Abstract
We have developed a system of mixed aggregates of cultured cells, to model in situ cell interactions. This three-dimensional (3D) system of floating cell aggregates, termed spheroids for their round shape, enables one to monitor their growth in both size and number of constituent clonogens and to measure survival curves for cells having 3D cell-cell interactions. This system was used to measure the three-dimensional cell-cell interactions on growth, and clonogenicity of either AG1522 fibroblasts, or HeLa cervical cancer cells (pure spheroids, or if both feeder and test cells are the same type, pseudohybrid spheroids), and/or of mixtures of both (hybrid spheroids). By following the increase or decrease in size of, or number of clonogens per, spheroid over time, one obtains growth or inhibition curves. By relating these clonogen numbers, one obtains, after a suitable growth period, relative survival. The system allows one to score the effects of irradiation and of other treatments, as well as the effect of interaction of the constituent cells on their survival. Floating pure, or pseudohybrid (composed of 10% live fibroblasts and 90% supralethally irradiated fibroblast feeder cells) spheroids, shrank to about 10–20% of their volume in three days and then remained at that size for up to six days. In contrast, pure spheroids composed of live HeLa cells increased their volume by an order of magnitude over the same period. Survival of cells in spheroids was measured by the ability of individual spheroids to grow beyond a size implying a ten-fold increase. A caveat to be observed is to correct survival for cellular multiplicity, i.e. reduce survival values to compensate for more than one colony former at the time of irradiation. The system of spheroids floating and growing in nutrient medium provides a selective system for evaluating growth of HeLa, and by implication, other neoplastic cells, without interference from (overgrowth by) normal fibroblasts. Thus it is possible to discriminate between normal and neoplastic cells by virtue of whether or not cells grow in suspension. Such a system seems ideal for testing novel strategies (radiation in combination with chemicals), in an in vivo-like environment.
Tumor cells from surgical samples or biopsies have exceedingly low plating efficiencies, such that only a miniscule fraction, probably unrepresentative of the tumor as a whole, will grow in culture. However, knowledge of the sensitivity of each patient's tumor to various chemotherapies and radiation treatments is essential to treating each patient as an individual rather than the average of all clinical experience. The heterogeneity of tumors and of patient responses to therapy is well-known, so the ability to individualize patient treatment plans, taking into account the inherent sensitivities of their tumor(s) could be an important advance in improving the cure rates of various cancers.
The first attempt to measure the clonogenic survival of tumors in culture as an assay system was that of Salmon and Hamburger Citation[1]. Their system of tumor cell growth in soft agar produced sufficient cells to perform an assay in only about 25% of their patient samples and for each cell to form a colony, 100 000 had to be plated. This low plating efficiency (PE = 10−5) made it difficult to believe that the results were representative of the tumor in situ. An improvement on this system was based on the Courtney et al. double layer soft-agar method Citation[2], used by West et al. Citation[3] to measure the sensitivity of cervical cancers to a single dose of 2 Gy. This method obtained results from about three quarters of patients and had a PE of about 0.1% (10−3). A major benefit of the soft-agar methods was supposed to have been that they prevented the growth of stromal fibroblasts, which would otherwise take over the culture, so that only the tumor cells were thought to grow in agar. Similarly, the cell adhesive matrix assay (CAM) was thought to be selective for fibroblasts. However, Lawton et al. Citation[4] and Stausbol-Gron et al. Citation[5] showed that this assumption was incorrect; stromal fibroblasts also proliferate on the matrix plates Citation[4] and in agar Citation[5], so that the results were for fibroblasts (or possibly both mixed fibroblasts and tumor cells), and not the tumors. Moreover, like all single-cell plating assays, there is a fundamental problem that soft-agar assays lack physiological relevance to in situ tumors, because they lack the three-dimensional (3D) contact seen in tumors. It may be for exactly this reason that no one has yet correctly predicted individual patient outcomes using these assays Citation[3]. To alleviate these problems, we have developed an in vivo-like system, the hybrid spheroid assay Citation[6–10], suitable for testing primary tumor cells. Our system exhibits a much higher PE∼1–10%, with almost all samples producing sufficient colonies for assay Citation[6]. Furthermore, in the hybrid spheroid system, cells are enveloped in a 3D agglomerate of cells exhibiting all the mutual influences on survival after treatment. This is important, as it is becoming clear that the survival of tumor cells and the functionality of various tissues surrounding tumors are determined not only by the direct impact of inactivating agents, but also by the now well recognized Bystander Effect (BE) (see review in Citation[11]).
Hybrid spheroids were first shown to form from mixtures of unirradiated HeLa test cells and supralethally irradiated HeLa feeder cells Citation[6]. Using this system with test cells directly from human tumor surgical samples, it was shown that the PE was considerably higher than that in other clonogenicity-based putative predictive assays for patient tumor radiosensitivity Citation[7]. Unlike monolayer assays, this system appeared to maintain the G0 fraction in chemosensitivity assays Citation[8], and was the only one to reliably provide radiation survival curves Citation[6–10]. However, the radiosensitivity of HeLa test cells to each daily dose fraction was shown to increase exponentially with increasing number of fractions Citation[9], rather than remain constant, as previously assumed. This was shown to be a form of BE Citation[11]. Since test cell contact with supralethally irradiated feeder cells produces an artifactually large BE effect (rather than the much smaller one due to the feeder cells receiving the same dose as the test cells), this led us to examine the use of fibroblasts as feeder cells in hybrid spheroids. This use of fibroblast feeder cells also can block the growth of stromal fibroblasts (which could otherwise overgrow the culture). The results of some of the studies using HeLa test cells (as a repeatable standard) are presented here.
Until recently, the effects of ionizing radiations on cells were considered to be independent for each cell, with no interaction between neighbors. The analysis of in vitro single cell survival curves was based on this assumption, as were in vitro models for tumor radiosensitivity. However, there is a phenomenon of collective response to irradiation known by the term “Bystander Effect” (BE, Citation[11–20]). It deals with the fact that cells in culture (and in whole animals) which had not been traversed by a photon or ionizing radiation track, are able to be affected by their traversed neighbors via either a direct contact Citation[12], Citation[15–17], Citation[19], Citation[20] or a medium-transmitted effect Citation[14], Citation[18]. From all appearances, this phenomenon may be of considerable significance in clinical situations, notably during protracted treatment regimens. The text below briefly summarizes the phenomenon, and highlights possibilities of modulating clinical practices, with a view to obtain a therapeutic gain in tumor control.
We became aware that BE may be operating in our system of PseudoHybrid Spheroids composed of agglomerated supralethally irradiated HeLa feeder and live test HeLa cells, when we described exponentially increased radiosensitivity in the course of multifraction irradiation Citation[8]. A much smaller radiosensitizing effect was seen when a similar combination of HeLa cells in monolayer, with or without feeder cells, was repeatedly irradiated Citation[9]. Since such findings indicated an effect of close cellular contact in BE, it was reasoned that three-dimensional cell-cell contact maintained for longer periods of time, could be more revealing of the effect of cellular contact and more like the situation in situ. Consequently, we have measured the importance of cellular contact on test cell survival in long-term spheroids composed of test and irradiated or unirradiated feeder cells. This approach may provide a better in vitro model for in vivo cellular radiation responses.
Materials and methods
Types of spheroids
Three types of spheroids are used in this study: (1) Pure spheroids, in which all the cells are of the same type, as in pure fibroblast or pure HeLa spheroids; (2) Hybrid spheroids, in which two cell types are mixed (e.g., HeLa and fibroblasts), one type being the test cells whose growth and viability are being measured (1% or 10% of the initial spheroid cells), while the other type consists of nonclonogenic feeder cells (99% or 90% of the initial spheroid cells; rendered such by irradiation or culture condition); and (3) Pseudohybrid spheroids, in which all the cells are of the same type, but the feeder cells (99% or 90%) are supralethally irradiated.
Spheroid formation and maintenance
The method of spheroid formation has been described previously Citation[6–10]. Briefly, a mixed cell suspension composed of cells to be tested (test cells) and carrier cells (feeder cells) is co-incubated overnight, in a bacteriological Petri dish, to which cells do not adhere. Under these conditions, hybrid (or pseudohybrid or pure, depending on the mixture of cells used) spheroids are formed, the composition of which closely corresponds to the input of the original cell mixture Citation[6].
In the present study, two cell lines were used: AG1522 fibroblasts of human origin (early passage, obtained from Coriell Cell Repositories, Camden, NJ, and retrieved from liquid nitrogen storage shortly before use), and HeLa cells (maintained in this laboratory for more than a decade; periodically removed from cryogenic storage to minimize genetic drift).
To form hybrid spheroids with AG1522 feeder cells and HeLa test cells, a mixture of 3–5×106 AG1522 fibroblasts and 1/10th that of test cells for cell growth assays, or 1/100th that for cell radiosensitivity assays were incubated in a 100 mm bacteriological Petri dish (hydrophobic surface) with 10 ml of Eagle's Minimal Essential Medium with glutamine and non-essential amino acids, supplemented with 15% fetal bovine serum, streptomycin (100 µg/ml), and sodium bicarbonate (0.22% w/v) (complete MEM) (all Invitrogen Life Technologies Gibco Products, Carlsbad, CA; below noted as Gibco). The reason for the different average numbers of test cells used for the growth and radiosensitivity assays is that for the former, the time to see changes in spheroid volume and test cell number is shorter (the larger number of test cells providing a head start), while for the latter, having an average of about one test cell per spheroid makes it easier to perform multiplicity corrections and determine surviving fractions of test cells (if there is only one test cell in a spheroid, there will be no growth if that cell is sterilized or there will be growth if it survives).
For spheroids made with HeLa feeder cells and AG1522 test cells, the inverse of the above, the same number of fibroblast test cells was used for both viability and radiosensitivity assays. The test cell to feeder cell ratio used was 1/10, because the fibroblasts have about a 10% plating efficiency (a 10 times higher concentration for growth curves would mimic pure spheroids rather than pseudohybrid or hybrid spheroids; see below). In a variant of this procedure, spheroids made exclusively of fibroblasts, or of HeLa cells (pure spheroids), were also used. After agglomeration, spheroids of a desired size were selected by passing the entire harvest of cell agglomerates through a system of nylon sieves, and spheroids were then eluted from a selected sieve. We have found most satisfactory results with spheroids passing the 125 µm pore sieve, and arrested on the 88 µm pore sieve (with most of the harvested spheroids falling in the range of 100–110 µm in diameter and containing about 170 cells; this is almost twice the number previously reported for HeLa feeder cells grown with bromodeoxyuridine Citation[6], presumably due to the larger size of the analog-containing cells. We took the time of harvesting spheroids as zero time for our subsequent growth and survival measurements. Spheroids which were selected in the 250 µm diameter size range contained about 2 700 cells.
Principal features of the modified hybrid spheroid assay
The principal feature of our modified, improved, hybrid spheroid assay, as used in the present study, was to monitor the capacity of spheroids to grow beyond an arbitrary chosen size (equivalent to 10 divisions by the test cells), assumed to denote the ability of unlimited proliferation. The improved assay also prevents fibroblasts (potentially emanating from surgical specimens) from being scored in survival experiments. In order to obtain growth of neoplastic cells, hybrid (i.e., containing both fibroblastic feeder cells, as well as neoplastic HeLa test cells) spheroids were incubated over a 10–12 day period in bacteriological dishes, in which there is no cell attachment. Under such conditions, unirradiated AG1522 fibroblastic feeder cells fail to grow in floating spheroids; they could also be easily distinguished from HeLa cells on the basis of morphological appearance upon dispersal and replating. This feature enabled the system of floating hybrid spheroids to be used to monitor the radiation response of test HeLa cells, whereby growth beyond a certain size signifies survival, in a manner analogous to colony formation.
Dispersal of spheroids for cellular content studies
To determine the number of cells per spheroid, either in order to follow the growth pattern of spheroids, or for the purpose of measuring cellular multiplicity (see below), we trypsinized a known number of spheroids. This was accomplished by spinning spheroids in a centrifuge tube, aspirating supernatant medium, washing spheroids in 0.05% trypsin in EDTA (Gibco), and incubating them in 0.05% trypsin in EDTA for 10 min to disperse spheroids into individual cells. After dispersal of spheroids, the trypsin in the resultant cell suspension was inactivated by the addition of an equal volume of complete MEM, the cells counted in a hemacytometer, and an appropriate dilution plated in tissue culture Petri dishes or flasks for colony formation. From these data (cell numbers, colonies formed per spheroid) one obtains either the growth of spheroids as a function of time, or the average colony forming cell number per spheroid, at the time of irradiation, the so called cellular multiplicity (M). One can obtain the value of M in the original mixed cell suspension by allowing a sufficient growth period for constituent cells to form microcolonies. HeLa cells in microcolonies are easily distinguished from AG1522 cells in a culture dish in which both cell types are attached and grow.
X-irradiation and measurement of survival of spheroids
For irradiation studies, spheroids eluted from the selected sieve were distributed in equal aliquots into a series of 60 mm bacteriological Petri dishes, and irradiated with graded 2 Gy doses of 250 kVp X-rays from a Philips RT 250 therapy machine (15 mA, 2 mm Al inherent filtration, HVL 0.475 mm Cu, 50 cm FSD, dose rate 2.7 Gy/min). At the end of the incubation period allowed for scorable spheroid formation (10–12 days), spheroids over 250 µm in diameter were counted under a dissecting microscope, using an underlying transparent sheet with a grid to facilitate counting in the dish. These spheroids are now composed chiefly of HeLa test-cells; reflect growth from one or a few clonogen cells to a spheroid with a diameter more than double that of the original. Since the 250 µm diameter spheroids contained about 2 700 cells, the test cells in hybrid or pseudohybrid spheroids which had grown from 100 µm to 250 µm from their test cells, must have undergone about 10 to 11 doublings (divisions) per original clonogen. Survival of whole spheroids was determined by relating the number of such enlarged spheroids in the treated (irradiated) dish, to that in the control dish.
Survival curves were not measured for HeLa cells in pure spheroids because, with about 170 clonogens to start, very large doses (∼14 Gy) would have been required to reduce the surviving fraction to < 1 cell/spheroid and thus have no colony of surviving cells. Thus, at doses comparable to those used for the other spheroid conditions, almost all spheroids would have produced colonies. If all 170 cells were colongenic then only about 4 divisions would have been necessary to produce spheroids of 250 µm diameter, while the standard for survival of clonogenicity is 10 divisions in 10 days. Spheroids much larger than this could not be used since they have hypoxic centers and no longer behave the same as the 100–250 µm spheroids used in these studies.
Cellular multiplicity corrections of spheroid survival
To obtain single cell survival curves, fractional survival of whole spheroids was corrected for cellular multiplicity Citation[6], Citation[21], Citation[22] by using the equation1 where SF(s.c.) is the surviving fraction of single clonogens, SF(spher) is the surviving fraction of whole spheroids, and M is the average multiplicity of test cells in the control (unirradiated) series at the beginning of the irradiation procedure. In order to obtain M, an aliquot of same size control spheroids was dispersed by trypsinization at the time of irradiation and plated for colony formation. The cellular multiplicity, M, was determined as the ratio of test-cell colony numbers obtained from dispersed spheroids divided by the number of spheroids counted in unirradiated Petri dishes at the end of the time of trypsinization. Dispersal of spheroids served only this limited purpose. It should be noted that multiplicity denotes the clonogen content per spheroid at the time of irradiation, and not at the time of spheroid scoring at the end of the incubation period for spheroids served only this limited purpose. It should also be understood that the much smaller proportion of test cells for survival studies was necessitated by the method of multiplicity correction at the time of irradiation, which would be impractical or impossible with the higher input hybrid spheroids.
X-Ray survival curves of monolayer cells and cells from dispersed spheroids
Survival curves were obtained from dispersed cells plated in the conventional manner, by relating numbers of colonies formed 10–12 days following irradiation (or Plating Efficiencies) in the treated versus the control group; no multiplicity corrections were needed in these cases, since M was near unity.
Spheroid growth measurements
This was achieved by measuring spheroid diameters under a dissecting microscope using an ocular reticle, with the individual spheroid volume calculated as a function of time. To assure the round state of spheroids, we used conditions discouraging attachment, such as growth in bacteriological dishes or placing spheroids in 24 well plates coated with 0.5% agarose. In other situations, growth was measured by dispersal of spheroids by trypsinization, followed by counting cells in the dispersed cell suspension.
Statistical methods
The data points shown in all figures are the means with standard errors of the mean of the results of multiple flasks/dishes, normalized to unity within each experiment, prior to combining the data of replicate experiments. The standard error is the standard deviation divided by the square root of the number of observations, and is properly used when comparing mean values with each other rather than data points with the mean (for which the standard deviation should be used). The standard errors of ratios (as in plating efficiencies and surviving fractions) are determined using the chain rule for equipartition of variance, which takes into account the proper weight of the uncertainty contributions of both the numerator and the denominator Citation[23], Citation[24].
Results
Growth characteristics of spheroids composed of fibroblasts and/or HeLa cells
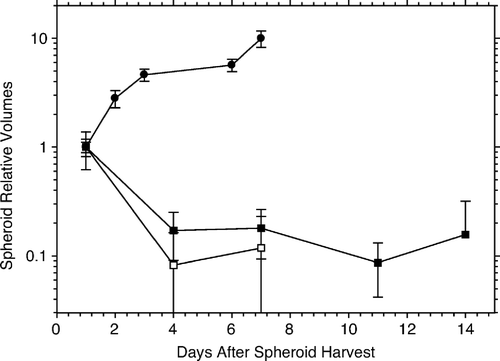
In order to verify the utility of AG1522 fibroblasts as (non-growing) feeder cells in hybrid spheroids, spheroids composed entirely of either live fibroblasts, or of supralethally irradiated fibroblasts, were incubated in suspension over a period of several days, and the growth (change in volume of spheroids) measured. The results in show that both types of spheroids shrank to about 10–20% of their original size within three days (day four after creation), after which, they remain the same reduced size for the rest of the period of observation (up to two weeks). In contrast, pure spheroids of HeLa cells grow, increasing in size (volume) by an order of magnitude over a six day period. The latter is consistent with the previously reported Gompertzian growth of these cells in spheroids Citation[10].
Figure 1. Effects of time held in suspension on spheroid volume. Relative volumes of spheroids composed exclusively of live (black squares), and supralethally irradiated (20 Gy, open squares) AG1522 fibroblasts are plotted for successive days after spheroid selection. Both types of spheroids shrank to about 10–20% of their original size within three days (day four after creation), after which, they remain the same reduced size for the rest of the period of observation (up to two weeks). In contrast, pure spheroids of HeLa cells (black circles) grow, increasing in size (volume) by an order of magnitude over a 6-day period. Data obtained from four experiments. Error bars in this and in other graphs are standard errors of the mean.
shows the increases in the relative number of clonogens with time in culture for HeLa cells in either attached or floating pure spheroids; i.e., spheroids composed of HeLa cells only. For comparison, a growth curve of HeLa cells in monolayer is also shown. Their respective doubling times are 15.7, 13.4, and 18.1 hours. In all three cases, the growth rates are similar.
Figure 2. Increases in the relative number of clonogens with time in culture. The top two curves with solid lines are for HeLa cells in either attached (open squares) or floating (open circles) pure spheroids. For comparison, a growth curve of HeLa cells in monolayer is also shown (open stars, dashed line). The next two curves represent the growth of HeLa cells in hybrid spheroids (with irradiated AG1522 fibroblast feeder cells; open triangles) and of unirradiated AG1522 fibroblasts grown in monolayer (black stars). The bottom curve (black circles) shows the initial stasis, and then growth, of AG1522 fibroblasts as test cells in attached hybrid spheroids with irradiated HeLa feeder cells; the final growth seen between days 3 and 4 is similar to that of the HeLa cells in floating or attached pure spheroids or in monolayer.
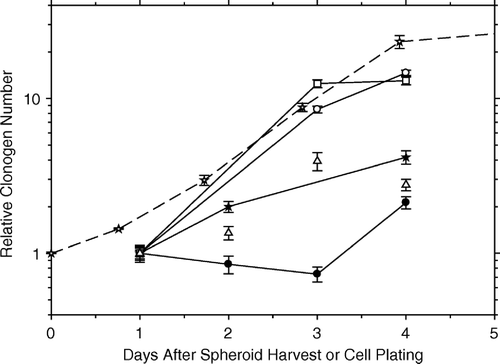
The next two sets of data (triangles and black stars) represent the growth of HeLa cells in hybrid spheroids (with irradiated AG1522 fibroblast feeder cells) and of unirradiated AG1522 fibroblasts grown in monolayer. Their common doubling time is 31.6 hours, about half the rate of the HeLa cells in pure spheroids or monolayer. For HeLa cells in hybrid spheroids with unirradiated fibroblast feeder cells (data not shown), we can deduce a HeLa cell doubling time of ≤24 hours. This is based on the survival curve data in and the survival criterion that the test cells undergo at least 10 doublings (>1024 cells) in 10 days with a dose-dependent delay in the start of growth. Such a doubling time is consistent with that observed for the other three conditions.
The bottom curve (black circles) represents the initial stasis and then growth of AG1522 fibroblasts as test cells in attached hybrid spheroids with irradiated HeLa feeder cells. The final growth seen between days three and four has a doubling time of 16.3 hours, similar to that of the HeLa cells in floating or attached pure spheroids or in monolayer.
shows the rapid loss of clonogenic AG1522 fibroblasts in floating hybrid spheroids with irradiated HeLa feeder cells. A similar curve is seen for AG1522 fibroblasts in floating pure spheroids. Within three days after harvest, only about 1–2 per cent of fibroblasts remain clonogenic; within four days, <3 per thousand remain clonogenic. In both cases, the spheroids were trypsinized before plating for colony formation.
Figure 3. The loss of clonogenic AG1522 fibroblasts maintained in spheroids. The bottom two curves show the rapid loss of clonogenic fibroblasts in: (1) floating hybrid spheroids with irradiated HeLa feeder cells (black circles) and (2) in floating pure spheroids (black squares). Within three days after harvest, only about 1–2 percent of fibroblasts remain clonogenic; within four days < 3 per 1 000 remain clonogenic. In both cases, the spheroids were trypsinized before plating for colony formation. The top curve (black triangles) shows the loss of clonogenicity with time for AG1522 fibroblasts in pseudohybrid spheroids trypsinized prior to plating for colonies.
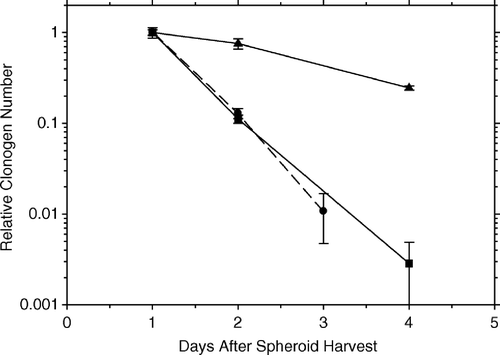
The top curve shows the loss of clonogenicity with time for AG1522 fibroblasts in pseudohybrid spheroids (unirradiated fibroblasts as test cells in a spheroid composed predominantly of irradiated fibroblast feeder cells) trypsinized prior to plating for colonies. Thus, irradiated fibroblast feeder cells in psuedohybrid spheroids are less effective in shutting down the clonogenicity of unirradiated test fibroblasts than are unirradiated fibroblasts in pure spheroids.
shows the slow loss of clonogenicity of AG1522 fibroblasts in pure and in pseudohybrid spheroids left intact for plating for colonies to grow out of each marked spheroid, i.e., NOT trypsinized. The top two curves show the results for pure spheroids. One curve is for attached spheroids, the locations of which have been marked, and clonogenicity was calculated from the fraction which did not form a colony Citation[6]. The other is for floating pure spheroids, which were plated at the indicated time and scored for how many colonies they had produced. There seems to be no difference between these two conditions for the pure fibroblast spheroids.
Figure 4. The slow loss of clonogenicity of AG1522 fibroblasts in pure and in pseudohybrid spheroids left intact for plating for colonies to grow out of each marked spheroid, i.e., NOT trypsinized. The top two curves show the results for pure spheroids. One curve is for attached spheroids, the positions of which had been marked, and clonogenicity was calculated from the fraction which did not form a colony (open square). The other is for floating pure spheroids (black square), which were plated at the indicated time and later scored for how many colonies they had produced. The lower two curves (solid lines) are for pseudohybrid fibroblast spheroids. The upper one (open circles) is for floating spheroids (as above), while the lower one (black circles) is for attached spheroids (as above). The curve for trypsinized floating pseudohybrid fibroblast spheroids is shown for comparison (from , black triangles, dashed line), and is essentially the same as that for attached pseudohybrid spheroids.
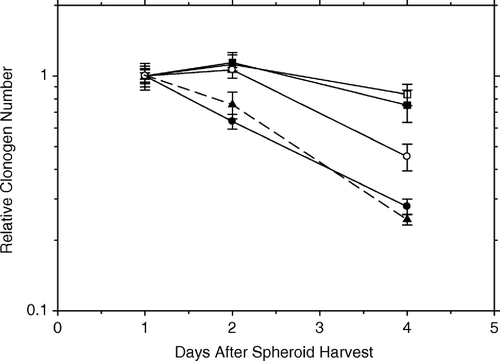
The lower two curves (solid lines) are for pseudohybrid fibroblast spheroids. The upper one of these is for floating spheroids (measured as in the paragraph above), while the lower one is for attached spheroids (as above). Thus, the presence of irradiated cells in the pseudohybrid spheroids brings clonogenicity down faster than in their absence (pure spheroids). The curve for trypsinized floating pseudohybrid fibroblast spheroids is shown for comparison (from , dashed line), and is essentially the same as that for attached pseudohybrid spheroids.
Thus, several factors affect the survival of test cells in spheroids (): (1) the type of test cells (fibroblast vs HeLa) and the prior treatment (irradiated vs unirradiated) of the feeder cells in the spheroids — where the proximity of different cells may affect their survival differently, (2) the attachment status of spheroids — floaters vs. attached, (3) dispersal of spheroids — trypsinization diminished survival of clonogens more in hybrid spheroids than in pure spheroids, and (4) trypsinization diminished clonogenicity much faster in floating spheroids than it did in attached spheroids.
The negative growth characteristics (shrinkage) of spheroids in suspension, composed of fibroblasts (), indicated that when hybrid spheroids grew, it was due to the proliferation of HeLa test cells, not of AG1522 fibroblast feeder cells. It follows for hybrid spheroids, that fibroblasts did not make a contribution to the growth of (hybrid) spheroids, as further evidenced from the following experiment. Upon termination of the spheroid growth experiment (ten days), resulting spheroids were trypsinized, plated in a tissue culture dish, and stained after a suitable growth period (ten additional days), as detailed in the Methods section. It was found that the only colonies (>99.9%) to grow from these dispersed hybrid spheroids were HeLa cells, even though the spheroids initially consisted predominantly of fibroblasts (data not shown).
Survival curves of X-irradiated hybrid spheroids maintained in suspension
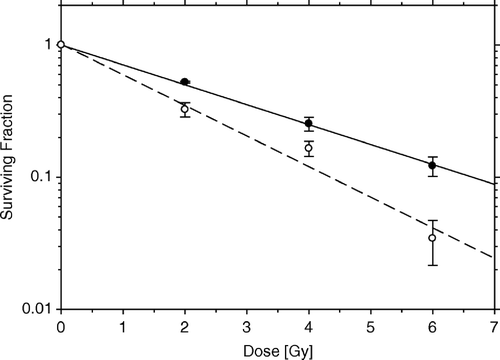
In the next experiment, two types of fibroblastic feeder cells were used, unirradiated and supralethally irradiated, encasing HeLa test cells. In order to obtain survival curves using the improved spheroid assay, hybrid spheroids with the two types of feeder cells were placed in 60 mm bacteriological Petri dishes (in which spheroids remain floating), X-irradiated in 2 Gy increments up to a dose of 6 Gy, and maintained over a period of 10–12 days. Survival was determined as described in Methods, scoring only spheroids >220 µm in diameter (which corresponds to 10 divisions by a HeLa test cell in the spheroid). The results of these measurements are presented in , after appropriate corrections for cellular multiplicity as described in Methods. Both survival curves were fitted to an exponential equation, even when continuous bending of the lower curve (with irradiated feeder cells) remained a possibility Citation[22], Citation[25]. The important observation is that the radiosensitivities of the two series were different, with the lower curve being steeper by a factor of 1.54±0.13. Evidently, lethally irradiated fibroblast feeder cells confer radiosensitivity to tumor test cells in spheroids, with the difference in survival between the two conditions (live vs. dead feeders) most pronounced at the highest dose used (6 Gy). [Unfortunately, it is not practical to deliver higher radiation doses in the system presented here in order to detect larger differences in survival levels, for reasons of both a diminishing statistical accuracy with higher doses (i.e., fewer scorable spheroids), and of problems associated with spheroids overloaded with test cells (when occasional breakup of spheroids could produce spurious carriers of clonogens).] Thus, the use of irradiated feeder cells carries with it the problem of a Bystander effect on the test cells, while the use of unirradiated fibroblasts as feeder cells, which will not proliferate when in 3-D contact as in spheroids, avoids this problem.
Figure 5. Survival curves of HeLa cells irradiated in hybrid spheroids, corrected for clonogen multiplicity. Hybrid spheroids were maintained in suspension over a period of 10–12 days before scoring surviving spheroids. Data obtained from four experiments in each series. Black circles: live fibroblast feeder cells, Do=2.95±0.08 Gy (α = 0.339±0.009 Gy−1). White circles: supralethally (20 Gy) irradiated fibroblast feeder cells, Do=1.92±0.15 Gy (α = 0.52±0.04 Gy−1). The ratio of D0 values = 1.54±0.13.
Survival of monolayer HeLa cells and cells from dispersed spheroids
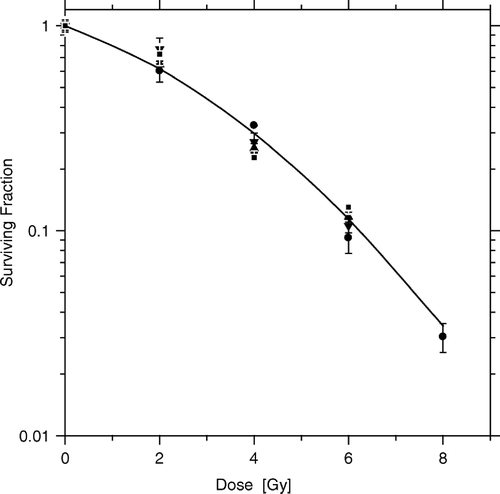
Unlike HeLa cells in floating spheroids, monolayer HeLa cells, obtained either from conventional stock cultures, or from cultures from dispersed hybrid spheroids, displayed identical radiation responses when tested for their colony forming ability 12 days after irradiation, irrespective of the kind of feeder cells present (). It appears therefore, that for the radiosensitizing effect by supralethally irradiated AG1522 fibroblasts, and a close 3-dimensional contact with test cells must take place during and for prolonged periods of time following irradiation.
Figure 6. Fibroblast feeder cells (dead or alive) do not affect the radiosensitivity of HeLa test cells in spheroids dispersed before irradiation. HeLa cell survival curves were obtained for cells dispersed, irradiated and then incubated for 10–12 days before scoring colonies and fitting the Linear-Quadratic equation to the data. Dispersed monolayer cultures with (squares) and without (circles) irradiated feeder cells, dispersed hybrid spheroids containing live fibroblast feeders (triangles), or dispersed hybrid spheroids containing supralethally irradiated fibroblast feeders (inverted triangles). Parameters from common curve, α = 0.18±0.03 Gy−1, β = 0.030±0.008 Gy−2.
Discussion
We were able to use unirradiated normal (diploid) AG1522 human fibroblasts as feeder cells after demonstrating that fibroblasts do not grow in spheroids suspended in nutrient medium (see ). This non-growth feature of normal fibroblasts is essential for scoring the response of neoplastic cells to cytocidal agents: only the latter will grow and respond to various treatments in our system in an easily detectable fashion. Undoubtedly, a suitable model is important; recall that the failure of a number of predictive tests for tumor control could be traced to their inability to differentiate between normal and neoplastic cells Citation[4], Citation[5], Citation[26], Citation[27].
Our system of floating hybrid spheroids is ideally suited for the measurement of radiation effects in HeLa cells, in a three-dimensional, in vivo-like environment. We applied the same principle of measuring survival as in the conventional method, relying on an appropriate increase in the size of proliferating spheroids after a 10–12 day incubation period (i.e., corresponding to ∼10 divisions by an initial HeLa clonogen). This was performed at a higher stringency than in surface attached colonies (10 vs. 5.5 divisions to form a 50 cell colony Citation[25]). As in conventional systems, cellular multiplicities were obtained for spheroids at the time of irradiation.
One of the interesting findings in the present report is seen in the series of floating spheroids with supralethally irradiated fibroblasts (). The introduction of irradiated fibroblastic feeder cells to HeLa test cells significantly reduces growth, when compared to HeLa cells growing free of feeder cells (). In addition, there seems to be a direct effect of irradiated feeders on survival, as seen in . This strongly indicates a kind of Bystander Effect, hinted at previously in conjunction with incremental doses in a fractionated radiation regimen Citation[9]. Apparently, not all incremental doses have the same effect; this effect increases with the accumulation of irradiated neighbors. Since a post-irradiation, possibly long term, cell contact is required for this type of radiation response modification; a Bystander Effect (BE) appears to be involved.
The mechanism of the BE remains to be determined, but several different mechanisms have been proposed Citation[12–20]. Some involve plasma-membrane gap-junctional phenomena Citation[15–17], Citation[19], Citation[20], and others act through diffuse vectors Citation[14], Citation[18]. Not all radiation doses during a multi-fraction treatment have the same efficacy: as we have shown previously Citation[9], the BE may critically increase with accumulated dose and fraction number, with increased duration of cell contact, and with increasing numbers of sterilized neighbors. Thus, the mechanism of action of genotoxic agents may not be as clear-cut as is generally assumed, and survival may be modified at several time-points after the initial damage.
One may speculate that the plating efficiency (PE) may be influenced by autocrine and/or paracrine mechanisms, as found in tumors in situ Citation[28–30]. The same interactions may occur in spheroids too. It is our hope that before long we may be able to solve this problem. It is tempting to contemplate that beneficial modifications of clinical practices may be achieved through the predictive power of our Hybrid Spheroid assay, after necessary modifications are made to make the system compatible with fractionated schedules extending over longer periods of time. In this fashion, protocols which presently are either difficult to compare, or were otherwise incompatible, could be tested in parallel and the best variant selected for patient treatment. It is envisaged, that when the system becomes fully operational, it will be applied to surgical or biopsy tumor samples and the best protocol selected in a clinical setting.
References
- Salmon SE, Hamburger AW. Primary bioassay of human tumor stem cells. Science 1977; 197: 461–3
- Courtney VD, Selby PJ, Smith JE, Mills J, Peckham MJ. Growth of human tumor cell colonies from biopsies using two soft-agar techniques. Brit J Cancer 1978; 38: 77–81
- West CML, Davidson SE. The independence of intrinsic radiosensitivity as a prognostic factor for patient response to radiotherapy for carcinoma of the cervix. Br J Cancer 1997; 76: 1184–90
- Lawton PA, Hodgkiss RJ, Eyden BP, Joiner MC. Growth of fibroblasts as a potential confounding factor in soft agar clonogenic assays for tumor cell radiosensitivity. Radiother Oncol 1994; 32: 218–25
- Stausbol-Gron B, Nielsen OS, Moller Bentzen S, Overgaard J. Selective assessment of in vitro radiosensitivity of tumor cells and fibroblasts from single tumor biopsies using immunocytochemical identification of colonies in the soft agar clonogenic assay. Radiother Oncol 1995; 37: 87–99
- Djordjevic B, Lange CS. Clonogenicity of mammalian cells in hybrid spheroids: A new assay method. Radiat Environ Biophys 1990; 29: 31–46
- Lange CS, Djordjevic B, Brock WA. The hybrid spheroid clonogenic assay for the intrinsic radio- and chemo-sensitivities of human tumors. Int J Radiat Oncol Biol Phys 1992; 24: 511–8
- Djordjevic B, Lange CS. Measurement of sensitivity to adriamycin in hybrid spheroids. Cancer Invest 1991; 9: 505–12
- Djordjevic B, Lange CS, Rotman MZ, Torres C, Zheng Z. Increasing radiosensitivity in the course of fractionated×irradiation: The effect of contact with dead and dying cells. Radiat Res 1998; 150: 275–82
- Djordjevic B, Lange CS. Hybrid spheroids as a tool for prediction of radiosensitivity in tumor therapy. Indian J Exper Biol 2004; 42: 443–7
- Djordjevic B. Bystander effect: A concept in need of clarification. BioEssays 2000; 22: 286–90
- Azzam EI, Little JB. The radiation-induced bystander effect: Evidence and significance. Hum Exp Toxicol 2004; 23: 61–5
- Iyer R, Lehnert BE. Effects of ionizing radiation in targeted and nontargeted cells. Arch Biochem Biophys 2000; 376: 14–25
- Seymour CB, Mothersill C. Relative contribution of bystander and targeted cell killing to the low-dose region of the radiation dose-response curve. Radiat Res 2000; 153: 508–11
- Ishii K, Watanabe M. Participation of the gap-junctional cell communication on the adaptive response in human cells induced by low dose of X-rays. Int J Radiat Biol 1996; 69: 291–9
- Bishayee A, Rao DV, Howell RW. Evidence for pronounced bystander effects caused by nonuniform distributions of radioactivity using a novel three-dimensional tissue culture model. Radiat Res 1999; 152: 88–97
- Azzam EI, De Toledo SM, Gooding T, Little J. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Radiat Res 1998; 150: 497–504
- Hill HZ, Trizna Z, Hill GJ. A radiation resistance factor in cultured Cloudman mouse melanoma cells. Radiat Res 1992; 129: 43–7
- Iyer R, Lehnert BE. Factors underlying the cell growth-related bystander response to α particles. Cancer Res 2000; 60: 1290–8
- Prise KM, Belyakov OV, Folkard M, Michael BD. Studies of bystander effects in human fibroblasts using a charged particle microbeam. Int J Radiat Biol 1998; 74: 793–8
- Sinclair WK, Morton RA. Recovery following x-irradiation of synchronized Chinese hamster cells. Nature 1964; 203: 247–50
- Elkind MM, Whitmore GF. In: The Radiobiology of Cultured Mammalian Cells. New York: Gordon and Breach; 1967. p 69–74.
- Hald A. In: Statistical Theory with Engineering Applications. New York: John Wiley & Sons, Inc: 1952. See sections 5.7–5.9 and 5.16–5.17, esp. Eq.5.17.7.
- Lange CS, Liberman DF, Clark RW, Ferguson P. The organization and repair of DNA in the mammalian chromosome. I. Calibration procedures and errors in the determination of the molecular weight of a native DNA. Biopolymers 1977;1063–81.
- Puck TT, Markus PI. Action of X-rays on mammalian cells. J Exp Med 1956; 103: 653–66
- Brock WA, Brown DW, Goepfert H, Peters LJ. In vitro radiosensitivity of tumor cells and local tumor control by radiotherapy. In: Dewey WC, Edington M, Fry RJM, Hall EJ, Whitmore GF, editors. Radiation Research: A Twentieth Century Perspective. San Diego: Academic Press; 1992. p 696–9.
- Parkins CS, Steel GG. Growth and radiosensitivity testing of human tumor cells using the adhesive tumor cell culture system. Br J Cancer 1990; 62: 935–41
- Edelman M, Gamara F, Kemp da Silva A, Hornung V, Castro M, Passlick B, et al. Cell cycle effects of radiation on human bronchial epithelium and lung carcinoma cells in monolayer cultures and a three-dimensional co-culture system. Radiat Res 2005; 164: 391–9
- Muerkoster S, Wegehenkel K, Arlt A, Witt M, Bence S, Kruse M, et al. Tumor stroma interactions induce chemoresistance in pancreatic ductal carcinoma cells involving increased secretion and paracrine effects of nitric oxide and interlukin-1β. Cancer Res 2004; 64: 1331–7
- Sung SY, Chung LW. Prostate tumor–stroma interaction: Molecular mechanisms and opportunities for molecular targeting. Differentiation 2002; 70: 506–21
