherapy without biopsy and without chemotherapy (). Starting in 1992, patients negative for tumor markers such as alpha-fetoprotein (AFP) and human chorionic gonadotropin (HCG) underwent surgery to assess the histology of their tumors; those with histologically confirmed germinomas (n = 30) were treated with combined platinum-based chemotherapy and reduced-volume radiotherapy.
Table I. QOL analysis in patients with germinomas who received the whole brain radiotherapy between 1976 and 1991.
Treatment Protocols
Chemotherapy consisted of 450 mg/m2 carboplatin (CBDCA) or 20 mg/m2 cisplatin (CDDP) on day 1 and 150 mg/m2 or 100 mg/m2 etoposide (VP-16) on Days 1 to 3 in patients with pure germinomas (n = 26). Some of those with germinomas with STGCs or multiple lesions received 900 mg/m2 ifosphamide (IFOS), 20 mg/m2 CDDP, and 60 mg/m2 VP-16 on 5 consecutive days (n = 4). Patients treated between 1992 and 1995 underwent one of these chemotherapy regimens and simultaneously they received 30 Gy extended local radiotherapy that covered the lateral ventricles, third ventricle, and upper third of the fourth ventricle, and 20 Gy delivered locally. Since 1996, the administration of two or three courses of chemotherapy was followed by radiotherapy. Patients treated between 1996 and 1998 who, after the administration of two or three courses of chemotherapy manifested a complete response (CR, n = 7), were treated with 24 Gy of local radiation. In 1998 we started the delivery of 24 Gy of extended local radiation to the lateral ventricles, third ventricle, pineal, and neurohypophyseal region, and upper third of the fourth ventricle. Patients who failed to exhibit a complete response after three courses of chemotherapy were treated with 30 Gy of extended local and 20 Gy of local radiotherapy.
Patient Characteristics
As shown in Table 26 patients were operated (biopsy n = 22: partial removal n = 3, total removal n = 1); four patients refused surgery. Chemotherapy consisting of CBDCA (450 mg/m2) or CDDP (100 mg/m2) and VP-16 (150 mg/m2 or 100 mg/m2) was administered to 26 patients; four patients received IFOS (4500 mg/m2), CDDP (100 mg/m2), and VP-16 (300 mg/m2). Local irradiation (24 Gy) was delivered to seven patients, two received 30 Gy whole-brain- plus 20 Gy local radiation, 16 were treated with extended local irradiation (30 Gy) covering the lateral ventricles, third ventricle, pineal and neurohypophyseal region, and upper third of the fourth ventricle, plus 20 Gy delivered locally, and five underwent extended local irradiation with 24 Gy.
Table II. Characteristics of patients with germinoma or germinoma STGC since 1992 (total 30 cases).
All 52 patients were registered in the data bank of the Department of Neurosurgery, Kumamoto University Medical School. Pathological specimens were reviewed by two neuropathologists (JK and MK) who had at least 15 years of experience.
Results
Germinoma patients treated at our institute between 1975 and 1991 (n = 22) received whole-brain irradiation without biopsy or chemotherapy. To investigate the effect of whole-brain irradiation on their quality of life (QOL), two factors, i.e. their post-treatment level of social activity and their need for hormone replacement were studied. Of the 22 patients, seven (31.8%) were unable to work and one patient had died. The tumor was located in the neuro-hypothalamic region in 16 patients (72.7%); 14 (63.6%) required hormone replacement at least once (). Based on this experience we changed our protocol for treating patients with germinomas with or without STGCs. Since 1992, we have employed a reduced radiation volume in combination with chemotherapy. By 2005 we had tried four different radiotherapy protocols.
Recurrence was confirmed in four of the 30 (1992–) patients (13.3%), three had received local irradiation with 24 Gy (). The other had undergone extended local (30 Gy)- and local irradiation (20 Gy) and when we re-examined the irradiated area we were surprised to find that the field of extended local radiation was not sufficiently large and that in this, and the other three patients, the recurrent tumor arose outside the radiation field. In the other 20 patients with extended local radiation all ventricles were covered and there was no tumor recurrence.
Table III. Germinoma Recurrence rate in the treatment of each Radiotherapy.
Illustrative Cases
Case 1 ()
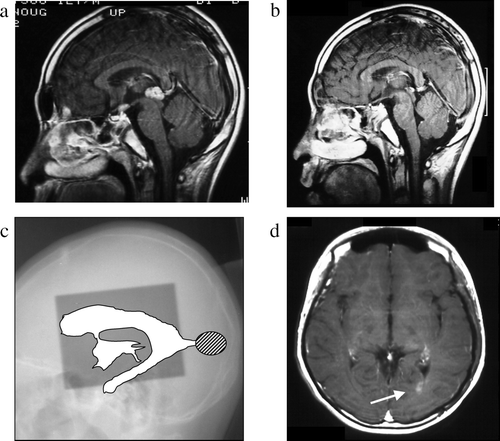
This 12-year-old boy complained of headache and general fatigue in December 1991. One week later he experienced vomiting and polyuria and a brain CT scan obtained at a local hospital revealed a mass lesion in the pineal and suprasellar region. Chemotherapy with CDDP (100 mg/m2) and VP-16 (300 mg/m2) and simultaneous radiotherapy were started. The delivery of extended local (30 Gy)- and local (20 Gy) irradiation proved inadequate because the posterior horn of the lateral ventricle was not covered. After 6 courses of chemotherapy and 50 Gy of radiotherapy he was discharged in April 1992 in good condition and recorded as having made a complete recovery. He was apparently free of tumor recurrence until September 1996 when follow-up MRI revealed a mass lesion in the left posterior horn of the lateral ventricle. Examination of a biopsy specimen returned a histological diagnosis of germinoma. He again received six courses of chemotherapy (IFOS 4500 mg/m2, CDDP 100 mg/m2, and VP-16 300 mg/m2) and radiotherapy (whole brain 30 Gy + local 10 Gy). On a subsequent MRI scan the tumor had decreased but not disappeared. In the course of chemotherapy dissemination to the medulla was found and he underwent γ-knife treatment of the medulla. However, he died of adrenal crisis in March, 1999.
Figure 1. Case 1, a 12-year-old boy. a: Pre-treatment MRI scan revealing a pineal mass. b: Post-treatment MRI scan revealing disappearance of the pineal mass. c: On this x-radiograph, the field of irradiation (gray area) and ventricles (white area) on MRI scans are traced. The relapse site is identified by a hatched circle. d: This post-treatment MRI scan shows an enhanced lesion in the posterior horn of the left lateral ventricle (arrow).
Case 2 ()
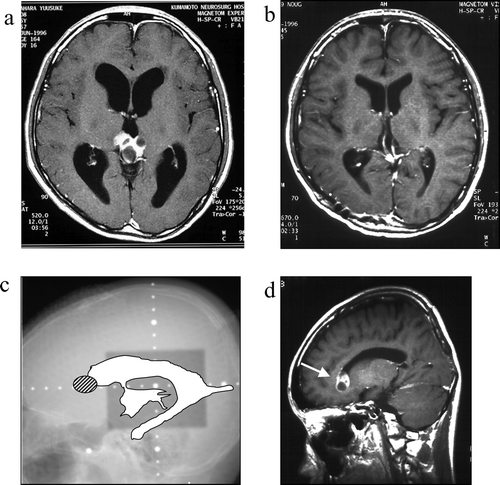
This 15-year-old boy complained of double vision in May 1996. A brain CT scan obtained at a local hospital revealed a pineal mass with calcification and hydrocephalus. He was negative for all tumor markers examined. Biopsy performed via the occipital transtentorial approach returned a histological diagnosis of germinoma and he received three courses of CBDCA & VP-16 followed by local irradiation with 24 Gy. He was discharged in satisfactory condition and complete recovery was recorded. A brain MRI scan obtained in April 1998 revealed tumor recurrence in the anterior horn of the right lateral ventricle. He received four courses of chemotherapy consisting of IFOS (4500 mg/m2), CDDP (100 mg/m2), and VP-16 (300 mg/m2) and extended local (24 Gy)- plus local (24 Gy) radiotherapy. The recurrent tumor disappeared and he was discharged in good condition. He subsequently graduated from school and is currently working with no neurological deficits.
Figure 2. Case 2, a 15-year-old boy. a: Pre-treatment MRI scan revealing a pineal mass. b: Post-treatment MRI scan reveals complete disappearance of the pineal mass. c: On this x-radiograph, the field of irradiation (gray area) and the ventricles (white area) on MRI scans are traced. The relapse site is identified by a hatched circle. d: This post-treatment MRI scan reveals an enhanced lesion in the anterior horn of the right lateral ventricle (arrow).
Case 3 ()
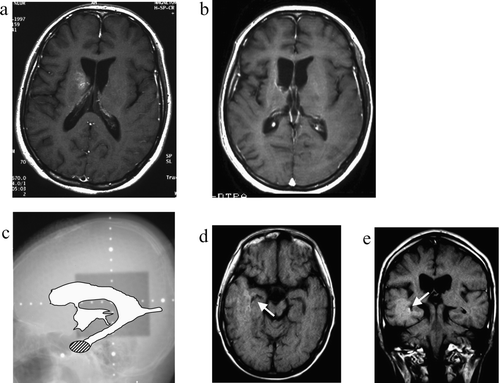
This 16-year-old boy complained of mild leg weakness in January 1995. His activity had decreased and his symptoms gradually worsened. An MRI scan obtained at a local hospital in July 1995 revealed a low-intensity area in the right basal ganglia. The lesion was slightly enhanced with gadolinium. Under a diagnosis of multiple sclerosis he received several steroid administrations, however, his symptoms continued to progress. In January 1997 he was seen at our hospital where stereotactic biopsy returned a histological diagnosis of germinoma. He received three courses of CBDCA & VP-16 followed by local irradiation with 24 Gy. His symptoms improved only little although the lesion in the right basal ganglia disappeared. In March 1999, MRI revealed tumor recurrence at the inferior horn of the right lateral ventricle. He received seven courses of chemotherapy consisting of IFOS (4500 mg/m2), CDDP (100 mg/m2), and VP-16 (300 mg/m2) and whole-brain (30 Gy) irradiation. The recurrent tumor disappeared and he is currently undergoing rehabilitation. There has been no further tumor recurrence.
Figure 3. Case 3, a 16-year-old boy. a: Pre-treatment MRI scan revealing an enhanced lesion in the right basal ganglia. b: Post-treatment MRI scan shows complete disappearance of the lesion. c: On this x-radiograph, the field of irradiation (gray area) and the ventricles (white area) on MRI scans are traced. The relapse site is identified by a hatched circle. d: This post-treatment MRI scan (axial view) reveals an enhanced lesion in the inferior horn of the right lateral ventricle (arrow). e: MRI scan obtained at the time of tumor recurrence (coronal view).
Case 4 ()
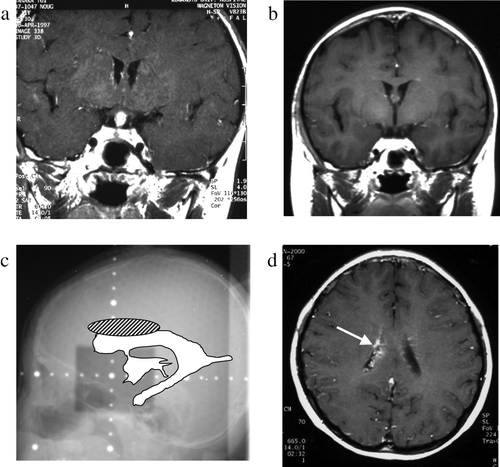
This 11-year-old girl was found to be of unusually short stature at an examination performed at her school in 1995. In July 1996 she started to complain of polyuria and thirst. An MRI scan obtained in April 1997 revealed a mass lesion involving the pituitary stalk and hypothalamus. Biopsy returned a histological diagnosis of germinoma. She received three courses of CBDCA & VP-16 and local (24 Gy) radiotherapy. The gadolinium-enhanced lesion subsequently disappeared, however, in March 1999; there was gadolinium enhancement in the pituitary stalk. She received eight courses of chemotherapy consisting of IFOS (4500 mg/m2), CDDP (100 mg/m2), and VP-16 (300 mg/m2). The lesion subsequently disappeared and she was in good condition without neurological deficits. She underwent GH and cortisol administration. In February 2002 a new gadolinium-enhanced lesion appeared in the body of the right lateral ventricle. She received local radiotherapy with 30 Gy and 3 courses of chemotherapy (CBDCA, VP-16, and bleomycin). The recurrent tumor subsequently disappeared and she is currently working with no neurological deficits.
Figure 4. Case 4, an 11-year-old boy. a: Pre-treatment MRI scan revealing a hypothalamic enhanced lesion. b: Post-treatment MRI scan shows complete disappearance of the hypothalamic mass. c: On this x-radiograph, the field of irradiation (gray area) and the ventricles (white area) on MRI scans are traced. The relapse site is identified by a hatched circle. d: MRI scan obtained at the time of tumor recurrence shows an enhanced lesion in the body of the right lateral ventricle (arrow).
Discussion
Several clinical trials have shown that central nervous system (CNS) germinomas are highly responsive to chemotherapy Citation[13–16]. Treatment of these tumors with volume-reduced radiotherapy decreases the rate of complications such as neurocognitive dysfunction and neuro-endocrine disorders. Patients treated with chemotherapy alone, despite its initial effectiveness and high response rate, are reportedly at high risk for tumor recurrence Citation[10], Citation[17]. Chemotherapy alone is not sufficient treatment in the patients with even pure germinomas. Therefore, patients with germinomas with or without STGCs are currently treated with combined radiotherapy and platinum-based chemotherapy. We studied the effects of alternative approaches to reduce the volume of radiotherapy. Tumor recurrence was confirmed in four germinoma patients treated with chemo- and radiotherapy. All recurrent tumors arose within the ventricles and all recurrence sites were outside the initial radiation field. This suggests that the radiation field should cover the whole ventricles. Although some have suggested that patients with germinoma should be treated with local radiotherapy, this treatment is associated with a high recurrence rate Citation[18–21]. Aoyama et al. Citation[22] pointed out that local radiotherapy that does not cover the whole ventricles yielded 10-year relapse-free survival rates as low as 22%.
While the optimal irradiation dose to the primary tumor remains controversial, it has been suggested that 40 – 50 Gy irradiation is usually required to cure intracranial germinomas Citation[23]. In our series, none of the patients who received at least 24 Gy of extended local irradiation and chemotherapy suffered tumor recurrence.
The mechanism(s) underlying recurrence remain unclear and it is currently not known whether recurrence in the ventricles is due to tumor dissemination. In case 3 of our patients the primary tumor site was the right basal ganglia and the relapse site was the inferior horn of the lateral ventricles. This is suggestive of tumor cell dissemination via the cerebrospinal fluid and raises the question of whether the initial treatment contributes to the dissemination of germinoma cells. Among our 30 patients with germinomas with or without STGCs none manifested dissemination when they visit our hospital. Review of our database turned up no germinoma patients with dissemination. On the other hand, we found dissemination in several patients with non-germinomatous malignant germ cell tumors. In the present series, four patients treated with combined chemo- and radiotherapy suffered tumor recurrence, suggesting that their treatment may have contributed to tumor cell dissemination. We posit that tumor cells addressed by chemo- and radiotherapy die at different times and that while directly targeted cells die early, this results in the release and potential propagation of remaining viable cells. The interval to tumor recurrence may be determined by the volume of irradiated (i.e. killed) tumor cells. Based on these considerations we suggest that whole-ventricle irradiation be performed to prevent the subsequent dissemination of germinoma tumor cells.
Therapy for Recurrent Tumors
The treatment of recurrent germinomas remains controversial. We treat these patients with additional combined radiotherapy and chemotherapy. In our series, all four patients with tumor recurrence received whole-brain or whole-ventricle radiotherapy; γ-knife surgery was added in patient one because his lesions remained recalcitrant to treatment. Three patients received combined chemotherapy with ifosphamide/cisplatin/etosposide. Patient one, treated with carboplatin/etoposide/bleomycin died (), and the other three achieved a complete response. Some Citation[24], Citation[25] used high-dose chemotherapy followed by autologous stem-cell rescue in patients with relapsed or progressive CNS germ cell tumors. This treatment modality may be useful not only in patients with non-germinomatous malignant germ cell tumors but also in those with relapsed or progressive germinoma.
Table IV. Characteristics of the patients with recurrence of the germinoma at the outside of the initial radiation field.
Concerning spinal irradiation, the dissemination to spinal seems to be rare in germinomas or germinomas with STGC. Therefore, the examination of CSF cytology is not always necessary if the negative findings can be obtained in the examination of spinal MRI. Also, spinal irradiation to prevent the spinal dissemination is not necessary in the patients with intracranial germinomas.
A recent review Citation[26] has been in accordance with our conclusion that in combination with chemotherapy, whole-ventricle radiotherapy is highly effective for reducing the rate of relapse in patients with germinomas with or without STGCs.
This work was supported by a research grant from Nippon Gakujutu Shinkoukai/Kiban Kenkyuu No. 17591520. We are indebted to Masayo Obata for help with the immunohistochemical staining.
References
- Jenkin RD, Simpson WJ, Keen CW. Pineal and suprasellar germinomas. Results of radiation treatment. J Neurosurg 1978; 48: 99–107
- Rich TA, Cassady JR, Strand RD, Winston KR. Radiation therapy for pineal and suprasellar germ cell tumors. Cancer 1985; 55: 932–40
- Shibamoto Y, Abe M, Yamashita J, Takahashi M, Hiraoka M, Ono K, et al. Treatment results of intracranial germinoma as a function of the irradiated volume. Int J Radiat Oncol Biol Phys 1988; 15: 285–90
- Fields JN, Fulling KH, Thomas PR, Marks JE. Suprasellar germinoma: Radiation therapy. Radiology 1987; 164: 247–9
- Fuller BG, Kapp DS, Cox R. Radiation therapy of pineal region tumors: 25 new cases and a review of 208 previously reported cases. Int J Radiat Oncol Biol Phys 1994; 28: 229–45
- Kun LE, Mulhern RK, Jr. Neuropsychologic function in children with brain tumors: II. Serial studies of intellect and time after treatment. Am J Clin Oncol 1983; 6: 651–6
- Packer RJ, Sutton LN, Atkins TE, Radcliffe J, Bunin GR, D'Angio G, et al. A prospective study of cognitive function in children receiving whole-brain radiotherapy and chemotherapy: 2-year results. J Neurosurg 1989; 70: 707–13
- Schriock EA, Schell MJ, Carter M, Hustu O, Ochs JJ. Abnormal growth patterns and adult short stature in 115 long-term survivors of childhood leukemia. J Clin Oncol 1991; 9: 400–5
- Buckner JC, Peethambaram PP, Smithson WA, Groover RV, Schomberg PJ, Kimmel DW, et al. Phase II trial of primary chemotherapy followed by reduced-dose radiation for CNS germ cell tumors. J Clin Oncol 1999; 17: 933–40
- Balmaceda C, Heller G, Rosenblum M, Diez B, Villablanca JG, Kellie S, et al. Chemotherapy without irradiation–a novel approach for newly diagnosed CNS germ cell tumors: Results of an international cooperative trial. The First International Central Nervous System Germ Cell Tumor Study. J Clin Oncol 1996; 14: 2908–15
- Calaminus G, Bamberg M, Baranzelli MC, Benoit Y, di Montezemolo LC, Fossati-Bellani F, et al. Intracranial germ cell tumors: A comprehensive update of the European data. Neuropediatrics 1994; 25: 26–32
- Baranzelli MC, Patte C, Bouffet E, Couanet D, Habrand JL, Portas M, et al. Nonmetastatic intracranial germinoma: The experience of the French Society of Pediatric Oncology. Cancer 1997; 80: 1792–7
- Paulino AC, Wen BC, Mohideen MN. Controversies in the management of intracranial germinomas. Oncology (Williston Park) 1999; 13: 513–21
- Yoshida J, Sugita K, Kobayashi T, Takakura K, Shitara N, Matsutani M, et al. Prognosis of intracranial germ cell tumours: Effectiveness of chemotherapy with cisplatin and etoposide (CDDP and VP-16). Acta Neurochir (Wien) 1993; 120: 111–7
- Matsutani M, Sano K, Takakura K, Fujimaki T, Nakamura O. Combined treatment with chemotherapy and radiation therapy for intracranial germ cell tumors. Childs Nerv Syst 1998; 14: 59–62
- Diez B, Balmaceda C, Matsutani M, Weiner HL. Germ cell tumors of the CNS in children: Recent advances in therapy. Childs Nerv Syst 1999; 15: 578–85
- Nakajima T, Kumabe T, Jokura H, Yoshimoto T. Recurrent germinoma in the optic nerve: Report of two cases. Neurosurgery 2001;48:214–7; discussion 217–8.
- Chao CK, Lee ST, Lin FJ, Tang SG, Leung WM. A multivariate analysis of prognostic factors in management of pineal tumor. Int J Radiat Oncol Biol Phys 1993; 27: 1185–91
- Matsutani M, Sano K, Takakura K, Fujimaki T, Nakamura O, Funata N, et al. Primary intracranial germ cell tumors: A clinical analysis of 153 histologically verified cases. J Neurosurg 1997; 86: 446–55
- Shibamoto Y, Takahashi M, Abe M. Reduction of the radiation dose for intracranial germinoma: A prospective study. Br J Cancer 1994; 70: 984–9
- Shirato H, Nishio M, Sawamura Y, Myohjin M, Kitahara T, Nishioka T, et al. Analysis of long-term treatment of intracranial germinoma. Int J Radiat Oncol Biol Phys 1997; 37: 511–5
- Aoyama H, Shirato H, Kakuto Y, Inakoshi H, Nishio M, Yoshida H, et al. Pathologically-proven intracranial germinoma treated with radiation therapy. Radiother Oncol 1998; 47: 201–5
- Dattoli MJ, Newall J. Radiation therapy for intracranial germinoma: The case for limited volume treatment. Int J Radiat Oncol Biol Phys 1990; 19: 429–33
- Modak S, Gardner S, Dunkel IJ, Balmaceda C, Rosenblum MK, Miller DC, et al. Thiotepa-based high-dose chemotherapy with autologous stem-cell rescue in patients with recurrent or progressive CNS germ cell tumors. J Clin Oncol 2004; 22: 1934–43
- Tada T, Takizawa T, Nakazato F, Kobayashi S, Koike K, Oguchi M, et al. Treatment of intracranial nongerminomatous germ-cell tumor by high-dose chemotherapy and autologous stem-cell rescue. J Neurooncol 1999; 44: 71–6
- Rogers SJ, Mosleh-Shirazi MA, Saran FH. Radiotherapy of localized intracranial germinoma: Time to sever historical ties?. Lancet Oncol 2005; 6(7)509–19
