Abstract
High dose methotrexate (HDMTX) with folinic acid rescue is widely used to treat osteosarcoma, which predominantly afflicts children; the study investigated HDMTX pharmacokinetics (pk) in adult subjects in neoadjuvant/adjuvant settings. Twenty five patients with advanced osteosarcoma (11 females – 14 males, median age 26.0 years) were treated by 12 g/m2 HDMTX 4 hour iv infusion (64 total courses, range 1 – 7 courses). Pk was determined by non-compartmental analysis and population pk modeling. Median (range) bioavailability pk parameters were: Cmax (maximum MTX concentration) 1149.5 µM (692 – 2 200), AUCtot (total area under curve) 6 955.1 µmol*h/l (3 477 – 12 681). Cmax>1 000 µM gave increased histological responses (p < 0.05). Six covariates (height-weight-hemoglobin-AST-ALT-creatinine) were found to influence MTX volume of distribution (V) and elimination rate constant (Kel). Toxicity was mild: only two reversible G4 events were observed, related to AUCtot >12 000 µmol*h/l (p < 0.001). HDMTX pk and interpatient variability in adults are comparable to those in children. No correlation between Cmax/AUCtot and subject age/sex was found, even in the population pk model. The excretion mechanism is not affected by sex/age differences. HDMTX can safely be administered to adults: as in younger patients, a good clinical response can be predicted by Cmax, while severe toxicity depends on highest AUCtot values.
Osteosarcoma is a rare disease generally affecting children and adolescents; many studies have addressed the efficacy, clinical and pathological response and pharmacokinetics of methotrexate (MTX) in these patients. High dose MTX (HDMTX) intravenous infusions followed by folinic acid (LV) rescue are frequently used in the treatment of osteosarcoma and response is generally correlated with MTX dose and end-of-infusion concentration (Cmax, maximum MTX concentration), whereas severe toxicity depends on highest MTX AUCtot (total area under concentration-time curve) values.
The rationale for the use of HDMTX is to overcome all known mechanisms of resistance to MTX by increasing cell uptake, tumor cell polyglutamate formation, MTX intracellular residence and LV rescue selectivity. Some resistance mechanisms to MTX are: a) decreased drug uptake, b) defective polyglutamylation, c) expanded dihydrofolate reductase (DHFR) activity, d) defective decrease in MTX binding to DHFR Citation[1]. HDMTX can theoretically overcome these mechanisms, increasing plasma drug concentration and duration of exposure. LV rescue from the antimetabolic effects of HDMTX opens a therapeutic window, protecting normal cells from the toxic effects while tumor cells remain unprotected Citation[1–4].
Many different HDMTX regimens have been studied in osteosarcoma treatment, in all of which MTX is combined with other drugs, such as doxorubicin (ADM), cisplatin (DDP) and ifosfamide (IFO). MTX optimal dosage per cycle fluctuates widely, ranging from 8 to 24 g/m2, the usual dose being 12 – 15 g/m2Citation[5]. HDMTX (200–500 mg/kg] with LV rescue was first used by Djerassi in 1967. In1972 Jaffe introduced it for osteosarcoma therapy, followed two years later by Rosen and Frei Citation[6–9].
HDMTX chemotherapy requires individual monitoring, due to high MTX dosage, large interpatient pk variability and the demonstrated relationship between safety, efficacy and serum drug concentrations. Pharmacokinetic monitoring can significantly help patient management, since the efficacy of HDMTX has been shown to depend on a Cmax level of 700 – 1 000 µM (serum levels observed at the end of 6 and 4 hour iv infusion, respectively), in terms both of histological response and of overall and disease-free patient survival Citation[3], Citation[10–12].
Osteogenic sarcoma typically occurs in children or adolescents, so that few data are available relating to HDMTX pk in adult patients Citation[13,14]; information concerning HDMTX disposition and excretion is limited and unconclusive for this group. There is some indication that children excrete MTX more effectively than adults, and on this basis most protocols exclude patients aged above 40 from HDMTX treatment. The present study investigates pk behavior in HDMTX and clinical response of adult patients to this regimen in neoadjuvant and adjuvant settings.
Material and methods
Clinical protocols
Since 1990, we have been cooperating with the Istituti Ortopedici Rizzoli (IOR) Bologna-Italy in treating patients afflicted with primary osteosarcoma, who are undergoing three different IOR protocols: IOR-OS 5 (1995–1997), ISG/SSG I (1998–2001) and OS/ISG1 (2002 – today) Citation[5,10,15]. All protocols comprised HDMTX, ADM, DDP and IFO but, depending on response to preoperative chemotherapy (rated as good/complete: >90% or poor/incomplete: <90%), patients were given different postoperative cycles.
Thirty-one patients were treated at our center by one of these three protocols. The specific nature of our institution, which is a clinical oncology division, meant that we exclusively treated adult patients. MTX was administered at different dosages in the three protocols (from 8 to 20 g/m2) by 4 or 6 h iv infusions, with 0.5 h pre-hydration. LV rescue started 24 hours after the beginning of HDMTX iv infusion (LV 8 mg/m2 q 6 h for 3 days). Post-hydration was protracted for 48 hours or more, in case of delayed MTX elimination. No protocol contemplated dose reduction.
Pharmacokinetic protocol
Twenty-five patients (of the initial 31) were enrolled in the pharmacokinetic study, whereby HDMTX was administered at 12 g/m2 by 4 h iv infusion, (standardized dose and infusion time); all other study characteristics were the same as those described above. Blood samples for the pk study were drawn at the following times: before start of iv infusion (0 h), at end of iv infusion (4 h) and 12, 24, 48 and 72 hours from start of iv infusion. Analyses were performed at the pharmacology and toxicology laboratory, Gradenigo Hospital, Torino-Italy; MTX concentrations were estimated by MTX II TDX (Abbott, USA), using a fluorescence polarization immunoassay.
Patients inclusion criteria
At accrual, only patients with histologically proved non-metastatic osteosarcoma were included. Staging procedures were by clinical and X-ray examination, CT scan, MRI and open biopsy of the primary tumor. Bone scan and lung CT scan were compulsory to exclude secondary involvement. These procedures were performed before starting chemotherapy and repeated at the conclusion of preoperative treatment, after surgery, at 3rd and 6th cycle and at the end of postoperative chemotherapy. Surgery was performed at the IOR, as were histo-pathological examination and necrosis evaluation.
Prior to entry into the pharmacokinetic study, patients had to fulfil the following eligibility criteria: diagnosis of non-metastatic osteosarcoma, measurable disease according to WHO criteria, age <40 years, ECOG Performance Status ≤2, absence of prior cancer history, serum creatinine ≤1.2 mg/dl, creatinine clearance >60 ml/min, serum bilirubin <1.2 mg/dl, AST/ALT <twice normal value, no severe or uncontrolled cardiovascular, metabolic, neurological or infectious diseases. HDMTX course was administered only when absolute neutrophils count >1 000/µl and platelets >60 000/µl. The Local Ethical Committee approved the protocol; all patients gave their informed consent in line with the Helsinki-Tokyo Declaration. Patient characteristics are summarized in .
Table I. Patient characteristics.
Pharmacokinetic and statistical analysis
The pharmacokinetic study entailed two different approaches: non-compartmental analysis (NCA) and population pk. NCA is a typical model-free approach, whose purpose is to provide an estimate of the kinetic parameters of a drug, like AUC or MRT (mean residence time), based on statistical moment theory. These parameters are determined by a numerical integration procedure, such as the trapezoidal rule; the only assumption is that the terminal elimination phase of the drug can be described by a monoexponential equation, following a first order kinetics Citation[16].
NCA was performed using Kinetica 3.0 software (InnaPhase Corp., USA), with the following parameters: Cmax (maximum concentration), AUCtot (total area under concentration-time curve), Kel (elimination rate constant), t1/2 (elimination half-life), MRT (mean residence time), Cltot (total clearance), Vz (volume of distribution of the late elimination phase) and Vss (volume of distribution at the steady state).
Population pharmacokinetics (pop pk) is the study of the variability in drug disposition between individuals when standard dosage regimens are administered. This methodology is capable of analyzing even sparse data, leading to a better prediction of the dose-response relationships. Population pk investigates how the so-called covariates, i.e. interindividual variability in demographic characteristics (age, sex, height, weight, race etc.) or physiopathological characteristics (transaminases, creatinine and other clinical laboratory parameters) can affect drug pk behavior, modifying pk parameters like volume of distribution (V) and elimination rate constant (Kel). Our modeling technique was based on an expectation-maximization algorithm (EM) for a non-linear mixed-effect model. This model takes into account both fixed and random effects, which are pk model parameters that respectively do not vary/vary between and within subjects Citation[17]. Such a model can predict MTX pk behavior in future patients and future cycles.
Population pk modeling was performed using Kinetica 4.1.1 software (InnaPhase Corp., USA), with the following parameters: V (volume of distribution), Kel (elimination rate constant), K12 (transfer rate constant from the central to the tissue compartment) and K21 (transfer rate constant from the tissue to the central compartment).
Descriptive statistics were performed using Instat 3.05 software (Graphpad, USA) and survival analysis by the Kaplan-Meier method, using SPSS 8.0 software (SPSS Inc., USA).
Results
A total of 31 patients (11 females and 20 males, median age 27.7 years; total 97 courses, course range 1 – 7; 27 pts neoadjuvant\adjuvant, 4 pts adjuvant-only setting) were enrolled in one of the three IOR protocols at our center. HDMTX therapy was generally well tolerated: only two cases of hematological and renal G4 toxicity were reported, both recovering in seven days. AUCtot was found to be closely correlated to these adverse events: in both subjects, AUCtot exceeded 12 000 µmol*h/l, significantly higher than it was in the no-severe-toxicity group (p < 0.001). On the contrary, these adverse reactions were not related with MTX Cmax, elimination rate constant or with total clearance.
In patients receiving HDMTX as neoadjuvant treatment (27/31), the degree of osteosarcoma necrosis correlated with the MTX pk parameters. Following Huvos’ classification, two degrees of necrosis were distinguished, good (>90%) and poor (< 90%) response Citation[18]: there were 13 good and 14 poor responders. MTX Cmax was significantly higher in the good responders than in the poor ones (p < 0.05).
Twenty-five patients of the total 31 (11 females and 14 males, median age 26.0 years; total 64 courses, course range 1 – 7; 22 pts neoadjuvant\adjuvant, 3 pts adjuvant-only setting) entered the pk study and were treated with HDMTX (12 g/m2 by 4 h iv infusion) and folinic acid rescue (8 mg/m2 q 6 h for 3 days). MTX median dose/cycle was equal to 19.8 g (range 16 – 23.2 g). A total of 64 HDMTX courses were administered (all evaluable), during which a total of 391 blood samples were collected.
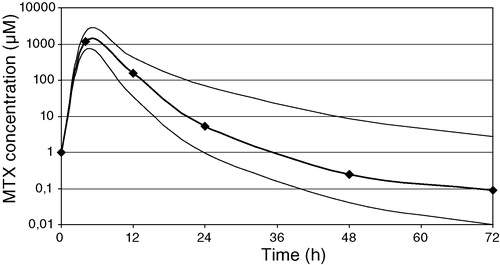
HDMTX disposition can be described in full through NCA (). Median Cmax was 1 149.5 µM, with large interpatient variability (range 692 – 2 200 µM). Cmax was >1 000 µM at the end of 4 h iv infusion in 75% of courses (48/64) and was found to be associated with increased histological response (p < 0.05), as has been suggested Citation[11]; higher serum concentrations did not offer any clinical advantage, confirming reported findings Citation[3], Citation[10–12]. At the same time, no difference in pk parameters was found when either MTX Cmax/AUCtot vs. neoadjuvant/adjuvant setting (p > 0.05) or MTX Cmax/AUCtot vs. patient age/sex (p > 0.05) were compared. AUCtot values were similarly scattered (range 3 477 – 12 681 µmol*h/l), with a median value of 6 955 µmol*h/l, very close to those reported in child studies. Cmax and AUCtot showed a linear relationship (r2 0.68). Median MTX t½ (late elimination phase half-time) was 4.02 hours, while median Cltot (total clearance) was 5.95 l/h, ranging from 3.03 to 11.00 l/h. The median (range) MTX concentration-time curve is plotted in .
Table IIa. NCA results.
A two-compartment pop pk model without covariates was first applied to the same data set. V and Kel mean values were 24.07 l and 0.32 1/h, while K12 and K21 mean values were 0.011 and 0.070 1/h, respectively. Ten covariates were then added to the model (sex-age-height-weight-hemoglobin-white blood cells-platelets-AST-ALT-creatinine) to test their possible effect on the pk parameters (V and Kel). As shown by model equations (), only six covariates affected pk behavior (height-weight-hemoglobin-AST-ALT-creatinine); the remaining four covariates (sex-age-white blood cells-platelets) did not contribute to parameter variation, which confirmed the NCA findings.
Table IIb. Population pk results.
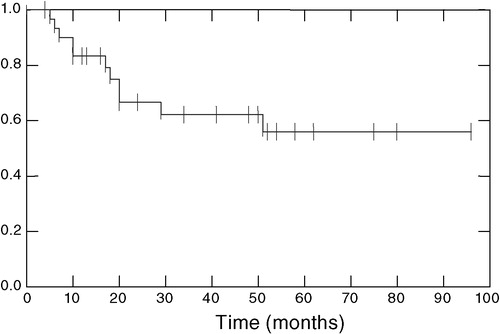
The overall survival curve (Kaplan-Meier method), measured from start of chemotherapy to patient follow-up/death, is presented in . At present, 20 patients are alive and disease-free and 11 have died from the disease.
Discussion
Osteogenic sarcoma typically affects children and most clinical and pk studies have involved adolescents; there is little pk information relating to HDMTX in adults Citation[3,13], and only one study suggested that children excrete MTX more effectively than adults Citation[4]. Consequently, many protocols exclude patients above 40 years of age from HDMTX treatment. The present study provides some data relating to HDMTX pk in adult subjects, who were entered into one of three different IOR Bologna protocols; 31 adult osteosarcoma patients were treated at our Institution with HDMTX regimens, 25 of them entering the pk study.
From a pharmacokinetic perspective, our results do not significantly differ from previous evidence from child studies. Interpatient variability with regard to the bioavailability pk parameters (Cmax and AUCtot) was similar to that reported for pediatric patients. Cmax and AUCtot were related neither to patient age/sex nor to neoadjuvant/adjuvant setting. Cmax was the only statistically significantly different pk parameter between good and poor responders; at the end of 4 h iv infusion, Cmax >1 000 µM predicted an optimal histological response without compromising the toxicity profile. These data are in good agreement with those reported for children Citation[11].
The population pk study revealed that the EM algorithm (a two-step Expectation-Maximization procedure) is an interesting iterative procedure for accurate estimation of individual MTX exposure; in our model, it is based on a two-compartment (central and tissutal) model. While the E-step produces an expectation of individual pk parameters, the M-step estimates population mean and variance of the same pk parameters (V and Kel) from the individual ones. A recent study by Rousseau applied a two-compartment pop pk model to a group of children (mean age 14.8 years±4.5) Citation[19], while the median age was about double in our group (26.0 years, range 16 – 39). The results from our pop pk model are in good agreement with the results of the NCA. In adult patients, MTX V and Kel values depend on the interindividual physiopathological variations of six different covariates (height-weight-hemoglobin-AST-ALT-creatinine). Since V * Kel=total clearance, these six covariates may modify the MTX excretion profile in adult subjects: from the regression coefficients in the model equations, it appears that the largest contribute to V modification is due to variations in weight, while creatinine levels are mainly responsible for alterations in Kel. On the contrary, sex and age play no direct role in MTX excretion, even when the drug is administered to adults at high dosages, thus confirming similar findings in younger patients.
From the clinical standpoint, contrasting results have been observed with regard to the efficacy of various HDMTX regimens. Delepine suggested MTX doses from 8 to 24 g/m2, considering dosage to be the most important factor for tumor response, whereas Jaffe found no difference in tumor necrosis with doses ranging from 7.5 to 12.5 g/m2Citation[7,20]. In our pharmacokinetic research, with HDMTX at 12 g/m2, no correlation was found between MTX Cmax, Kel, Cltot and protocol toxicity, confirming previous findings in adult patients Citation[13,14].
Toxicity was generally mild, although two severe adverse reactions (G4 neutropenia and renal impairment) occurred, clearly related to the very high AUCtot values. Most adverse events (nausea, vomiting, mucositis, diarrhea, transaminase increase) were low grade and successfully rescued by LV and forced hydration. There were neither toxic deaths nor any cases of chronic/late toxicity (renal or hepatic impairment, pulmonary fibrosis, CNS disturbances) after a median follow-up of 43.7 months.
In conclusion, HDMTX plays a key role in osteosarcoma treatment, in which knowledge of MTX pk can extend its safe use to an adult patient population. NCA and pop pk evaluation appear to be significant in predicting MTX sensitivity/resistance of osteosarcoma, forecasting and controlling interindividual variations in HDMTX excretion profile, optimizing dosage for future chemotherapy cycles and increasing our knowledge of the efficacy-exposure relationship.
References
- Bertino JR. Karnofsky memorial lecture. Ode to methotrexate. J Clin Oncol 1993; 11: 5–14
- Bertino JR. Clinical use of methotrexate with emphasis on use of high doses. Cancer Treat Rep 1981; 65(Suppl 1)131–5
- Graf N, Winkler K, Betlemovic M, Fuchs N, Bode U. Methotrexate pharmacokinetics and prognosis in osteosarcoma. J Clin Oncol 1994; 12: 1443–51
- Nirenberg A, Mosende C, Mehta BM, Gisolfi AL, Rosen G. High dose methotrexate with citrovorum factor rescue: predictive value of serum methotrexate concentrations and corrective measures to avert toxicity. Cancer Treat Rep 1997; 61: 779–83
- Ferrari S, Sassoli V, Orlandi M, Strazzari S, Puggioli C, Battistini A, et al. Serum methotrexate (MTX) concentrations and prognosis in patients with osteosarcoma of the extremities treated with a multidrug neoadjuvant regimen. J Chemother 1993; 5: 135–41
- Djerassi I, Farber S, Abir E, Neikirk W. Continuous infusion of methotrexate in children with acute leukemia. Cancer 1967; 20: 233–42
- Jaffe N. Recent advances in the chemotherapy of metastatic osteogenic sarcoma. Cancer 1972; 30: 1627–31
- Rosen G, Suwansirikul S, Kwon C, Tan C, Wu SJ, Beattie EJ, Jr, et al. High dose methotrexate with citrovorum factor rescue and adriamicyn in childhood osteogenic sarcoma. Cancer 1974; 33: 1151–63
- Frei E, 3rd, Jaffe N, Tattersall MH, Pitman S, Parker L. New approaches to cancer chemotherapy with methotrexate. N Engl J Med 1975; 292: 846–51
- Bacci G, Ferrari S, Delepine N, Bertoni F, Picci P, Mercuri M, et al. Predictive factors of histological response to primary chemotherapy in osteosarcoma of the extremity: study of 272 patients preoperatively treated with high dose methotrexate, doxorubicin and cisplatin. J Clin Oncol 1998; 16: 658–63
- Delepine N, Delepine G, Jasmin C, Desbois JC, Cornille H, Mathe G. Importance of age and methotrexate dosage: prognosis in children and young adults with high-grade osteosarcomas. Biomed Pharmacother 1988; 42: 257–62
- Gorlick R, Meyers P, Kellick M, Bertino JR. Monitoring methotrexate levels in osteosarcoma. J Clin Oncol 1998; 16: 2290–1
- Pignon T, Lacarelle B, Duffaud F, Guillet P, Catalin J, Durand A, et al. Pharmacokinetics of high-dose methotrexate in adult osteogenic sarcoma. Cancer Chemother Pharmacol 1994; 33: 420–4
- Raude E, Oellerich M, Weinel P, Freund M, Schrappe C, Riehm H, et al. High dose methotrexate: pharmacokinetics in children and young adults. Intl J Clin Pharmacol Ther Toxicol 1988; 26: 364–70
- Bacci G, Ferrari S, Bertoni F, Rueeieri P, Picci P, Longhi A, et al. Long-term outcome for patients with non-metastatic osteosarcoma of the extremity treated at the Istituto Ortopedico Rizzoli according to IOR/Osteosarcoma-2 protocol: an updated report. J Clin Oncol 2000; 18: 4016–27
- Shargel, L, Yu, A. Applied biopharmaceutics & pharmacokinetics. Appleton & Lange, New York 1999.
- Food and Drug Administration. Guidance for Industry Population Pharmacokinetic. U.S. Dept. of Health, Rockville 1999.
- Huvos AG, Rosen G, Marcove RC. Primary osteogenic sarcoma: pathologic aspects in 20 patients after treatment with chemotherapy en bloc resection, and prosthetic bone replacement. Arch Pathol Lab Med 1977; 101: 14–8
- Rousseau A, Sabot C, Delepine N, Delepine G, Debord J, Lachatre G, et al. Bayesian estimation of methotrexate pharmacokinetic parameters and area under the curve in children and young adults with localised osteosarcoma. Clin Pharmacokinet 2002; 41: 1095–104
- Jaffe N, Link MP, Cohen D, Traggis D, Frei E, 3rd, Wattis H, et al. High dose methotrexate in osteogenic sarcoma. NCI Monogr 1981; 56: 201–6