Abstract
Time trends in the incidence of glioma may reflect changes in the prevalence of environmental risk factors for glioma. We therefore investigated trends in the incidence of childhood and adult glioma in the Netherlands from 1989 to 2003. We used population-based incidence data from the Netherlands Cancer Registry. We calculated European standardised incidence rates for glioma, and stratified for age, gender and glioma subgroups. Changes in the incidence were estimated by calculating the Estimated Annual Percentage Change. Similar to other countries, the overall incidence of glioma was fairly stable in the Netherlands during the period 1989 to 2003, for both children and adults. In adult astrocytic glioma, a significantly increasing incidence of high-grade astrocytoma was balanced by simultaneous decreases of low-grade astrocytoma, astrocytoma with unknown malignancy grade and glioma of uncertain histology. Most of these time trends can be explained by improving detection and diagnostic precision. Stable incidence rates of adult and childhood glioma suggest that no major changes in environmental risk factors have occurred, which influenced the incidence of glioma in the studied period.
Approximately 85% of all histologically verified primary cancers of the central nervous system (CNS) are gliomas Citation[1], malignant brain tumours of neuroepithelial origin Citation[2]. Although gliomas are the most common type of primary brain tumours they are still relatively rare. In the Netherlands, world-standardised incidence rates of glioma are 6.5 per 100 000 person-years for males and 4.4 for females with a male/female ratio of 1.5:1.8 Citation[1]. The male predominance, which is consistently observed in different countries, remains yet unexplained Citation[3]. Several authors reported that the incidence of glioma is stable or slightly decreasing in almost every age group since the late 1980s Citation[4–7]. In the most recent years, an increase in incidence is particularly apparent among the elderly in Western countries Citation[4–7], which is probably the result of better detection.
Many environmental risk factors for glioma have been studied. Only for ionising radiation has an aetiological role been established, and no other major risk factors have been identified Citation[3]. Temporal trends can therefore not be explained on the basis of known environmental risk factors. However, the monitoring of time trends and the early detection of changing patterns in the incidence of glioma may reveal changes in the prevalence of environmental risk factors and provide new hypotheses for glioma aetiology. For example, attempts have been made to link trends in the incidence of glioma to the increasing prevalence of mobile phone users Citation[6].
In this study we investigated trends in the incidence of childhood and adult glioma and of glioma subgroups in the Netherlands, in the period 1989 to 2003. We compared these with the observed trends in Europe and the United States.
Material and methods
All epidemiological data were obtained from the Netherlands Cancer Registry (NCR) from the Association of Comprehensive Cancer Centres (ACCC). This population-based nationwide cancer registry records data of all malignant neoplasms. Information is available on date of incidence, histology, topography, invasiveness, grade, stage and basis of diagnosis Citation[8]. Histology is coded according to the International Classification of Diseases for Oncology (ICD-O), first edition (until 1992), second edition (1993–2000) and third edition (since 2001) Citation[9–11]. Pathological diagnoses were derived from the nationwide network and registry of histo- and cytopathology (PALGA) containing data of all histological, cytological and autopsy examinations in the Netherlands. Diagnoses were also derived from the Dutch Medical Register (LMR) that comprises data from all hospital admissions in the Netherlands, including discharge diagnoses. Since 1989, all hospitals in the Netherlands are linked to one of the regional cancer registries that submit their data to the NCR. During the study period there were no major changes in health care facilities in the Netherlands, nor major improvements in diagnostic methods. Neurosurgery, neuroradiology and neuropathology are concentrated in large centres with close links to radiotherapy departments. Computed tomography (CT), magnetic resonance imaging (MRI) and stereotactic biopsies were introduced and already widely available before 1989 Citation[1].
Six histological groups were defined for analysis following a cluster scheme for CNS tumours Citation[12]. Astrocytic gliomas were divided in low-grade tumours (ICD-O grade 1, 2), high-grade tumours (ICD-O grade 3, 4), and tumours with unspecified malignancy grade (ICD-O grade 9). Oligodendrogliomas and ependymal gliomas were analysed without considering the malignancy grade, as numbers in these groups were considered too small for subgroup analysis. A sixth group (glioma with uncertain histology) consisted of clinically diagnosed CNS tumours without histopathological confirmation. We also classified ‘glioma not otherwise specified’ (glioma NOS) into this group. ICD-O codes for the six groups are given in . Tumours with codes 9383 (subependymal glioma), 9384 (subependymal giant cell astrocytoma), 9393 (papillary ependymoma) and 9394 (myxopapillary ependymoma) were only registered since 1999 and were therefore excluded from the analyses.
Table I. International Classification of Diseases for Oncology (ICD-O) codes for the analysed glioma groups.
Annual incidence rates were calculated per 100 000 person-years, using the average annual population as obtained from Statistics Netherlands. Rates were age adjusted by standardisation to the European standard population (European Standardised Rates, ESR) and calculated as 3-year moving averages. Trends were estimated by calculating the Estimated Annual Percentage Change (EAPC). A regression line was fitted to the natural logarithm of the rates using calendar year as a regressor variable, i.e. y = mx + b where y = ln(rate) and x = calendar year. Then the EAPC = 100*(em-1). Testing the hypothesis that the EAPC is equal to zero is equivalent to testing the hypothesis that the slope of the line in the above equation is equal to zero. The latter hypothesis was tested two-sided using the t-distribution of m/SEm, while the number of degrees of freedom equals the number of calendar years minus two. The standard error of m, SEm, was obtained from the fit of the regression line Citation[13]. This calculation assumes that the rates increase or decrease at a constant rate over the entire period.
Incidence rates and trends were calculated for males and females separately. Age was stratified into four groups: 0–14 years, 15–44 years, 45–64 years and 65+ years. Analyses were performed using Statistical Analysis System v8.2 and Microsoft Excel 2003. All data were analysed respecting the privacy legislation that applies in the Netherlands.
Results
All glioma
Between 1989 and 2003, 9290 newly diagnosed gliomas were registered in the Netherlands, 5402 in males and 3888 in females. An additional 1312 males and 1210 females were registered with a clinically diagnosed tumour or a glioma NOS. The numbers of registered glioma patients per diagnosis group and age category are shown in . Age-adjusted incidence rates for all glioma combined and including gliomas of uncertain histology were stable between 1989 and 2003, for males (EAPC −0.2%, p = 0.57) and females (EAPC 0.3%, p = 0.49) (). Within age categories, small and non-significant trends in the incidence of all glioma could be seen (). Incidence rates in the elderly (aged 65+ years) showed a small increase (EAPC 0.7%, p = 0.26 for males and EAPC 1.4%, p = 0.03 for females), whereas in children, incidence rates were slightly decreasing (EAPC −1.0%, p = 0.34 for boys and EAPC −1.3%, p = 0.26 for girls).
Figure 1. Incidence of all glioma according to gender, European Standardised Rates, 3-year moving average, with Estimated Annual Percentage Change (EAPC).
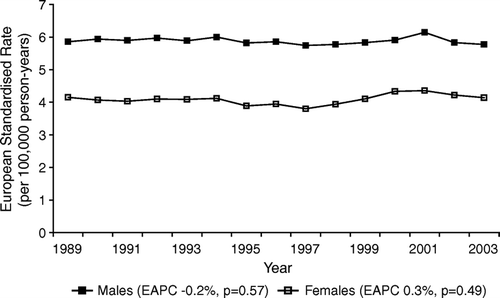
Figure 2. Incidence of all glioma according to gender and age, European Standardised Rates, 3-year moving average, with Estimated Annual Percentage Change (EAPC).
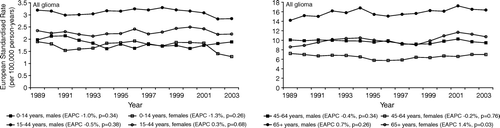
Table II. Total number of registered glioma patients in the Netherlands during the period 1989–2003, for diagnosis groups and age categories.
Astrocytoma
In an all ages analysis, age-adjusted incidence rates of high-grade astrocytoma increased significantly for males (EAPC 1.5%, p = 0.005), accompanied by a significantly decreasing trend in low-grade astrocytoma (EAPC −1.9%, p = 0.02), in astrocytoma with unknown malignancy grade (EAPC −12.5%, p < 0.001) and in glioma of uncertain histology (EAPC −1.7%, p = 0.06) (). The incidence of astrocytic glioma in females showed a similar pattern although the accompanying decrease in low-grade astrocytoma was less pronounced (EAPC −0.8%, p = 0.46) (). Within age categories, a significantly rising incidence of high-grade astrocytoma was seen in young adults and elderly, but not in adults aged 45–64 years (). The incidence of low-grade astrocytoma showed no consistent pattern. A marked decrease was seen in adult astrocytoma with unknown malignancy grade (EAPC −7.0% to −16%). The incidence of glioma of uncertain histology showed a modest and mostly borderline significant decreasing trend in all adult age categories ().
Figure 3. Incidence of astrocytic glioma and glioma of uncertain histology according to gender, European Standardised Rates, 3-year moving average, with Estimated Annual Percentage Change (EAPC).
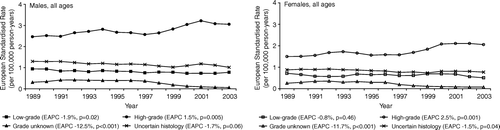
Figure 4. Incidence of astrocytic glioma and glioma of uncertain histology according to gender and age, European Standardised Rates, 3-year moving average, with Estimated Annual Percentage Change (EAPC).
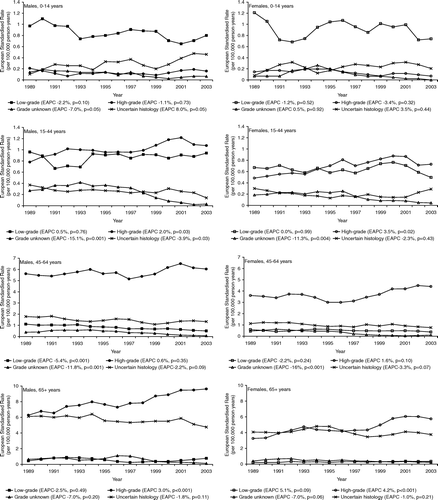
In children aged 0–14 years, decreasing incidence was seen for all astrocytoma groups (). This was balanced by glioma of uncertain histology (EAPC 8.0%, p = 0.05 for boys and EAPC 3.5%, p = 0.44 for girls). None of these trends were statistically significant.
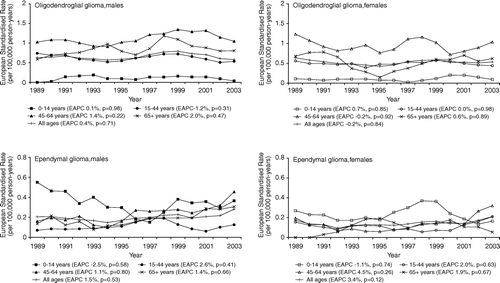
Oligodendroglioma and ependymoma
The overall incidence of oligodendroglioma was stable for males (EAPC 0.4%, p = 0.71) and females (EAPC −0.2%, p = 0.84) (). Also within age categories, no clear trends in the incidence could be seen. The overall incidence of ependymoma showed a small but non-significant increase between 1989 and 2003 (EAPC 1.5%, p = 0.53 for males and EAPC 3.4%, p = 0.12 for females). In general, incidence rates were decreasing in children and increasing in adults. None of these trends however were statistically significant and rates varied widely over the studied period, which is probably caused by the small numbers in each age category ().
Male/female ratios
The male/female ratio remained constant with an average of 1.44 for all glioma, 1.41 for ependymal glioma, 1.25 for oligodendroglial glioma and 1.49 for astrocytic glioma (including low-grade astrocytoma, high-grade astrocytoma and astrocytoma with unknown malignancy grade) (not shown). Within age groups of all glioma and astrocytic glioma, the sex-ratios were fairly constant as well. Other analyses within age groups were not performed because of small numbers of cases.
Discussion
In general, minor trends in the incidence of glioma were observed in the Netherlands during the period 1989 to 2003. Age-adjusted incidence rates were stable for all glioma combined and for oligodendroglioma/mixed glioma. In adult astrocytic glioma, a significant increase in high-grade astrocytoma was accompanied by a simultaneous decrease in low-grade astrocytoma, astrocytoma with unknown malignancy grade and glioma of uncertain histology. The incidence of astrocytoma and ependymoma in children showed a decreasing trend, which was accompanied by an increasing incidence of gliomas of uncertain histology. The overall incidence of glioma in children was stable.
Methodological considerations
The NCR is characterised by high quality incidence data and near complete ascertainment with less than 2% underregistration Citation[14]. Completeness of registration is achieved by combining data from different sources, including PALGA and the LMR. In the studied period, access to medical care in the Netherlands was good and less than 1% of the population was uninsured Citation[8]. We also verified registration procedures for different areas in the Netherlands, which did not change since 1989. The NCR does not have access to death records as a data source. However, patients with a clinically diagnosed tumour will be reported to the LMR. Since the cancer registry has access to the LMR, we do not think that the unavailability of death records is a relevant source of bias. In some of the studied glioma subgroups the numbers of cases are small and trend estimation may be difficult. We therefore evaluated the general pattern of incidence trends.
Evaluation of incidence rates over time is complicated by changes in histopathological classifications Citation[12]. We used a uniform cluster scheme for ICD-O coded primary CNS tumours Citation[12]. In this system, primary CNS tumours are clustered as clinically relevant entities, based on the second edition of the World Health Organisation (WHO) classification of CNS tumours Citation[15]. In our opinion, no essential changes in pathological practice have occurred in the studied period that could have greatly influenced the incidence statistics. Changes in classifications may have occurred within the analysed groups, most likely in the low-grade astrocytoma group. Pilocytic astrocytomas, for example, are characterised by a very distinct histology and prognosis Citation[16]. The recognition and classification of these tumours is improving Citation[16], resulting in more pilocytic astrocytomas that were separately classified from other low-grade astrocytomas (data not shown). However, these shifts have occurred within the group of low-grade astrocytoma and it is therefore unlikely that this has influenced incidence rates in this study. It is possible that some of the clinically diagnosed gliomas without histopathological confirmation will in fact be a different type of tumour. This may particularly be a problem for the primary central nervous system lymphomas (PCNSL) which can be difficult to distinguish from glioma, based on neuroimaging only. The world standardised incidence of PCNSL however is less than 0.3 per 100 000 person-years Citation[17]. This problem would also potentially apply to solitary cerebral metastases from undiagnosed primary sites. Cerebral metastases usually show characteristics that are different from gliomas: metastases are mostly well circumscribed and show a different pattern of deposition in the brain.
Comparison with other countries
The incidence of glioma has been remarkably stable over the past decades in Europe and the United States. A temporal increase in incidence has been observed in the late 1970s and the early 1980s, but incidence was stabilising or slightly decreasing in almost every age group in more recent years Citation[4–7]. This suggests that these increases in incidence were artifactual, probably owing to the introduction of CT and MRI in this period Citation[7], Citation[18]. In the most recent years, increasing incidence of glioma was particularly apparent among the elderly Citation[4–7]. This was probably the result of increasing efforts to obtain histopathological diagnosis. A simultaneous decrease in clinically diagnosed tumours and in glioma NOS is compatible with this view Citation[6], Citation[19]. In addition, physicians treating elderly patients are increasingly willing to use more diagnostics in the elderly, revealing malignancies that otherwise would have gone undetected Citation[5], Citation[7], Citation[20]. An observed increase in high-grade gliomas and simultaneous decrease in low-grade tumours was probably caused by improving techniques in neuroimaging and neurosurgery resulting in less sampling error and better characterisation of malignancy grade Citation[6], Citation[7], Citation[20].
In the Netherlands, similar patterns were seen as those described in the literature. Not only was the incidence of glioma stable, also similarly occurring shifts in astrocytoma subgroups suggests improving detection and diagnostic precision. The small increase in incidence of glioma in patients aged over 65 years is most likely the result of a changing attitude towards the elderly in which diagnosis and therapy were more persistently pursued Citation[5], Citation[7], Citation[21]. This assumption is supported by a decreasing incidence of glioma with unknown malignancy grade and clinically diagnosed tumours. However, these trends were most marked in adults aged 15–64 years, suggesting that patients in this age category may benefit most from new techniques. The observed male/female ratios also correspond with figures previously reported in the literature.
Some authors argue that not all of the observed increases in incidence can be explained by better detection Citation[22–25]. For example, increasing trends in the incidence of childhood astrocytoma were largely confined to girls Citation[23], and an observed increase in the incidence of ependymoma could not be explained by diagnostic practice Citation[19]. However, good explanations for these trends cannot be offered. In the present study, we noticed a decreasing instead of increasing trend of childhood astrocytoma. Ependymoma incidence possibly showed a modest increase in adults, but this increase was not statistically significant and numbers were small ().
In conclusion, the incidence of glioma in the Netherlands shows only minor trends between 1989 and 2003. Most variation can be explained by better detection, improving diagnostic precision and changing attitude towards the elderly. Stable incidence rates suggest that no major changes in environmental risk factors have occurred which influenced the incidence of glioma in the studied period.
We thank the Association of Comprehensive Cancer Centres in the Netherlands for the data used for this study. Dr. O. Visser is kindly acknowledged for his helpful comments on an earlier version of the manuscript. The Dutch Cancer Society provided financial support, grant EUR 2001-2454.
References
- van der Sanden GA, Schouten LJ, van Dijck JA, van Andel JP, Coebergh J. Incidence of primary central nervous system cancers in South and East Netherlands in 1989–1994. Neuroepidemiology 1998; 17: 247–57
- Kleihues P, Cavenee WK, editors. Pathology & genetics of tumours of the central nervous system. World Health Organization classification of tumours. LyonFrance: IARC press; 2000.
- Wrensch M, Minn Y, Chew T, Bondy M, Berger MS. Epidemiology of primary brain tumors: Current concepts and review of the literature. Neuro-oncol 2002; 4: 278–99
- Ahsan H, Neugut AI, Bruce JN. Trends in incidence of primary malignant brain tumors in USA, 1981–1990. Int J Epidemiol 1995; 24: 1078–85
- Hess KR, Broglio KR, Bondy ML. Adult glioma incidence trends in the United States, 1977–2000. Cancer 2004; 101: 2293–9
- Lonn S, Klaeboe L, Hall P, Mathiesen T, Auvinen A, Christensen HC, et al. Incidence trends of adult primary intracerebral tumors in four Nordic countries. Int J Cancer 2004; 108: 450–5
- Legler JM, Ries LA, Smith MA, Warren JL, Heineman EF, Kaplan RS, et al. Brain and other central nervous system cancers: Recent trends in incidence and mortality. J Natl Cancer Inst 1999; 91: 1382–90
- van Dijck JAAM, Coebergh JWW, Siesling S, Visser O. Trends of cancer in the Netherlands 1989–1998. Utrecht: Vereniging van Integrale Kankercentra; 2002.
- Percy C, van Holten V, Muir C. International Classification of Diseases for Oncology. 2nd ed. World Health Organization, Geneva 1990
- International Classification of Diseases for Oncology. 1st edition. Geneva: World Health Organization; 1976.
- International Classification of Diseases for Oncology (ICD-O-3). 3rd edition. Geneva: World Health Organization; 2000.
- van der Sanden GA, Wesseling P, Schouten LJ, Teepen HL, Coebergh J. A uniform histological cluster scheme for ICD-O-coded primary central nervous system tumors. Neuroepidemiology 1998; 17: 233–46
- Kleinbaum DG, Kupper LL, Muller KE. Applied regression analysis and other multivariable methods. PWS-KENT Publishing Company, Boston 1988
- Schouten LJ, Hoppener P, van den Brandt PA, Knottnerus JA, Jager JJ. Completeness of cancer registration in Limburg, The Netherlands. Int J Epidemiol 1993; 22: 369–76
- Kleihues P, Burger PC, Scheithauer BW. The new WHO classification of brain tumours. Brain Pathol 1993; 3: 255–68
- Perry A. Pathology of low-grade gliomas: An update of emerging concepts. Neuro-oncol 2003; 5: 168–78
- van der Sanden GA, Schouten LJ, van Dijck JA, van Andel JP, van der Maazen RW, Coebergh JW. Primary central nervous system lymphomas: Incidence and survival in the Southern and Eastern Netherlands. Cancer 2002; 94: 1548–56
- Helseth A. The incidence of primary central nervous system neoplasms before and after computerized tomography availability. J Neurosurg 1995; 83: 999–1003
- Jukich PJ, McCarthy BJ, Surawicz TS, Freels S, Davis FG. Trends in incidence of primary brain tumors in the United States, 1985–1994. Neuro-oncol 2001; 3: 141–51
- Modan B, Wagener DK, Feldman JJ, Rosenberg HM, Feinleib M. Increased mortality from brain tumors: A combined outcome of diagnostic technology and change of attitude toward the elderly. Am J Epidemiol 1992; 135: 1349–57
- Marijnen CA, van den Berg SM, van Duinen SG, Voormolen JH, Noordijk EM. Radiotherapy is effective in patients with glioblastoma multiforme with a limited prognosis and in patients above 70 years of age: A retrospective single institution analysis. Radiother Oncol 2005; 75: 210–6
- Fleury A, Menegoz F, Grosclaude P, Daures JP, Henry-Amar M, Raverdy N, et al. Descriptive epidemiology of cerebral gliomas in France. Cancer 1997; 79: 1195–202
- Hjalmars U, Kulldorff M, Wahlqvist Y, Lannering B. Increased incidence rates but no space-time clustering of childhood astrocytoma in Sweden, 1973–1992: A population-based study of pediatric brain tumors. Cancer 1999; 85: 2077–90
- Polednak AP. Interpretation of secular increases in incidence rates for primary brain cancer in Connecticut adults, 1965–1988. Neuroepidemiology 1996; 15: 51–6
- Werner MH, Phuphanich S, Lyman GH. The increasing incidence of malignant gliomas and primary central nervous system lymphoma in the elderly. Cancer 1995; 76: 1634–42